Abstract
Currently, human and veterinary medicine are threatened worldwide by an increasing resistance to carbapenems, particularly present in opportunistic Enterobacterales pathogens (e.g., Klebsiella spp.). However, there is a lack of comprehensive and comparable data on their occurrence in wastewater, as well as on the phenotypic and genotypic characteristics for various countries including Germany. Thus, this study aims to characterize carbapenem-resistant Klebsiella spp. isolated from municipal wastewater treatment plants (mWWTPs) and their receiving water bodies, as well as from wastewater and process waters from poultry and pig slaughterhouses. After isolation using selective media and determination of carbapenem (i.e., ertapenem) resistance using broth microdilution to apply epidemiological breakpoints, the selected isolates (n = 30) were subjected to WGS. The vast majority of the isolates (80.0%) originated from the mWWTPs and their receiving water bodies. In addition to ertapenem, Klebsiella spp. isolates exhibited resistance to meropenem (40.0%) and imipenem (16.7%), as well as to piperacillin-tazobactam (50.0%) and ceftolozan-tazobactam (50.0%). A high diversity of antibiotic-resistance genes (n = 68), in particular those encoding β-lactamases, was revealed. However, with the exception of blaGES-5-like, no acquired carbapenemase-resistance genes were detected. Virulence factors such as siderophores (e.g., enterobactin) and fimbriae type 1 were present in almost all isolates. A wide genetic diversity was indicated by assigning 66.7% of the isolates to 12 different sequence types (STs), including clinically relevant ones (e.g., ST16, ST252, ST219, ST268, ST307, ST789, ST873, and ST2459). Our study provides information on the occurrence of carbapenem-resistant, ESBL-producing Klebsiella spp., which is of clinical importance in wastewater and surface water in Germany. These findings indicate their possible dissemination in the environment and the potential risk of colonization and/or infection of humans, livestock and wildlife associated with exposure to contaminated water sources.
1. Introduction
Antimicrobial resistance (AMR) is currently considered one of the major threats to public health and modern healthcare worldwide [1]. In 2015, more than 670,000 infections in the European Union (EU) and European Economic Area (EEA) countries were caused by bacteria resistant to antibiotics, resulting in an estimated 33,000 deaths [2]. Of those, K. pneumoniae resistant to third-generation cephalosporins and carbapenems accounted for 84,500 infections in healthcare settings with approx. 6,000 attributable deaths, where this pathogen was most frequently associated with bloodstream and ventilator-associated pneumonia infections [2]. Furthermore, K. pneumoniae and K. oxytoca/K. michiganensis, which represent the most important clinical species, have been associated with community-acquired infections such as UTIs, meningitis, pneumonia and bacteraemia [3]. In addition, Klebsiella spp. are ubiquitous in the environment and have been recovered from surface water, soil, and plants [4].
At 11.3%, K. pneumoniae was one of the most commonly reported bacterial species in EU/EEA countries in 2019 among invasive isolates originating from blood or cerebrospinal fluid [5]. In Germany in 2019, less than 1% of clinical K. pneumoniae isolates exhibited resistance to carbapenems (i.e., imipenem and/or meropenem); meanwhile, several Southern and East European countries reported rates of more than 10% [6]. However, an increase in carbapenem resistance in K. pneumoniae isolates in Germany is clearly noticeable, with a rise from 0.1% in 2015 to 0.9% in 2019 [6]. Furthermore, significantly increasing trends are seen in EU/EEA population-weighted mean percentages of carbapenem resistance among K. pneumoniae isolates, from 6.8% in 2015 to 7.9% in 2019 [6].
Currently, the most clinically used carbapenems are meropenem and imipenem [7]. They are the sole, or one of the few, safe and efficacious therapies available for people affected by severe and polymicrobial infections caused by critical priority Gram-negative pathogens, such as multidrug-resistant (MDR) A. baumannii, P. aeruginosa, and various bacteria of the Enterobacteriaceae family [8]. However, amid increasing rates of resistance to carbapenems, e.g., due to the production of carbapenemases, the effectiveness of most β-lactam antimicrobials is compromised. Genes encoding clinically relevant carbapenemases (i.e., KPC, NDM, IMP, OXA-48-like, and VIM), are often located on mobile genetic elements such as plasmids, transposons and intergrons and can be exchanged between Enterobacteriaceae and other Gram-negative bacteria, contributing to their spread [9].
While the incidence of carbapenem-resistant Enterobacteriaceae (CRE) in the general population is still low (0.3–2.93 infections per 100,000 person-years in USA), they show a high potential to cause outbreaks in healthcare settings [10]. Through clinical wastewater, such high-risk bacterial pathogens are introduced into municipal wastewater systems and are discharged into surface water due to inadequate wastewater treatment. In Germany, a study by Kehl et al. (2021) demonstrated the discharge of a high-risk K. pneumoniae clone, ST147, carrying blaNDM and blaOXA-48 from a hospital into surface water [11]. Similar findings of carbapenem-resistant K. pneumoniae in rivers with high genetic concordance to clinical isolates have also been reported in other European countries [12,13,14,15].
Carbapenems are restricted to human use only and are not approved for use in veterinary medicine [8,16]. However, the risk of co-resistance to carbapenems, through the use of other antimicrobials in livestock or through horizontal gene transfer from human pathogens, cannot be ruled out [16]. CRE have been sporadically reported in the food chain in various European countries [17]. Carbapenem-resistant, and carbapenemase-producing K. pneumoniae have already been detected in poultry, chicken meat, cows and fish in countries that lack strict antimicrobial stewardship in livestock production [18,19,20,21]. However, the transmission of CRE from non-human sources is still limited. Nevertheless, antibiotic resistance remains a notable One Health problem, since not only animals and humans but also the environment is affected by CRE. The aquatic environment is of particular importance, since it provides a basic resource for all ecosystems, including agroecosystems, and holds a crucial role in the dissemination of AMR and their propagation between the natural environment, humans and other animals.
In Germany, the data are still lacking regarding the occurrence of the most clinically relevant species of Klebsiella spp. (i.e., K. pneumoniae and K. oxytoca) with resistance to carbapenems in wastewater, as well as their phenotypic and genotypic characteristics. Thus, the aim of this study is to evaluate the occurrence of carbapenem-resistant Klebsiella spp. in municipal wastewater treatment plants (mWWTPs) and their receiving water bodies, as well as in wastewater and process waters from poultry and pig slaughterhouses. In order to better assess their clinical relevance to public and environmental health, we also aim to characterize the recovered Klebsiella spp. isolates by applying phenotypic and genotypic methods.
2. Results
An overview of the phenotypic antimicrobial resistance of the investigated Klebsiella spp. isolates is presented in Figure 1. The isolates exhibit various phenotypic resistances to antimicrobials, inter alia to those highly and critically important for humans (Figure 1).
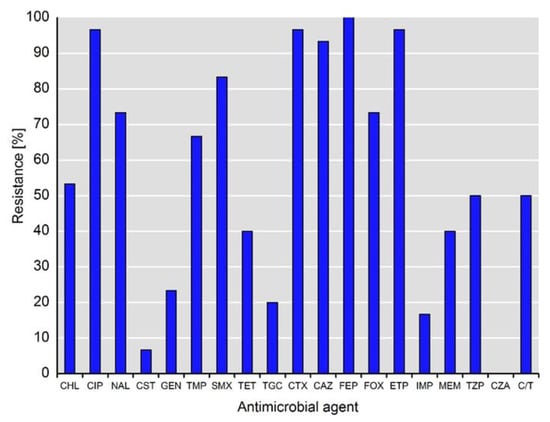
Figure 1.
Phenotypical resistance to antimicrobial agents detected among isolates of Klebsiella spp. (n = 30). Abbreviations for antimicrobial agents: CHL, chloramphenicol; CIP, ciprofloxacin; NAL, nalidixic acid; CST, colistin; GEN, gentamicin; TMP, trimethoprim; SMX, sulfamethoxazole; TET, tetracycline; TGC, tigecycline; CTX, cefotaxime; CAZ, ceftazidime; FEP, cefepime; FOX, cefoxitin; ETP, ertapenem; IMI, imipenem; MEM, meropenem; TZP, piperacillin-tazobactam; CZA, ceftazidime-avibactam; C/T, ceftolozane-tazobactam.
As expected, the resistance rates to third- and fourth-generation cephalosporins (i.e., cefotaxime, ceftazidime, cefepime) were high and ranged from 93.3% (28/30) to 100%, whereas the rate of resistance to cefoxitin was lower at 73.3% (22/30). In addition to ertapenem resistance (96.7%, 29/30), as a selection criterion for this study, 16.7% (5/30) and 40.0% (12/30) of the isolates were resistant to imipenem and meropenem, respectively. Notably, 50% (15/30) of the isolates showed resistance to piperacillin-tazobactam and ceftolozan-tazobactam, whereas all of the isolates were susceptible to ceftazidime-avibactam. Almost all of the isolates (96.7%, 29/30) exhibited resistance to fluoroquinolones (i.e., ciprofloxacin), whereas only two isolates (6.7%) were resistant to colistin. Of note, the resistance rates to tigecycline and gentamicin were 20.0% (6/30) and 23.3% (7/30), respectively. The phenotypic resistance patterns of individual isolates are shown in Table 1.

Table 1.
Selected phenotypic and genotypic characteristics of carbapenem-resistant Klebsiella spp. isolates recovered from municipal WWTPs and their receiving water bodies as well as from process waters of poultry and pig slaughterhouses.
Klebsiella spp. isolates represented a reservoir for 68 different ARGs (antimicrobial resistance genes) conferring resistance to antimicrobials belonging to 11 different classes (Table 2).

Table 2.
Prevalence of antibiotic-resistance genes detected in carbapenem-resistant Klebsiella spp. isolates recovered from municipal WWTPs and their receiving water bodies as well as from process waters of poultry and pig slaughterhouses.
Of the detected ARGs, 25 encoded β-lactamases which, as expected, were found in all isolates. They encoded enzymes of seven families: blaCTX-M, blaTEM, blaSHV, blaOXA, blaOXY, blaGES, and blaOKP. The most abundant were blaCTX-M-15, blaTEM-1B and blaOXY-2-8-like, accounting for 36.7% (11/30), 30.0% (9/30) and 20.0% (6/30) of the isolates, respectively; and blaOXA-1, blaSHV-1 and blaOXA-10 were each detected in 16.7% (5/30) of the isolates. Of note, combinations of up to four β-lactamases were detected in K. pneumoniae isolates from both in- and effluent of mWWTP. Interestingly, blaOXA-1 and blaOXA-10, in combination with other extended spectrum β-lactamases of SHV and TEM families, were found to a large extent in isolates with resistance to piperacillin-tazobactam and ceftolozan-tazobactam (Table 1). No carbapenemases were detected, with the exception of blaGES-5-like carried by a K. pneumoniae isolate recovered from the effluent of mWWTP. Thus, resistance could be mediated by chromosomal alterations.
Among the Klebsiella spp. isolates of this study, only some of the genes could be backed to mobile genetic elements. Using the MobileElementFinder tool (version 1.0) from the Center for Genomic Epidemiology, some of the genes could be associated with plasmid or insertion sequences (IS) (Table S1). Interestingly, IncR plasmids often comprise a combination of the genes aph(3″)-Ib-aph(6)-Id, leading to a streptomycin resistance phenotype, while some other resistance genes coding for resistances against extended-spectrum β-lactamase antibiotics, tetracycline and sulphonamides were found on other plasmid types (based on the Inc-groups). Overall, the majority of the isolates exhibit a broad diversity of different IS, but mainly IS elements such as ISVsa3, ISAhy2, ISEc9, IS6100, ISKpn19 were found to be associated with resistance determinants. Based on the prevailing data, it cannot be excluded that further genes will be associated with plasmid or IS sequences, due to the use of short-read sequencing data for in silico analysis. Further information about the impact of the plasmids or IS elements on the spread of resistances needs to be determined in detail in another study.
The results of the multilocus sequence-typing (MLST), performed to identify high-risk clones of public health importance, showed that 66.7% (20/30) of the isolates could be assigned to 12 different sequence types (STs). Three isolates each belonged to ST503 and ST2459. ST873, ST458, ST268 and ST252 each accounted for two isolates, whereas the remaining six isolates were identified as ST789, ST441, ST307, ST219, ST1948 and ST16. The STs of seven K. oxytoca and three K. pneumoniae isolates (Table 1) could not be determined using the prevailing genotyping schemes, possibly indicating new STs.
Among known virulence factors, genes encoding various siderophores, and fimbriae were detected (Table 3). Almost all isolates (96.7%, 29/30) carried genes coding for the enterobactin siderophore system, whereas yersiniabactin, salmochelin, and aerobactin were less prevalent and accounted for 40.0% (12/30) and 33.3% (10/30) of the isolates, respectively. Fimbriae type 1 and fimbriae type 3 were detected in 90.0% (27/30) and 63.3% (19/30) of the isolates, respectively.

Table 3.
Virulence factors detected in carbapenem-resistant Klebsiella spp. isolates recovered from municipal WWTPs and their receiving water bodies as well as from process waters of poultry and pig slaughterhouses.
Surface polysaccharide locus typing, performed in order to determine the capsule (K antigen) serotypes, showed that the capsule polysaccharide (CPS) types of the vast majority of the isolates (80.0%, 24/30) could be assigned to 14 different types. Three isolates each accounted for KL9 and KL60. KL81, KL74, KL62, KL52, KL21 and KL20 were each represented by two isolates, whereas the remaining six isolates were assigned to KL51, KL24, KL18, KL151, KL114 and KL102.
3. Discussion
This study provides novel data on antimicrobial resistance, genetic lineages, virulence factors and CPS-types of carbapenem-resistant (CR) Klebsiella spp. from municipal WWTPs as well as process waters and wastewater from German poultry and pig slaughterhouses.
The occurrence of ESBL-producing and CR Klebsiella spp. in municipal WWTPs indicates its possible dissemination in the general population and the impact of clinical effluents on the municipal sewer system. Wielders and colleagues (2017) reported an overall prevalence of ESBL-producing K. pneumoniae in the general population in the Netherlands of 4.3%, with seasonal differences ranging from 2.6% to 7.4% [22]. Meijs and colleagues (2021) reported even higher levels of ESBL-producing K. pneumoniae carriage for veterinary healthcare workers in the Netherlands of 9.8%, emphasizing occupational contact with animals as a potential source of ESBL-producing K. pneumoniae in the general population [23]. Several studies have reported high abundances of carbapenemase-producing Klebsiella spp. in clinical wastewater and its discharge into the municipal sewer system [11,24,25,26]. The subsequent incidence of such bacteria in surface waters suggests that conventional biological treatment is insufficient in terms of eliminating microbial loads, and shows the negative impact of inadequately treated wastewater on surface waters. Similar findings on ESBL, and on carbapenemase-producing K. pneumoniae in Austrian and Swiss rivers mostly within urbanized areas, also highlight the anthropological pollution in aquatic environments [13,14]. Klebsiella spp. are known for their ability to survive under adverse conditions and are widely distributed in nature, including in surface water and nutrient rich wastewater [27]. Lepuschitz and colleagues (2019) recovered two multidrug-resistant K. pneumoniae ST985 isolates, which share the same cgMLST profile, from sampling sites on a river 200 km apart, demonstrating the possible survival distance of K. pneumoniae in river water [13]. A study by Rocha and colleagues (2022) suggests that Klebsiella spp. isolates in wastewater retain clinically relevant features, including those acquired through HGT, even after treatment. Thus, further dissemination of the CR isolates recovered in our study among animals and humans, and their colonization and/or infection cannot be ruled out [28]. The application of state-of-the-art wastewater treatment techniques based on oxidative, adsorptive, and membrane-based technologies, as well as the establishment of a surveillance system for clinically relevant antimicrobial-resistant bacteria in surface water should be encouraged.
In this study, almost all isolates exhibited resistance to ciprofloxacin, which is considered to be critically important in human medicine and is often administered to outpatients [29]. Thus, the use of (fluoro)quinolones may contribute to the selection of CR Klebsiella spp. in the general community. This finding is in line with the EDCD report indicating that resistance to carbapenems is almost always combined with resistance to other antimicrobial classes, severely narrowing the treatment options for invasive infections caused by “critical pathogens” (i.e., CR A. baumannii, P. aeruginosa and Enterobacteriaceae) and decreasing the likelihood of a positive outcome [6].
Resistance to carbapenems at a clinical level is most frequently caused by the production of carbapenemases, however, other mechanisms may also be involved in the development of such phenotypes [30]. In this study, the results of antimicrobial susceptibility testing were interpreted based on epidemiological cut-off values. This allows the detection of early changes in resistance patterns that could possibly lead to resistance at a clinical level. Nevertheless, no clinically relevant carbapenemases were detected, with only one K. pneumoniae isolate from the effluent of mWWTP carrying blaGES-5. Since no carbapenemases and AmpC β-lactamases were detected, possible mechanisms of resistance to carbapenems, and combinations of β-lactam–β-lactamase inhibitor (i.e., piperacillin-tazobactam and ceftolozane-tazobactam), could be: changes in membrane permeability due to mutations in the genes encoding efflux pump, alterations in the expression and function of porins, and the association of impermeability with the production of ESBL [30]. Furthermore, blaOXA-1, which encodes a penicillinase with weak affinity for inhibitors such as tazobactam, and hyperproduction of blaTEM-1 could also be responsible for resistance to piperacillin-tazobactam [31,32]. Narrow-spectrum oxacillinases, e.g., OXA-10-type class D β-lactamases, were previously shown to exhibit weak carbapenemase activity at a level comparable with that of OXA-58 [33,34]. Genes encoding class D β-lactamases are commonly found in P. aeruginosa; however, they are also detected in Enterobacteriaceae, albeit with lower abundance than in Pseudomonas spp., underlying the important role of horizontal gene transfer (HGT) in the spread of AMRs [35]. Resistance to ceftolozane-tazobactam, and ceftazidime-avibactam have been reported in clinical MDR/extensively-drug resistant (XDR) P. aeruginosa isolates due to mutations in blaOXA-10 which developed during antimicrobial treatment [36].
K. pneumoniae isolates belonging to the sequence types (STs) determined in this study (ST16, ST252, ST219, ST268, ST307, ST789, ST873, and ST2459) have been detected in various clinical settings across Europe and Asia, causing urinary and respiratory tract infections [37,38,39,40,41,42]. All of them carried different carbapenemases belonging to NDM, OXA, IMP, and KPC families, with some of them exhibiting an extensively drug-resistant (XDR) phenotype. In Europe, the spread of carbapenemases among K. pneumoniae is frequently linked to specific clonal lineages such as ST11, ST15, ST101, ST258/512 and their derivatives [43]. However, novel high-risk CR K. pneumoniae lineages are continuously emerging. Wyres and colleagues (2018) showed that some K. pneumoniae clones are generally better than others at acquiring genetic material via HGT [44]. Currently, comprehensive data on the mechanisms underlying this phenomenon are still lacking, and experimental studies are needed for its further investigation. Interestingly, K. pneumoniae ST789 carrying blaNDM-5 has been reported in neonates in China, and was classified as a novel high-risk CR lineage [45]. In our study K. pneumoniae ST789 was detected in wastewater from poultry eviscerators and was carrying blaSHV-25. However, given the potential of some Klebsiella spp. to become high-risk clonal lineage, the possible factors contributing to increased virulence and antimicrobial resistance that might occur in livestock production need to be investigated. This would help develop mitigation strategies in order to interrupt possible dissemination of CRE from livestock to humans. ESBL-producing Enterobacteriaceae can serve as a basic model for its spread, since the bacterial species involved are the same and the antimicrobial resistance genes are located on plasmids as well.
The contamination of food of animal and vegetable origin with ESBL-producing Enterobacteriaceae is already well described [46,47,48,49,50]. However, considering the risks of CRE to human health, there have been appeals for a zero-tolerance policy and an international ban on the sale of food contaminated with CRE [46]. The fact that carbapenems are not approved for use in veterinary medicine and are predominantly used in human hospital settings can explain the low incidence of carbapenem resistance among the isolates recovered from poultry and pig slaughterhouses. These findings are in line with other reports indicating the absence of or single cases of CRE in European livestock, in particular pigs and broilers [51]. Nevertheless, the risk of AMR transmission through horizontal gene transfer from human pathogens or of co-resistance through the use of other antimicrobials in agriculture cannot be ruled out.
According to epidemiological studies, the first step in the majority of K. pneumoniae infections is the colonization of the host’s gastrointestinal tract [52]. The recovered isolates carried genes encoding fimbrial adhesins (type 1 and type 3 fimbriae), which play an essential role in adhesion to the host’s mucosal surfaces and in biofilm formation, as well as genes encoding components of siderophore systems that mediate the uptake of ferric iron [53]. The presence of these virulence factors increases the probability of adherence to the host, colonization, and invasive infections. These data reinforce the recent trend of increasing occurrence of community-acquired K. pneumoniae infections in young and healthy individuals, rather than primarily nosocomial infections in immunocompromised patients [54]. However, in comparison to clinical isolates, all of the recovered isolates lacked the factors responsible for the hypermucoviscous phenotype that protects the bacteria against opsonization and phagocytosis.
4. Materials and Methods
The sampling sites, procedures and preparation of the samples have been previously described [55,56]. Briefly, the process waters and wastewater (n = 87) arising during the operation and cleaning of production facilities were collected from the delivery areas (transport trucks, transport crates, and holding pens) and unclean areas (stunning facilities, scalders, eviscerators, and aggregate wastewater from production facilities) of two poultry and two pig slaughterhouses. In- and effluents (n = 62) from their in-house wastewater treatment plants (WWTPs) were also sampled. Further samples (n = 36) were taken at two municipal WWTPs (mWWTPs) receiving pretreated wastewater from the pig slaughterhouses, including their on-site preflooders upstream and downstream from the discharge points [55]. At each sampling site, 1 L of water was collected using sterile Nalgene Wide Mouth Environmental Sample Bottles (Thermo Fisher Scientific, Waltham, MA, USA). For further information on selected characteristics of the sampled slaughterhouses, sampling sites and number of samples taken at each sampling site, please see [55,56].
Klebsiella spp. isolates with resistance to third-generation cephalosporins, and carbapenems were recovered from water samples by selective cultivation on CHROMagar ESBL and CHROMagar mSuperCarba plates (MAST Diagnostica, Reinfeld, Germany), as previously described [56]. Presumptive colonies of Klebsiella spp. were unselectively sub-cultured on Columbia Agar supplemented with 5% sheep blood (v/v) (Mast Diagnostics, Reinfeld, Germany). Species identification for the individual isolates was conducted using MALDI-ToF MS (bioMérieux, Marcy-l’Étoile, France) equipped with the Myla software.
Antimicrobial susceptibility testing was performed according to CLSI guidelines (M07-A10), using broth microdilution and applying the epidemiological cut-off values (ECOFFs) from the European Committee on Antimicrobial Susceptibility Testing (EUCAST). In order to assess the clinical relevance of the presumptive ESBL-producing and carbapenem-resistant Klebsiella spp. isolates for human medicine, they were tested against the newly approved β-lactam/β-lactamase inhibitor combinations ceftazidime-avibactam, ceftolozan-tazobactam, and piperacillin-tazobactam, by a microdilution method using the clinical cut-off values as previously described [56,57].
A total of 185 Klebsiella spp. (155 K. pneumoniae, 30 K. oxytoca) were isolated, of which 30 (16.2%), comprising 23 K. pneumoniae and 7 K. oxytoca, showed resistance to at least one of the tested carbapenems (i.e., ertapenem, imipenem, meropenem), and were further investigated in detail. The vast majority (80%, 24/30) originated from mWWTPs (influent, n = 12; effluent, n = 7) and their on-site preflooders upstream (n = 4) and downstream (n = 1) from the discharge points. Further isolates were recovered from the process waters and wastewater accruing in poultry (stunning facilities, n = 2; eviscerators, n = 1) and pig slaughterhouses (pig transporters, n = 1; holding pens, n = 1; influent in-house chemical-physical WWTP, n = 1).
Extraction of genomic DNA (gDNA) from the individual colonies of Klebsiella spp., DNA library preparation, and whole-genome sequencing (WGS) were performed as previously described [58]. Briefly, gDNA was extracted using PureLink® Genomic DNA Mini Kit (Invitrogen, Darmstadt, Germany) following the manufacturer’s instructions. Commercial DNA library preparation and WGS were conducted using LGC Genomics GmbH (Berlin, Germany) on an Illumina NextSeq 500/550 V2 (Illumina, CA, USA). De novo assembly of high-quality ~150 bp paired-end sequencing reads was conducted using the SPAdes algorithm of the PATRIC database (v. 3.5.27) [59]. ResFinder v 3.0 and MLST v 2.0 under default values, as well as MyDbFinder (release 1.1; parameters: 90% sequence identity, 60% sequence coverage) of the Center for Genomic Epidemiology (https://cge.cbs.dtu.dk/services/ (accessed on 10 December 2020)) were used for bioinformatics analysis of ARGs, sequence types (STs) and virulence factors, respectively [60]. The captive tool (https://kaptive-web.erc.monash.edu/ (accessed on 10 December 2020)) was used for surface polysaccharide locus typing and variant evaluation. The tool MobileElementFinder (Center for Genomic Epidemiology, version 1.0, default parameters; https://cge.cbs.dtu.dk/services/MobileElementFinder/ (accessed on 10 March 2022) was used for the prediction of mobile genetic elements (MGEs) in combination with plasmid or insertion sequences.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/antibiotics11040435/s1, Table S1: Mobile genetic elements detected in carbapenem-resistant Klebsiella spp. isolates recovered from municipal WWTPs and their receiving water bodies as well as from process waters of poultry and pig slaughterhouses.
Author Contributions
Conceptualization, M.S.; methodology, M.S. and J.A.H.; formal analysis, M.S., I.G.-M., S.S. and J.A.H.; investigation, M.S., I.G.-M., S.S. and J.A.H.; data curation, M.S.; writing—original draft preparation, M.S.; writing—review and editing, G.B., N.T.M., R.M.S., J.K., A.K. and J.A.H.; project administration, M.S. and J.A.H.; funding acquisition, J.K. and J.A.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financed by the BMBF (Federal Ministry of Education and Research) funding measure HyReKA [02WRS1377], internal grants from BfR (43-001, 43-002 and 1322-648), the European Joint Projects (EJP) “ARDIG” and “FULL_FORCE” funded by the European Union’s Horizon 2020 research and innovation programme (Grant Agreement No 773830), as well as by the project “GÜCCI” funded by the German Federal Ministry of Health. The funders had no role in the study design, data collection and interpretation, nor in the decision to submit the work for publication. I. García-Meniño acknowledges the Consellería de Cultura, Educación e Ordenación Universitaria, Xunta de Galicia for the post-doctoral grant (Grant Number: ED481B-2021-006).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data for this study have been uploaded to Sequence Read Archive (SRA). The accession number for the bioproject is PRJNA816413 and the individual biosamples are aslo available there. The temporary number will change as soon as the raw reads are processed by NCBI.
Acknowledgments
We thank the staff of the participating municipal WWTPs and slaughterhouses for their kind cooperation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- De Kraker, M.E.A.; Stewardson, A.J.; Harbarth, S. Will 10 Million People Die a Year due to Antimicrobial Resistance by 2050? PLoS Med. 2016, 13, e1002184. [Google Scholar] [CrossRef] [PubMed]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- Weiner, L.M.; Webb, A.K.; Limbago, B.; Dudeck, M.A.; Patel, J.; Kallen, A.J.; Edwards, J.R.; Sievert, D.M. Antimicrobial-Resistant Pathogens Associated With Healthcare-Associated Infections: Summary of Data Reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect. Control Hosp. Epidemiol. 2016, 37, 1288–1301. [Google Scholar] [CrossRef]
- Zumla, A. Mandell, Douglas, and Bennett’s principles and practice of infectious diseases. Lancet Infect. Dis. 2010, 10, 303–304. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Carbapenem-Resistant Enterobacteriaceae, Second Update—26 September 2019. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/carbapenem-resistant-enterobacteriaceae-risk-assessment-rev-2.pdf (accessed on 10 February 2022).
- European Centre for Disease Prevention and Control. Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report 2019. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/surveillance-antimicrobial-resistance-Europe-2019.pdf (accessed on 10 February 2022).
- Baughman, R.P. The use of carbapenems in the treatment of serious infections. J. Intensive Care Med. 2009, 24, 230–241. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for the Prevention and Control of Carbapenem-Resistant Enterobacteriaceae, Acinetobacter baumannii and Pseudomonas aeruginosa in Health Care Facilities. Available online: https://apps.who.int/iris/bitstream/handle/10665/259462/9789241550178-eng.pdf?sequence=1&isAllowed=y (accessed on 10 February 2022).
- Tzouvelekis, L.S.; Markogiannakis, A.; Psichogiou, M.; Tassios, P.T.; Daikos, G.L. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: An evolving crisis of global dimensions. Clin. Microbiol. Rev. 2012, 25, 682–707. [Google Scholar] [CrossRef]
- Livorsi, D.J.; Chorazy, M.L.; Schweizer, M.L.; Balkenende, E.C.; Blevins, A.E.; Nair, R.; Samore, M.H.; Nelson, R.E.; Khader, K.; Perencevich, E.N. A systematic review of the epidemiology of carbapenem-resistant Enterobacteriaceae in the United States. Antimicrob. Resist. Infect. Control 2018, 7, 55. [Google Scholar] [CrossRef]
- Kehl, K.; Schallenberg, A.; Szekat, C.; Albert, C.; Sib, E.; Exner, M.; Zacharias, N.; Schreiber, C.; Parčina, M.; Bierbaum, G. Dissemination of carbapenem resistant bacteria from hospital wastewater into the environment. Sci. Total Environ. 2022, 806, 151339. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.A.; Hellmark, B.; Ehricht, R.; Söderquist, B.; Jass, J. Related carbapenemase-producing Klebsiella isolates detected in both a hospital and associated aquatic environment in Sweden. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 2241–2251. [Google Scholar] [CrossRef]
- Lepuschitz, S.; Schill, S.; Stoeger, A.; Pekard-Amenitsch, S.; Huhulescu, S.; Inreiter, N.; Hartl, R.; Kerschner, H.; Sorschag, S.; Springer, B.; et al. Whole genome sequencing reveals resemblance between ESBL-producing and carbapenem resistant Klebsiella pneumoniae isolates from Austrian rivers and clinical isolates from hospitals. Sci. Total Environ. 2019, 662, 227–235. [Google Scholar] [CrossRef]
- Bleichenbacher, S.; Stevens, M.J.A.; Zurfluh, K.; Perreten, V.; Endimiani, A.; Stephan, R.; Nüesch-Inderbinen, M. Environmental dissemination of carbapenemase-producing Enterobacteriaceae in rivers in Switzerland. Environ. Pollut. 2020, 265, 115081. [Google Scholar] [CrossRef]
- Teban-Man, A.; Farkas, A.; Baricz, A.; Hegedus, A.; Szekeres, E.; Pârvu, M.; Coman, C. Wastewaters, with or without Hospital Contribution, Harbour MDR, Carbapenemase-Producing, but Not Hypervirulent Klebsiella pneumoniae. Antibiotics 2021, 10, 361. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. Drivers, Dynamics and Epidemiology of Antimicrobial Resistance in Animal Production. Available online: https://www.fao.org/3/i6209e/i6209e.pdf (accessed on 10 February 2022).
- The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2017. EFSA J. 2019, 17, e05598. [CrossRef]
- Hamza, E.; Dorgham, S.M.; Hamza, D.A. Carbapenemase-producing Klebsiella pneumoniae in broiler poultry farming in Egypt. J. Glob. Antimicrob. Resist. 2016, 7, 8–10. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Wang, Y.; Sun, L.; Pang, M.; Zhang, L.; Wang, R. Occurrence and characterization of blaNDM-5-positive Klebsiella pneumoniae isolates from dairy cows in Jiangsu, China. J. Antimicrob. Chemother. 2017, 72, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Diab, M.; Hamze, M.; Bonnet, R.; Saras, E.; Madec, J.-Y.; Haenni, M. OXA-48 and CTX-M-15 extended-spectrum beta-lactamases in raw milk in Lebanon: Epidemic spread of dominant Klebsiella pneumoniae clones. J. Med. Microbiol. 2017, 66, 1688–1691. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, H.M.; Reuland, E.A.; Wintermans, B.B.; Al Naiemi, N.; Koek, A.; Abdelwahab, A.M.; Ammar, A.M.; Mohamed, A.A.; Vandenbroucke-Grauls, C.M.J.E. Extended-Spectrum β-Lactamases and/or Carbapenemases-Producing Enterobacteriaceae Isolated from Retail Chicken Meat in Zagazig, Egypt. PLoS ONE 2015, 10, e0136052. [Google Scholar] [CrossRef]
- Wielders, C.C.H.; van Hoek, A.H.A.M.; Hengeveld, P.D.; Veenman, C.; Dierikx, C.M.; Zomer, T.P.; Smit, L.A.M.; van der Hoek, W.; Heederik, D.J.; de Greeff, S.C.; et al. Extended-spectrum β-lactamase- and pAmpC-producing Enterobacteriaceae among the general population in a livestock-dense area. Clin. Microbiol. Infect. 2017, 23, 120.e1–120.e8. [Google Scholar] [CrossRef]
- Meijs, A.P.; Gijsbers, E.F.; Hengeveld, P.D.; Dierikx, C.M.; De Greeff, S.C.; van Duijkeren, E. ESBL/pAmpC-producing Escherichia coli and Klebsiella pneumoniae carriage among veterinary healthcare workers in the Netherlands. Antimicrob. Resist. Infect. Control 2021, 10, 147. [Google Scholar] [CrossRef]
- Müller, H.; Sib, E.; Gajdiss, M.; Klanke, U.; Lenz-Plet, F.; Barabasch, V.; Albert, C.; Schallenberg, A.; Timm, C.; Zacharias, N.; et al. Dissemination of multi-resistant Gram-negative bacteria into German wastewater and surface waters. FEMS Microbiol. Ecol. 2018, 94, fiy057. [Google Scholar] [CrossRef] [PubMed]
- Kizny Gordon, A.E.; Mathers, A.J.; Cheong, E.Y.L.; Gottlieb, T.; Kotay, S.; Walker, A.S.; Peto, T.E.A.; Crook, D.W.; Stoesser, N. The Hospital Water Environment as a Reservoir for Carbapenem-Resistant Organisms Causing Hospital-Acquired Infections-A Systematic Review of the Literature. Clin. Infect. Dis. 2017, 64, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Surleac, M.; Czobor Barbu, I.; Paraschiv, S.; Popa, L.I.; Gheorghe, I.; Marutescu, L.; Popa, M.; Sarbu, I.; Talapan, D.; Nita, M.; et al. Whole genome sequencing snapshot of multi-drug resistant Klebsiella pneumoniae strains from hospitals and receiving wastewater treatment plants in Southern Romania. PLoS ONE 2020, 15, e0228079. [Google Scholar] [CrossRef] [PubMed]
- Cherak, Z.; Loucif, L.; Moussi, A.; Rolain, J.-M. Carbapenemase-producing Gram-negative bacteria in aquatic environments: A review. J. Glob. Antimicrob. Resist. 2021, 25, 287–309. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.; Ferreira, C.; Mil-Homens, D.; Busquets, A.; Fialho, A.M.; Henriques, I.; Gomila, M.; Manaia, C.M. Third generation cephalosporin-resistant Klebsiella pneumoniae thriving in patients and in wastewater: What do they have in common? BMC Genom. 2022, 23, 72. [Google Scholar] [CrossRef]
- WHO Advisory Group on Integrated Surveillance. Critically Important Antimicrobials for Human Medicine: 6th Revision 2018. Ranking of Medically Important Antimicrobials for Risk Management of Antimicrobial Resistance Due to Non-Human Use. Available online: https://www.who.int/publications/i/item/9789241515528 (accessed on 10 February 2022).
- Nordmann, P.; Poirel, L. Epidemiology and Diagnostics of Carbapenem Resistance in Gram-negative Bacteria. Clin. Infect. Dis. 2019, 69, S521–S528. [Google Scholar] [CrossRef]
- Hubbard, A.T.M.; Mason, J.; Roberts, P.; Parry, C.M.; Corless, C.; van Aartsen, J.; Howard, A.; Bulgasim, I.; Fraser, A.J.; Adams, E.R.; et al. Piperacillin/tazobactam resistance in a clinical isolate of Escherichia coli due to IS26-mediated amplification of blaTEM-1B. Nat. Commun. 2020, 11, 4915. [Google Scholar] [CrossRef]
- Livermore, D.M.; Day, M.; Cleary, P.; Hopkins, K.L.; Toleman, M.A.; Wareham, D.W.; Wiuff, C.; Doumith, M.; Woodford, N. OXA-1 β-lactamase and non-susceptibility to penicillin/β-lactamase inhibitor combinations among ESBL-producing Escherichia coli. J. Antimicrob. Chemother. 2019, 74, 326–333. [Google Scholar] [CrossRef]
- Antunes, N.T.; Fisher, J.F. Acquired Class D β-Lactamases. Antibiotics 2014, 3, 398–434. [Google Scholar] [CrossRef]
- Kotsakis, S.D.; Flach, C.-F.; Razavi, M.; Larsson, D.G.J. Characterization of the First OXA-10 Natural Variant with Increased Carbapenemase Activity. Antimicrob. Agents Chemother. 2019, 63, 4–6. [Google Scholar] [CrossRef]
- Maurya, A.P.; Dhar, D.; Basumatary, M.K.; Paul, D.; Ingti, B.; Choudhury, D.; Talukdar, A.D.; Chakravarty, A.; Mishra, S.; Bhattacharjee, A. Expansion of highly stable bla OXA-10 β-lactamase family within diverse host range among nosocomial isolates of Gram-negative bacilli within a tertiary referral hospital of Northeast India. BMC Res. Notes 2017, 10, 145. [Google Scholar] [CrossRef]
- Arca-Suárez, J.; Lasarte-Monterrubio, C.; Rodiño-Janeiro, B.-K.; Cabot, G.; Vázquez-Ucha, J.C.; Rodríguez-Iglesias, M.; Galán-Sánchez, F.; Beceiro, A.; González-Bello, C.; Oliver, A.; et al. Molecular mechanisms driving the in vivo development of OXA-10-mediated resistance to ceftolozane/tazobactam and ceftazidime/avibactam during treatment of XDR Pseudomonas aeruginosa infections. J. Antimicrob. Chemother. 2021, 76, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Heiden, S.E.; Hübner, N.-O.; Bohnert, J.A.; Heidecke, C.-D.; Kramer, A.; Balau, V.; Gierer, W.; Schaefer, S.; Eckmanns, T.; Gatermann, S.; et al. A Klebsiella pneumoniae ST307 outbreak clone from Germany demonstrates features of extensive drug resistance, hypermucoviscosity, and enhanced iron acquisition. Genome Med. 2020, 12, 113. [Google Scholar] [CrossRef] [PubMed]
- Bathoorn, E.; Rossen, J.W.; Lokate, M.; Friedrich, A.W.; Hammerum, A.M. Isolation of an NDM-5-producing ST16 Klebsiella pneumoniae from a Dutch patient without travel history abroad, August 2015. Euro Surveill. 2015, 20, 30040. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Papagiannitsis, C.C.; Dolejska, M.; Izdebski, R.; Dobiasova, H.; Studentova, V.; Esteves, F.J.; Derde, L.P.G.; Bonten, M.J.M.; Hrabák, J.; Gniadkowski, M. Characterization of pKP-M1144, a Novel ColE1-Like Plasmid Encoding IMP-8, GES-5, and BEL-1 β-Lactamases, from a Klebsiella pneumoniae Sequence Type 252 Isolate. Antimicrob. Agents Chemother. 2015, 59, 5065–5068. [Google Scholar] [CrossRef]
- Kiaei, S.; Moradi, M.; Hosseini-Nave, H.; Ziasistani, M.; Kalantar-Neyestanaki, D. Endemic dissemination of different sequence types of carbapenem-resistant Klebsiella pneumoniae strains harboring blaNDM and 16S rRNA methylase genes in Kerman hospitals, Iran, from 2015 to 2017. Infect. Drug Resist. 2019, 12, 45–54. [Google Scholar] [CrossRef]
- Van Duijkeren, E.; Wielders, C.C.H.; Dierikx, C.M.; van Hoek, A.H.A.M.; Hengeveld, P.; Veenman, C.; Florijn, A.; Lotterman, A.; Smit, L.A.M.; van Dissel, J.T.; et al. Long-term Carriage of Extended-Spectrum β-Lactamase-Producing Escherichia coli and Klebsiella pneumoniae in the General Population in The Netherlands. Clin. Infect. Dis. 2018, 66, 1368–1376. [Google Scholar] [CrossRef]
- Yoon, E.-J.; Kim, J.O.; Kim, D.; Lee, H.; Yang, J.W.; Lee, K.J.; Jeong, S.H. Klebsiella pneumoniae Carbapenemase Producers in South Korea between 2013 and 2015. Front. Microbiol. 2018, 9, 56. [Google Scholar] [CrossRef]
- David, S.; Reuter, S.; Harris, S.R.; Glasner, C.; Feltwell, T.; Argimon, S.; Abudahab, K.; Goater, R.; Giani, T.; Errico, G.; et al. Epidemic of carbapenem-resistant Klebsiella pneumoniae in Europe is driven by nosocomial spread. Nat. Microbiol. 2019, 4, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Wyres, K.L.; Holt, K.E. Klebsiella pneumoniae as a key trafficker of drug resistance genes from environmental to clinically important bacteria. Curr. Opin. Microbiol. 2018, 45, 131–139. [Google Scholar] [CrossRef]
- Wei, L.; Feng, Y.; Wen, H.; Ya, H.; Qiao, F.; Zong, Z. NDM-5-producing carbapenem-resistant Klebsiella pneumoniae of sequence type 789 emerged as a threat for neonates: A multicentre, genome-based study. Int. J. Antimicrob. Agents 2021, 59, 106508. [Google Scholar] [CrossRef]
- Kluytmans, J.A.J.W.; Overdevest, I.T.M.A.; Willemsen, I.; Kluytmans-van den Bergh, M.F.Q.; van der Zwaluw, K.; Heck, M.; Rijnsburger, M.; Vandenbroucke-Grauls, C.M.J.E.; Savelkoul, P.H.M.; Johnston, B.D.; et al. Extended-spectrum β-lactamase-producing Escherichia coli from retail chicken meat and humans: Comparison of strains, plasmids, resistance genes, and virulence factors. Clin. Infect. Dis. 2013, 56, 478–487. [Google Scholar] [CrossRef]
- Kola, A.; Kohler, C.; Pfeifer, Y.; Schwab, F.; Kühn, K.; Schulz, K.; Balau, V.; Breitbach, K.; Bast, A.; Witte, W.; et al. High prevalence of extended-spectrum-β-lactamase-producing Enterobacteriaceae in organic and conventional retail chicken meat, Germany. J. Antimicrob. Chemother. 2012, 67, 2631–2634. [Google Scholar] [CrossRef] [PubMed]
- Zarfel, G.; Galler, H.; Luxner, J.; Petternel, C.; Reinthaler, F.F.; Haas, D.; Kittinger, C.; Grisold, A.J.; Pless, P.; Feierl, G. Multiresistant bacteria isolated from chicken meat in Austria. Int. J. Environ. Res. Public Health 2014, 11, 12582–12593. [Google Scholar] [CrossRef] [PubMed]
- Ghodousi, A.; Bonura, C.; Di Noto, A.M.; Mammina, C. Extended-Spectrum ß-Lactamase, AmpC-Producing, and Fluoroquinolone-Resistant Escherichia coli in Retail Broiler Chicken Meat, Italy. Foodborne Pathog. Dis. 2015, 12, 619–625. [Google Scholar] [CrossRef]
- Egea, P.; López-Cerero, L.; Torres, E.; Del Gómez-Sánchez, M.C.; Serrano, L.; Navarro Sánchez-Ortiz, M.D.; Rodriguez-Baño, J.; Pascual, A. Increased raw poultry meat colonization by extended spectrum beta-lactamase-producing Escherichia coli in the south of Spain. Int. J. Food Microbiol. 2012, 159, 69–73. [Google Scholar] [CrossRef] [PubMed]
- The European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2017/2018. EFSA J. 2020, 18, e06007. [CrossRef]
- Russo, T.A.; Marr, C.M. Hypervirulent Klebsiella pneumoniae. Clin. Microbiol. Rev. 2019, 32, e00001-19. [Google Scholar] [CrossRef]
- Clegg, S.; Murphy, C.N. Epidemiology and Virulence of Klebsiella pneumoniae. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Paczosa, M.K.; Mecsas, J. Klebsiella pneumoniae: Going on the Offense with a Strong Defense. Microbiol. Mol. Biol. Rev. 2016, 80, 629–661. [Google Scholar] [CrossRef]
- Savin, M.; Bierbaum, G.; Hammerl, J.A.; Heinemann, C.; Parcina, M.; Sib, E.; Voigt, A.; Kreyenschmidt, J. Antibiotic-resistant bacteria and antimicrobial residues in wastewater and process water from German pig slaughterhouses and their receiving municipal wastewater treatment plants. Sci. Total Environ. 2020, 727, 138788. [Google Scholar] [CrossRef]
- Savin, M.; Bierbaum, G.; Hammerl, J.A.; Heinemann, C.; Parcina, M.; Sib, E.; Voigt, A.; Kreyenschmidt, J. ESKAPE Bacteria and Extended-Spectrum-β-Lactamase-Producing Escherichia coli Isolated from Wastewater and Process Water from German Poultry Slaughterhouses. Appl. Environ. Microbiol. 2020, 86, e02748-19. [Google Scholar] [CrossRef] [PubMed]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 11.0, 2021. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_11.0_Breakpoint_Tables.pdf (accessed on 10 February 2022).
- Savin, M.; Bierbaum, G.; Schmithausen, R.M.; Heinemann, C.; Kreyenschmidt, J.; Schmoger, S.; Akbaba, I.; Käsbohrer, A.; Hammerl, J.A. Slaughterhouse wastewater as a reservoir for extended-spectrum β-lactamase (ESBL)-producing, and colistin-resistant Klebsiella spp. and their impact in a “One Health” perspective. Sci. Total Environ. 2021, 804, 150000. [Google Scholar] [CrossRef] [PubMed]
- Wattam, A.R.; Abraham, D.; Dalay, O.; Disz, T.L.; Driscoll, T.; Gabbard, J.L.; Gillespie, J.J.; Gough, R.; Hix, D.; Kenyon, R.; et al. PATRIC, the bacterial bioinformatics database and analysis resource. Nucleic Acids Res. 2014, 42, D581–D591. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zheng, D.; Liu, B.; Yang, J.; Jin, Q. VFDB 2016: Hierarchical and refined dataset for big data analysis—10 years on. Nucleic Acids Res. 2016, 44, D694–D697. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).