The Effect of Toothpastes Containing Natural Extracts on Bacterial Species of a Microcosm Biofilm and on Enamel Caries Development
Abstract
:1. Introduction
2. Material and Methods
2.1. Ethical Aspects and Saliva Collection
2.2. Extracts and Toothpaste Compositions
2.3. Tooth Specimen Preparation
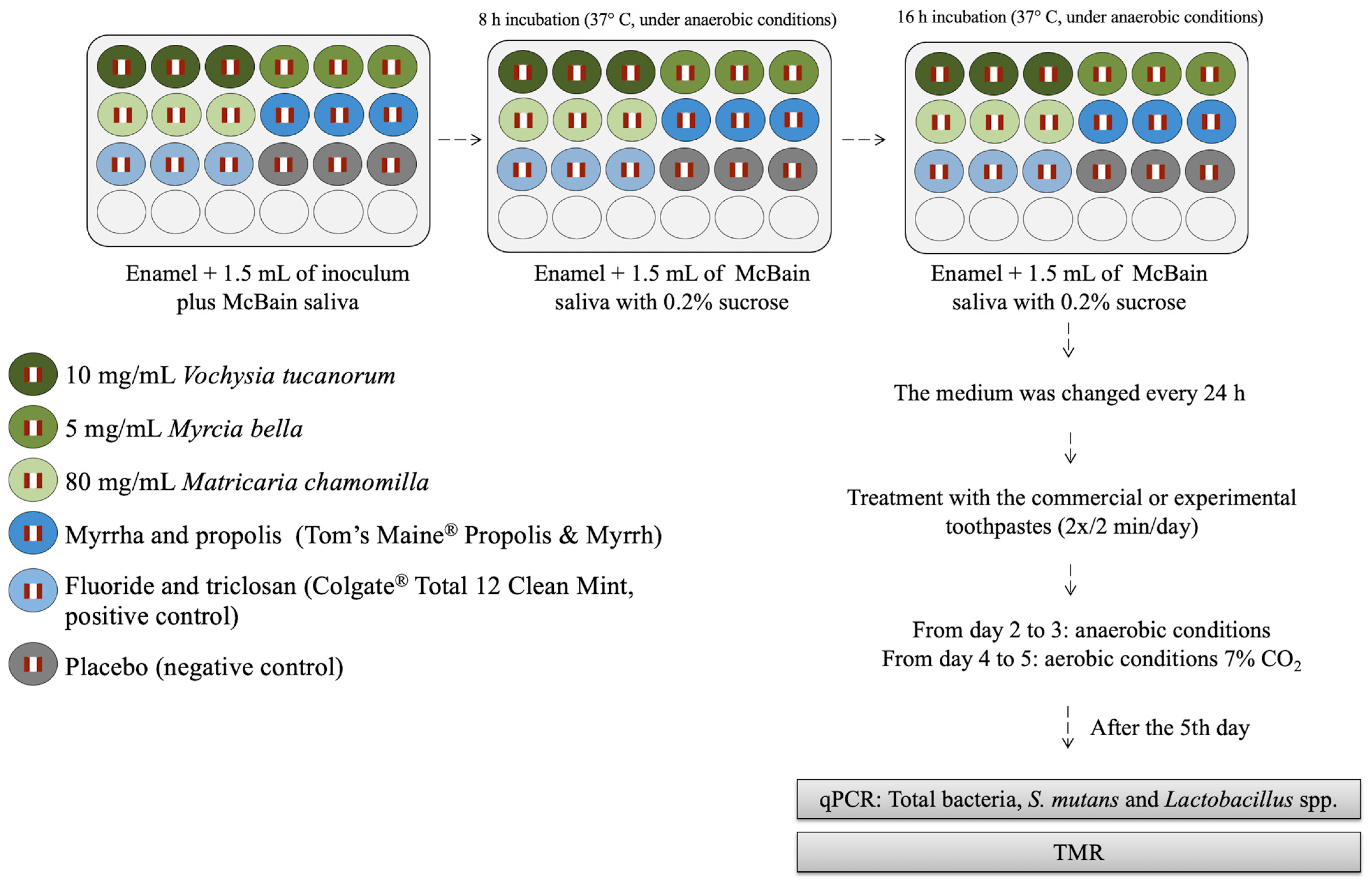
2.4. Microcosm Biofilm Formation and Treatments
2.5. pH Monitoring
2.6. Biofilm Analysis: Molecular Analyses (Genome Count Determination)—qPCR
2.7. Demineralization Analysis: TMR
2.8. Statistical Analysis
3. Results
3.1. pH Changes
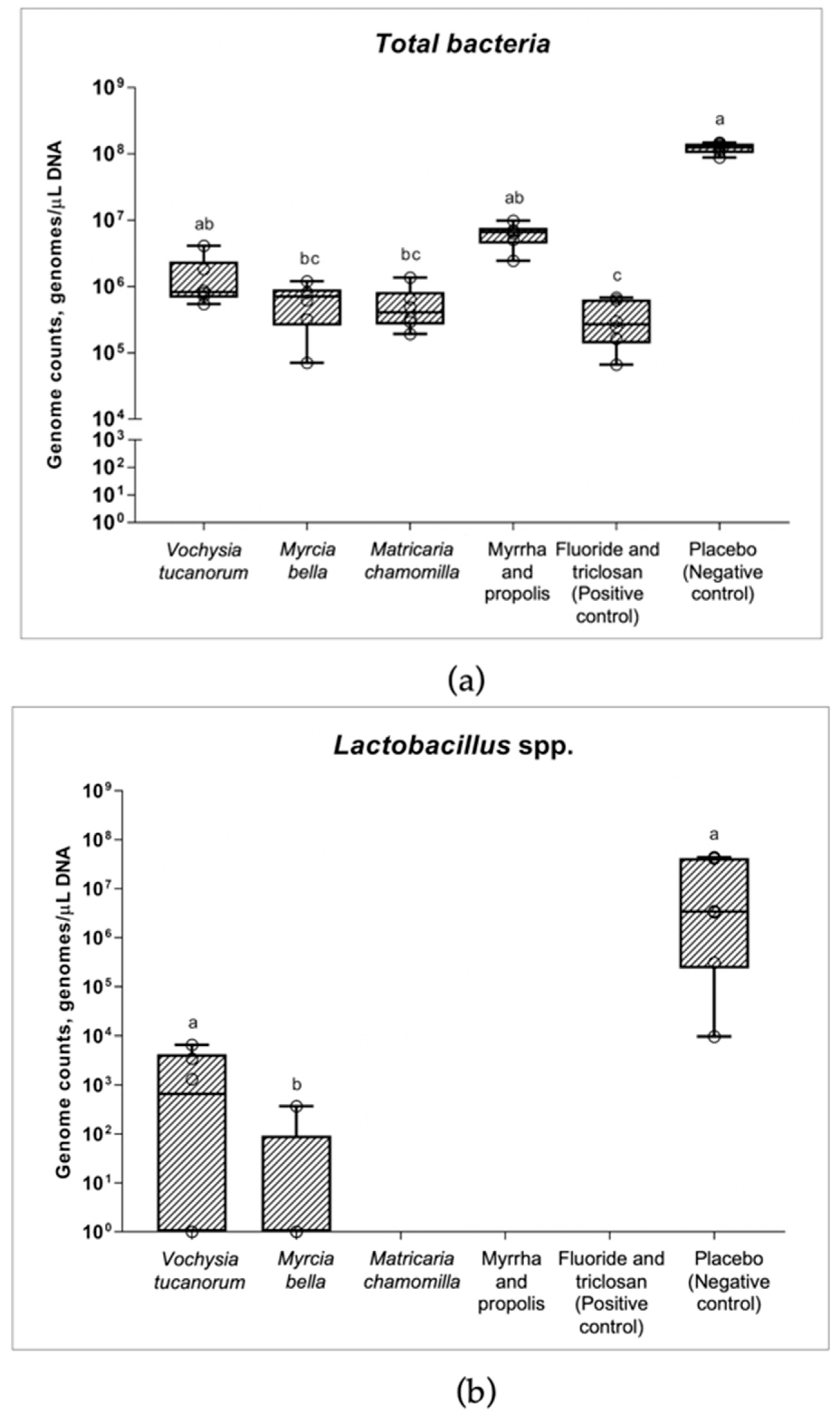
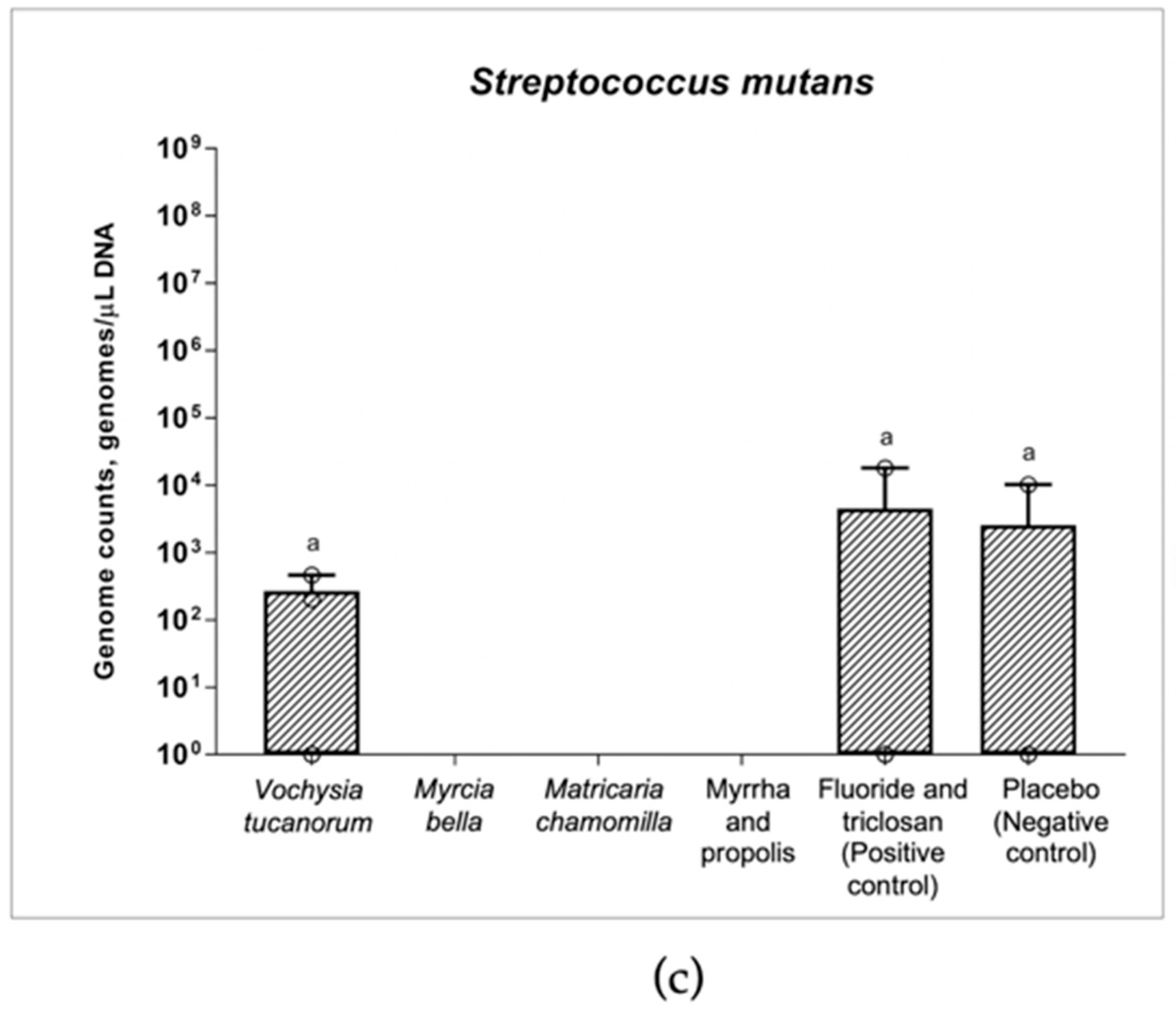
3.2. qPCR and Changes in Numbers of Bacteria
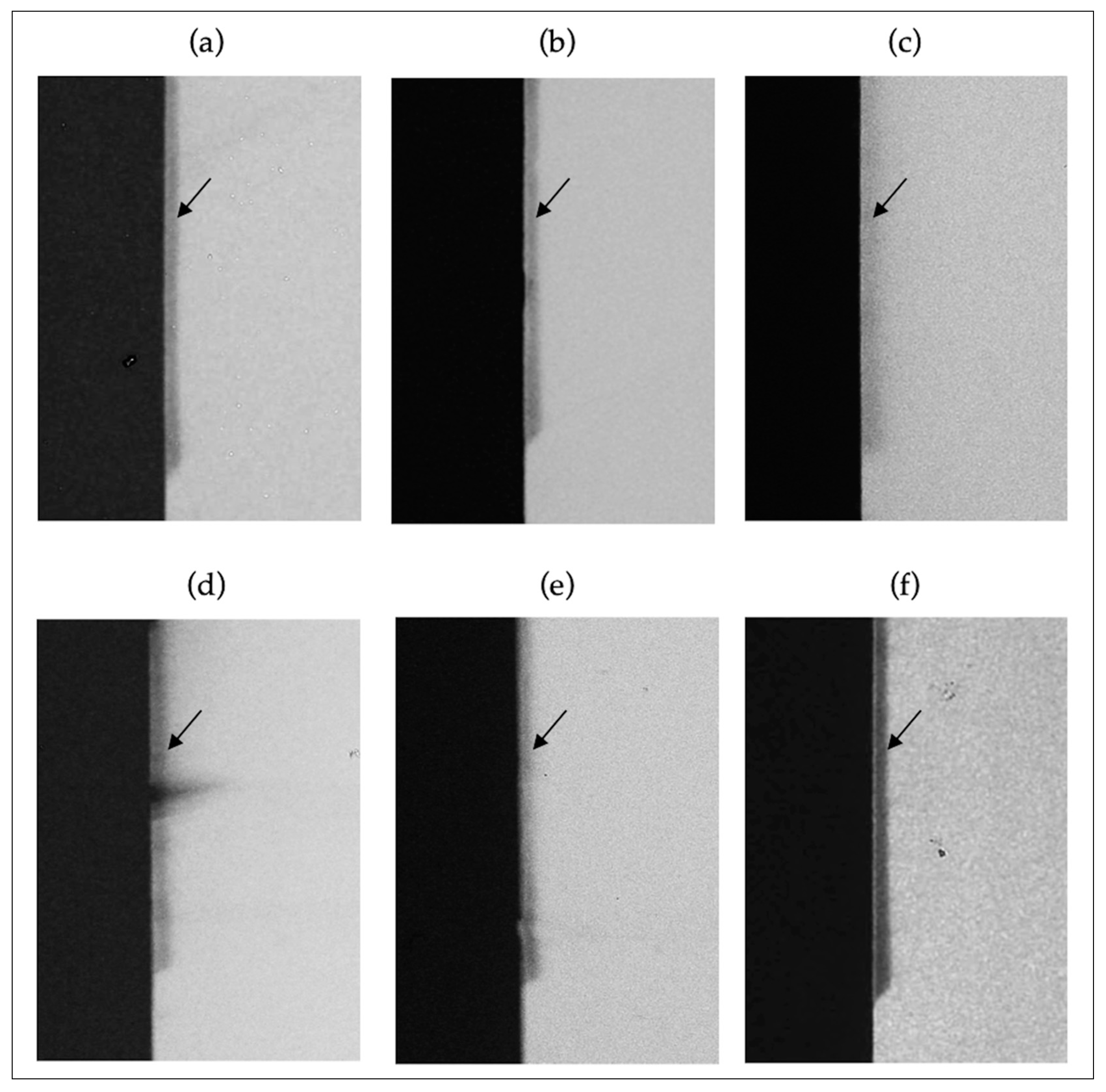
3.3. TMR and Enamel Demineralization
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marsh, P.D.; Zaura, E. Dental biofilm: Ecological interactions in health and disease. J. Clin. Periodontol. 2017, 44, S12–S22. [Google Scholar] [CrossRef] [PubMed]
- Rugg-Gunn, A. Dental caries: Strategies to control this preventable disease. Acta. Med. Acad. 2013, 42, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D. In sickness and in health-what does the oral microbiome mean to us? An ecological perspective. Adv. Dent. Res. 2018, 29, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Chenicheri, S.; Usha, R.; Ramachandran, R.; Thomas, V.; Wood, A. Insight into oral biofilm: Primary, secondary and residual caries and phyto-challenged solutions. Open Dent. J. 2017, 11, 312–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ancuceanu, R.; Anghel, A.I.; Ionescu, C.; Hovaneț, M.V.; Cojocaru-Toma, M.; Dinu, M. Clinical trials with herbal products for the prevention of dental caries and their quality: A scoping study. Biomolecules 2019, 9, 884. [Google Scholar] [CrossRef] [Green Version]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Hashimoto, G. Illustrated Cyclopedia of Brazilian Medicinal Plants; Aboc-Sha Press: Kamakura, Japan, 1996. [Google Scholar]
- Carnevale Neto, F.; Pilon, A.C.; Silva, D.H.S.; Bolzani, V.S.; Castro-Gamboa, I. Vochysiaceae: Secondary metabolites, ethnopharmacology and pharmacological potential. Phytochem. Rev. 2011, 10, 413–429. [Google Scholar] [CrossRef]
- Forzza, R.C.; Baumgratz, J.F.; Bicudo, C.E.; Canhos, D.A.; Carvalho, A.A., Jr.; Coelho, M.N.; Costa, A.F.; Costa, D.P.; Hopkins, M.G.; Leitman, P.M.; et al. New Brazilian floristic list highlights conservation challenges. BioScience 2012, 62, 39–45. [Google Scholar] [CrossRef]
- Munir, N.; Iqbal, A.S.; Altaf, I.; Bashir, R.; Sharif, N.; Saleem, F.; Naz, S. Evaluation of antioxidant and antimicrobial potential of two endangered plant species Atropa belladonna and Matricaria chamomilla. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Ledder, R.G.; Latimer, J.; Humphreys, G.J.; Sreenivasan, P.K.; McBain, A.J. Bacteriological effects of dentifrices with and without active ingredients of natural origin. Appl. Environ. Microbiol. 2014, 80, 6490–6498. [Google Scholar] [CrossRef] [Green Version]
- Braga, A.S.; de Melo Simas, L.L.; Pires, J.G.; Souza, B.M.; de Souza Rosa de Melo, F.P.; Saldanha, L.L.; Dokkedal, A.L.; Magalhãesa, A.C. Antibiofilm and anti-caries effects of an experimental mouth rinse containing Matricaria chamomilla L. extract under microcosm biofilm on enamel. J. Dent. 2020, 99, 103415. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.S.; de Melo, F.P.S.R.; Saldanha, L.L.; Dokkedal, A.L.; Meissner, T.; Bemmann, M.; Schulz-Kornas, E.; Haak, R.; Abdelbary, M.M.H.; Conrads, G.; et al. The effect of solutions containing extracts of Vochysia tucanorum Mart., Myrcia bella Cambess., Matricaria chamomilla L. and Malva sylvestris L. on cariogenic bacterial species and enamel caries development. Caries Res. 2021, 55, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.S.; Girotti, L.D.; de Melo Simas, L.L.; Pires, J.G.; Pelá, V.T.; Buzalaf, M.A.R.; Magalhães, A.C. Effect of commercial herbal toothpastes and mouth rinses on the prevention of enamel demineralization using a microcosm biofilm model. Biofouling 2019, 35, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Pratten, J.; Wilson, M.; Spratt, D.A. Characterization of in vitro oral bacterial biofilms by traditional and molecular methods. Oral Microbiol. Immunol. 2003, 18, 45–49. [Google Scholar] [CrossRef]
- Pires, J.G.; Zabini, S.S.; Braga, A.S.; de Cássia Fabris, R.; de Andrade, F.B.; de Oliveira, R.C.; Magalhães, A.C. Hydroalcoholic extracts of Myracrodruon urundeuva All. and Qualea grandiflora Mart. leaves on Streptococcus mutans biofilm and tooth demineralization. Arch. Oral Biol. 2018, 91, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Hostettmann, K.; Marston, A.; Hostettmann, M. Preparative Chromatography Techniques: Applications in Natural Product Isolation, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- McBain, A.J. Chapter 4: In vitro biofilm models: An overview. Adv. Appl. Microbiol. 2009, 69, 99–132. [Google Scholar]
- Conrads, G.; Wendt, L.K.; Hetrodt, F.; Deng, Z.L.; Pieper, D.; Abdelbary, M.M.H.; Barg, A.; Wagner-Döbler, I.; Apel, C. Deep sequencing of biofilm microbiomes on dental composite materials. J. Oral Microbiol. 2019, 11, 1617013. [Google Scholar] [CrossRef] [Green Version]
- Henne, K.; Rheinberg, A.; Melzer-Krick, B.; Conrads, G. Aciduric microbial taxa including Scardovia wiggsiae and Bifidobacterium spp. in caries and caries free subjects. Anaerobe 2015, 35, 60–65. [Google Scholar] [CrossRef]
- Henne, K.; Gunesch, A.P.; Walther, C.; Meyer-Lueckel, H.; Conrads, G.; Esteves-Oliveira, M. Analysis of bacterial activity in sound and cariogenic biofilm: A pilot in vivo study. Caries Res. 2016, 50, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Walther, C.; Meyer-Lueckel, H.; Conrads, G.; Esteves-Oliveira, M.; Henne, K. Correlation between relative bacterial activity and lactate dehydrogenase gene expression of co-cultures in vitro. Clin. Oral Investig. 2019, 23, 1225–1235. [Google Scholar] [CrossRef]
- Cheng, L.; Li, J.; He, L.; Zhou, X. Natural products and caries prevention. Caries Res. 2015, 49, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Walther, C.; Zumbülte, S.; Faerber, C.M.; Wierichs, R.J.; Meyer-Lueckel, H.; Conrads, G.; Henne, K.; Esteves-Oliveira, M. Analysis of relative bacterial activity and lactate dehydrogenase gene expression of caries-associated bacteria in a site-specific natural biofilm: An ex vivo study. Clin. Oral Investig. 2021, 25, 3669–3679. [Google Scholar] [CrossRef] [PubMed]
- Filoche, S.K.; Soma, K.J.; Sissons, C.H. Caries-related plaque microcosm biofilms developed in microplates. Oral Microbiol Immunol. 2007, 22, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Rosalen, P.L.; Cury, J.A.; Park, Y.K.; Bowen, W.H. Effects of compounds found in propolis on Streptococcus mutans growth and on glucosyltransferase activity. Antimicrob. Agents Chemother. 2002, 46, 1302–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, J.L.; Faustoferri, R.C.; Quivey, R.G., Jr. Acid-adaptive mechanisms of Streptococcus mutans-the more we know, the more we don’t. Mol. Oral Microbiol. 2017, 32, 107–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanner, A.C.R.; Kressirer, C.A.; Rothmiller, S.; Johansson, I.; Chalmers, N.I. The Caries Microbiome: Implications for Reversing Dysbiosis. Adv. Dent. Res. 2018, 29, 78–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dos Santos, C.; Galaverna, R.S.; Angolini, C.; Nunes, V.; de Almeida, L.; Ruiz, A.; De Carvalho, J.E.; Duarte, R.M.T.; Duarte, M.C.T.; Eberlin, M.N. Antioxidative, antiproliferative and antimicrobial activities of phenolic compounds from three Myrcia species. Molecules 2018, 23, 986. [Google Scholar] [CrossRef] [Green Version]
- Saldanha, L.L.; Allard, P.M.; Afzan, A.; de Melo, F.P.S.R.; Marcourt, L.; Queiroz, E.F.; Vilegas, W.; Furlan, C.M.; Dokkedal, A.L.; Wolfender, J.-L. Metabolomics of Myrcia bella populations in brazilian Savanna reveals strong influence of environmental factors on its specialized metabolism. Molecules 2020, 25, 2954. [Google Scholar] [CrossRef]
- Saldanha, L.L.; Vilegas, W.; Dokkedal, A.L. Characterization of flavonoids and phenolic acids in Myrcia bella Cambess. using FIA-ESI-IT-MS(n) and HPLC-PAD-ESI-IT-MS combined with NMR. Molecules 2013, 18, 8402–8416. [Google Scholar] [CrossRef] [Green Version]
- Cushnie, T.P.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356, Erratum in Int. J. Antimicrob. Agents 2006, 27, 181. [Google Scholar] [CrossRef]
- Puljula, E.; Walton, G.; Woodward, M.J.; Karonen, M. Antimicrobial Activities of Ellagitannins against Clostridiales perfringens, Escherichia coli, Lactobacillus plantarum and Staphylococcus aureus. Molecules 2020, 25, 3714. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A. Antimicrobial properties of tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Liu, X.L.; Hao, Y.Q.; Jin, L.; Xu, Z.J.; McAllister, T.A.; Wang, Y. Anti-Escherichia coli O157:H7 properties of purple prairie clover and sainfoin condensed tannins. Molecules 2013, 18, 2183–2199. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Luo, M.; Fu, Y.; Zu, Y.; Wang, W.; Zhang, L.; Yao, L.; Zhao, C.; Sun, Y. Effect of corilagin on membrane permeability of Escherichia coli, Staphylococcus aureus and Candida albicans. Phyther. Res. 2013, 27, 1517–1523. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, N.S.; Nazli, R.; Zafar, H.; Fatima, S. Effects of lipid based Multiple Micronutrients Supplement on the birth outcome of underweight pre-eclamptic women: A randomized clinical trial. Pak. J. Med. Sci. 2022, 38, 219–226. [Google Scholar]
- Salamon, I. Chamomile: A medicinal plant. Herb Spice. Med. Plant Dig. 1992, 10, 1–4. [Google Scholar]
- Singh, O.; Khanam, Z.; Misra, N.; Srivastava, M.K. Chamomile (Matricaria chamomilla L.): An overview. Pharmacogn. Rev. 2011, 5, 82–95. [Google Scholar] [CrossRef] [Green Version]
- Tolouee, M.; Alinezhad, S.; Saberi, R.; Eslamifar, A.; Zad, S.J.; Jaimand, K.; Taeb, J.; Rezaee, M.-B.; Kawachi, M.; Shams-Ghahfarokhi, M.; et al. Effect of Matricaria chamomilla L. flower essential oil on the growth and ultrastructure of Asper- gillus niger van Tieghem. Int. J. Food Microbiol. 2010, 139, 127–133. [Google Scholar] [CrossRef]
- Mendoza, L.; Wilkens, M.; Urzua, A. Antimicrobial study of the resinous exudates and of diterpenoids and flavonoids isolated from some Chilean Pseudognaphalium (Asteraceae) J. Ethnopharmacol. 1997, 58, 85–88. [Google Scholar] [CrossRef]
- Riley, P.; Lamont, T. Triclosan/copolymer containing toothpastes for oral health. Cochrane Database Syst. Rev. 2013, 5, CD010514. [Google Scholar]
- Williams, M.I.; Cummins, D. The technology behind colgate total advanced fresh. Compend. Contin. Educ. Dent. 2003, 24, 4–9. [Google Scholar] [PubMed]
- Buzalaf, M.; Pessan, J.P.; Honório, H.M.; Ten Cate, J.M. Mechanisms of action of fluoride for caries control. Monogr. Oral Sci. 2011, 22, 97–114. [Google Scholar] [PubMed]
- Ghanbarzadeh, B.; Almasi, H. Physical properties of edible emulsified films based on carboxymethyl cellulose and oleic acid. Int. J. Biol. Macromol. 2011, 48, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Simsek, M.; Eke, B.; Demir, H. Characterization of carboxymethyl cellulose-based antimicrobial films incorporated with plant essential oils. Int. J. Biol. Macromol. 2020, 163, 2172–2179. [Google Scholar] [CrossRef]
- Randall, J.P.; Seow, W.K.; Walsh, L.J. Antibacterial activity of fluoride compounds and herbal toothpastes on Streptococcus mutans: An in vitro study. Aust. Dent. J. 2015, 60, 368–374. [Google Scholar] [CrossRef] [Green Version]
| Experimental and Commercial Toothpastes | Company/City, Country | Composition/Extract Concentration |
|---|---|---|
| Vochysia tucanorum | Pharmacy Specifíca (Bauru/São Paulo, Brazil) | Carboxymethylcellulose, glycerin, sodium methyl p-hydroxybenzoate, saccharin, hydrated silica, titanium dioxide, sodium lauryl sulfate, water. Active component: Vochysia tucanorum (10 mg/g, 70% EtOH leaf extract). |
| Myrcia bella | Pharmacy Specifíca (Bauru/São Paulo, Brazil) | Carboxymethylcellulose, glycerin, sodium methyl p-hydroxybenzoate, saccharin, hydrated silica, titanium dioxide, sodium lauryl sulfate, water. Active component: Myrcia bella (5 mg/g, 70% EtOH leaf extract). |
| Matricaria chamomilla | Pharmacy Specifíca (Bauru/São Paulo, Brazil) | Carboxymethylcellulose, glycerin, sodium methyl p-hydroxybenzoate, saccharin, hydrated silica, titanium dioxide, sodium lauryl sulfate, water. Active component: Matricaria Chamomilla (80 mg/g, commercial aqueous flower and stalk extract). |
| Commiphora myrrha and propolis (Tom’s Maine® Propolis & Myrrh)—Myrrha and propolis toothpaste | Tom’s Maine®/Kennebunk, USA | Calcium carbonate, glycerin, water, hydrated silica, xylitol, sodium lauryl sulfate, xantan gum, benzyl alcohol, natural favor, Commiphora myrrha resin extract (myrrh), propolis extract. Active component: xylitol, Commiphora myrrha resin extract (myrrh), propolis extract. |
| Sodium Fluoride + 0.3% triclosan and sorbitol (Colgate® Total 12 Clean Mint, positive control)—Fluoride and triclosan toothpaste | Colgate-Palmolive/São Paulo, Brazil | Hydrated silica, sodium lauryl sulfate, PVM/ MA, copolymer, flavor, carrageenan, sodium hydroxide, sodium saccharin, titanium dioxide (CI 77891), dipentene, water. Active component: Sodium fluoride (1450 ppm F), 0.3% triclosan and sorbitol. |
| Placebo (Negative control) | Pharmacy Specifíca (Bauru/São Paulo, Brazil) | Carboxymethylcellulose, glycerin, sodium methyl p-hydroxybenzoate, saccharin, hydrated silica, titanium dioxide, sodium lauryl sulfate, water. Active component: none. |
| Treatment | pH Values (Mean ± SD) | ||||
|---|---|---|---|---|---|
| 8 h B | 24 h A | 72 h C | 96 h C | 120 h C | |
| Vochysia tucanorum a | 5.51 ± 0.02 | 4.27 ± 0.04 | 6.60 ± 0.07 | 6.82 ± 0.09 | 6.92 ± 0.10 |
| Myrcia bella a | 5.52 ± 0.01 | 4.23 ± 0.02 | 6.57 ± 0.03 | 6.79 ± 0.04 | 7.04 ± 0.01 |
| Matricaria chamomilla a | 5.50 ± 0.01 | 4.22 ± 0.03 * | 6.60 ± 0.02 | 6.76 ± 0.10 | 6.98 ± 0.06 |
| Myrrha and propolis a | 5.51 ± 0.03 | 4.24 ± 0.03 | 6.50 ± 0.06 | 6.55 ± 0.54 | 6.82 ± 0.10 |
| Fluoride + triclosan (positive control) ab | 5.52 ± 0.05 | 4.22 ± 0.01 * | 6.03 ± 0.21 | 5.89 ± 0.19 | 6.08 ± 0.04 |
| Placebo (negative control) b | 5.55 ± 0.02 | 4.18 ± 0.01 * | 5.03 ± 0.07 | 4.74 ± 0.09 | 5.88 ± 0.02 |
| Treatment | ΔZ (vol% × μm) | LD (μm) |
|---|---|---|
| Vochysia tucanorum | 1515.0 ± 358.1 a | 57.1 ± 14.0 a |
| Myrcia bella | 1460.0 ± 345.7 a | 52.7 ± 24.5 a |
| Matricaria chamomilla | 1540.0 ± 191.5 a | 74.2 ± 10.5 a |
| Myrrha and propolis | 1293.3 ± 293.2 a | 53.5 ± 22.6 a |
| Fluoride + triclosan (positive control) | 1310.0 ± 277.4 a | 49.3 ± 11.4 a |
| Placebo (negative control) | 2420.0 ± 699.0 b | 108.9 ± 21.2 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braga, A.S.; Abdelbary, M.M.H.; Kim, R.R.; Melo, F.P.d.S.R.d.; Saldanha, L.L.; Dokkedal, A.L.; Conrads, G.; Esteves-Oliveira, M.; Magalhães, A.C. The Effect of Toothpastes Containing Natural Extracts on Bacterial Species of a Microcosm Biofilm and on Enamel Caries Development. Antibiotics 2022, 11, 414. https://doi.org/10.3390/antibiotics11030414
Braga AS, Abdelbary MMH, Kim RR, Melo FPdSRd, Saldanha LL, Dokkedal AL, Conrads G, Esteves-Oliveira M, Magalhães AC. The Effect of Toothpastes Containing Natural Extracts on Bacterial Species of a Microcosm Biofilm and on Enamel Caries Development. Antibiotics. 2022; 11(3):414. https://doi.org/10.3390/antibiotics11030414
Chicago/Turabian StyleBraga, Aline Silva, Mohamed Mostafa Hefny Abdelbary, Rafaela Ricci Kim, Fernanda Pereira de Souza Rosa de Melo, Luiz Leonardo Saldanha, Anne Lígia Dokkedal, Georg Conrads, Marcella Esteves-Oliveira, and Ana Carolina Magalhães. 2022. "The Effect of Toothpastes Containing Natural Extracts on Bacterial Species of a Microcosm Biofilm and on Enamel Caries Development" Antibiotics 11, no. 3: 414. https://doi.org/10.3390/antibiotics11030414
APA StyleBraga, A. S., Abdelbary, M. M. H., Kim, R. R., Melo, F. P. d. S. R. d., Saldanha, L. L., Dokkedal, A. L., Conrads, G., Esteves-Oliveira, M., & Magalhães, A. C. (2022). The Effect of Toothpastes Containing Natural Extracts on Bacterial Species of a Microcosm Biofilm and on Enamel Caries Development. Antibiotics, 11(3), 414. https://doi.org/10.3390/antibiotics11030414







