The Association between icaA and icaB Genes, Antibiotic Resistance and Biofilm Formation in Clinical Isolates of Staphylococci spp.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Isolates
2.2. Species Identification of Clinical Isolates
2.3. Antibiotic Susceptibility Assessment
2.4. Studying Biofilm Formation via Microtiter Plate (MTP)
2.5. Detection of icaA and icaB Genes
2.5.1. Genomic DNA Extraction
2.5.2. PCR
2.6. Statistical Analysis
3. Results
3.1. Bacterial Isolates and Relative Distribution
3.2. Antibiotic Resistance Distribution
3.3. Detection of the Biofilm Formation via Microtiter Tissue Culture Plates
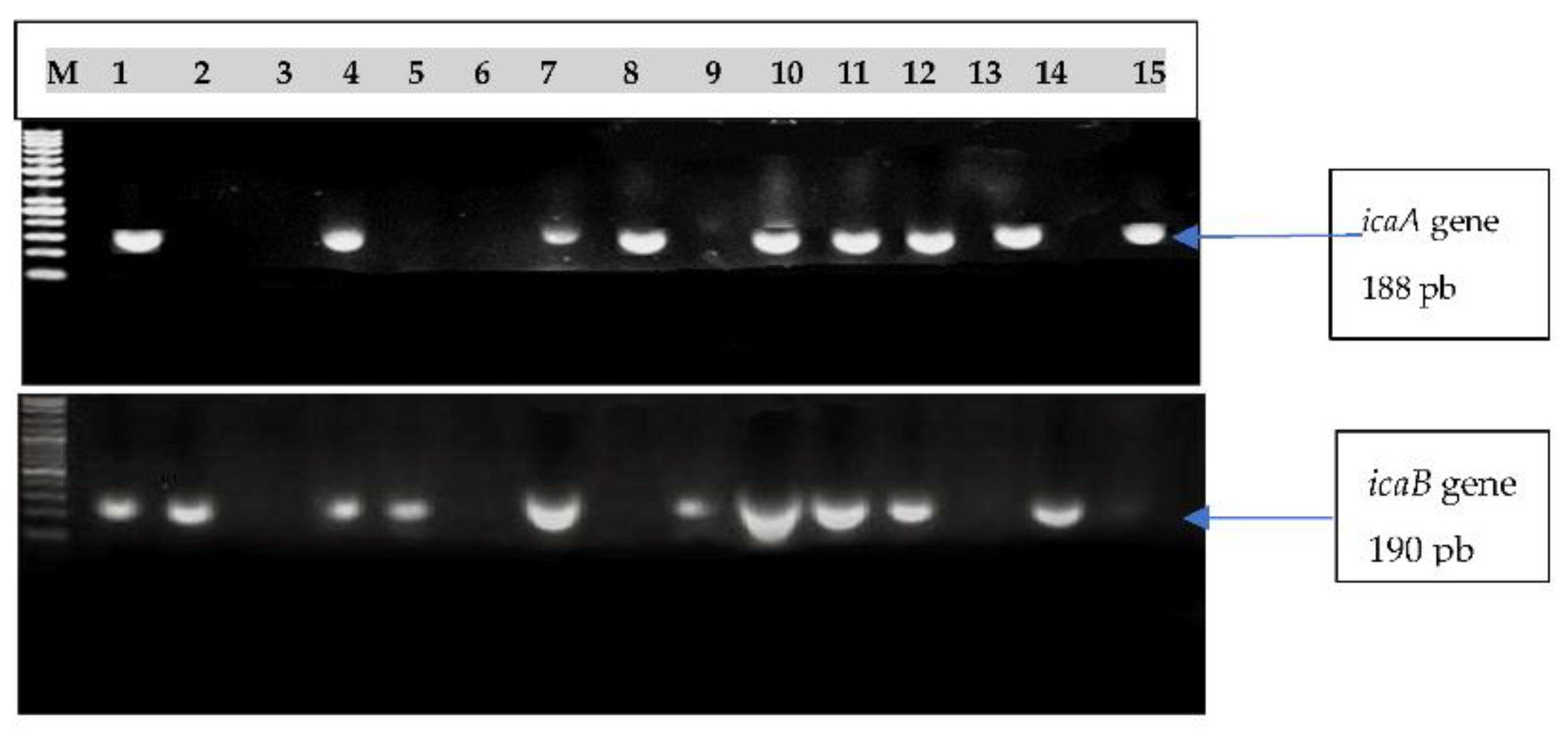
3.4. Detection of icaA and icaB Genes
3.5. The Association between Antibiotic Resistance, Biofilm Formation and the Biochemical Pattern
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, Y.; He, L.; Asiamah, T.K.; Otto, M. Colonization of Medical Devices by Staphylococci. Environ. Microbiol. 2018, 20, 3141–3153. [Google Scholar] [CrossRef] [Green Version]
- Otto, M. Staphylococcus Colonization of the Skin and Antimicrobial Peptides. Expert Rev. Dermatol. 2010, 5, 183–195. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.; Pillai, S.; Revsbech, N.P.; Schramm, A.; Bischoff, C.; Meyer, R.L. Biofilm Retention on Surfaces with Variable Roughness and Hydrophobicity. Biofouling 2011, 27, 111–121. [Google Scholar] [CrossRef]
- Khadije, R.K.; Aliyeh, S.; Mehdi, H.; Zahra, S. Detection of Intercellular Adhesion Genes (icaA and icaD) in Staphylococcus Aureus Clinical Isolates in Zabol-Iran. Res. Mol. Med. 2017, 5, 40. [Google Scholar]
- Namvar, A.E.; Asghari, B.; Ezzatifar, F.; Azizi, G.; Lari, A.R. Detection of the Intercellular Adhesion Gene Cluster (ica) in Clinical Staphylococcus Aureus Isolates. GMS Hyg. Infect. Control. 2013, 8, Doc03. [Google Scholar]
- Kot, B.; Sytykiewicz, H.; Sprawka, I.; Witeska, M. Effect of manuka honey on bioflm-associated genes expression during methicillin-resistant Staphylococcus aureus bioflm formation. Sci. Rep. 2020, 10, 13552. [Google Scholar] [CrossRef]
- Carla, R.A.; Davide, C.; Stefano, R.; Lucio, M. Polysaccharide Intercellular Adhesinin Biofilm: Structural and Regulatory Aspects. Front Cell Infect. Microbiol. 2015, 5, 1. [Google Scholar]
- Cue, D.; Lei, M.G.; Lee, C.Y. Genetic Regulation of the Intercellular Adhesion Locus in Staphylococci. Front. Cell. Infect. Microbiol. 2012, 2, 38. [Google Scholar] [CrossRef] [Green Version]
- Cheesbrough, M. District Laboratory Practice in Tropical Countries; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Patel, J.B. Performance Standards for Antimicrobial Susceptibility Testing. Clin. Lab. Stand. Inst. 2015, 100, 25. [Google Scholar]
- Torlak, E.; Korkut, E.; Uncu, A.T.; Şener, Y. Biofilm Formation by Staphylococcus Aureus Isolates from a Dental Clinic in Konya, Turkey. J. Infect. Public Health 2017, 10, 809–813. [Google Scholar] [CrossRef]
- Omidi, M.; Firoozeh, F.; Saffari, M.; Sedaghat, H.; Zibaei, M.; Khaledi, A. Ability of Biofilm Production and Molecular Analysis of spa and ica Genes among Clinical Isolates of Methicillin-Resistant Staphylococcus Aureus. BMC Res. Notes 2020, 13, 19. [Google Scholar] [CrossRef] [Green Version]
- Mirzaee, M.; Peerayeh, S.N.; Ghasemian, A. Detection of icaABCD Genes and Biofilm Formation in Clinical Isolates of Methicillin Resistant Staphylococcus Aureus. Iran. J. Pathol. 2014, 9, 257–262. [Google Scholar]
- Deighton, M.A.; Capstick, J.; Domalewski, E.; Van Nguyen, T. Methods for Studying Biofilms Produced by Staphylococcus Epidermidis. Methods Enzymol. 2001, 336, 177–195. [Google Scholar]
- Kifaya, A.; Walaa, Q.; Ziad, A. Screening of Genes Encoding Adhesion Factors and Biofilm Production in Methicillin Resistant Strains of Staphylococcus Aureus Isolated from Palestinian Patients. BMC Genom. 2019, 20, 578. [Google Scholar]
- Edwards, A.M.; Bowden, M.G.; Brown, E.L.; Laabei, M.; Massey, R.C. Staphylococcus Aureus Extracellular Adherence Protein Triggers TNF Alpha Release, Promoting Attachment to Endothelial Cells via Protein A. PLoS ONE 2012, 7, e43046. [Google Scholar] [CrossRef] [Green Version]
- Bello-Chavolla, O.Y.; Bahena-Lopez, J.P.; Garciadiego-Fosass, P.; Volkow, P.; Garcia-Horton, A.; Velazquez-Acosta, C.; Vilar-Compte, D. Bloodstream Infection Caused by S. Aureus in Patients with Cancer: A 10-Year Longitudinal Single-Center Study. Support Care Cancer 2018, 26, 4057–4065. [Google Scholar] [CrossRef]
- Rengaraj, R.; Velu, P.; Sekar, U. Prevalence of MRSA and Biofilm Associated Gene among Clinical Isolates of Staphylococci. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 745–752. [Google Scholar]
- Adeleye, I.A.; Akanmu, A.S.; Bamiro, B.S.; Obosi, A.C.; Unem, A.V. Bacterial Bloodstream Infections in HIV—Infected Adults Attending a Lagos Teaching Hospital. J. Health Popul. Nutr. 2010, 28, 318–326. [Google Scholar]
- Sánchez, C.J.; Mende, K.; Beckius, M.L.; Akers, K.S.; Romano, D.R.; Wenke, J.C.; Murray, C.K. Biofilm Formation by Clinical Isolates and the Implications in Chronic Infections. BMC Infect. Dis. 2013, 13, 47. [Google Scholar] [CrossRef] [Green Version]
- Solati, S.M.; Tajbakhsh, E.; Khamesipour, F.; Gugnani, H.C. Prevalence of Virulence genes of Biofilm Producing Strains of Staphylococcus Epidermidis Isolated from Clinical Samples in Iran. AMB Express. 2015, 5, 47. [Google Scholar] [CrossRef] [Green Version]
- Ghasemian, A.; Peerayeh, S.N.; Bakhshi, B.; Mirzaee, M. Comparison of Biofilm Formation between Methicillin-Resistant and Methicillin-Susceptible Isolates of Staphylococcus Aureus. Iran. Biomed. J. 2016, 20, 175–181. [Google Scholar] [PubMed]
- Osman, A.; El-Didamony, G.; Sitohy, M.; Khalifa, M.; Enan, G. Soybean Glycinin Basic Subunit Inhibits Methicillin Resistant-Vancomycin Intermediate Staphylococcus Aureus (MRSA-VISA) in Vitro. Int. J. Appl. Res. Nat. Prod. 2016, 9, 17–26. [Google Scholar]
- Al-Mohammadi, A.R.; Osman, A.; Enan, G.; Sitohy, M.; Taha, M.A. Powerful Antibacterial Peptides from Egg Albumin Hydrolysates. Antibiotics 2020, 9, 901. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.M.; Sitohy, M.Z.; Ahmed, A.I.; Soliman, M.M.; El-Saadony, M.T. Biochemical and Functional Characterization of Kidney Bean Protein Alcalase-Hydrolysates and Their Preservative Action on Stored Chicken Meat. Molecules 2021, 26, 4690. [Google Scholar] [CrossRef] [PubMed]
- Sitohy, M.; Mahgoub, S.; Osman, A. Controlling Psychrotrophic Bacteria in Raw Buffalo Milk Preserved at 4 °C with Esterified Legume Proteins. LWT-Food Sci. Technol. 2011, 44, 1697–1702. [Google Scholar] [CrossRef]
- Abdel-Shafi, S.; Osman, A.; Enan, G.; El-Nemer, M.; Sitohy, M. Antibacterial Activity of Methylated Egg White Proteins against Pathogenic G+ and G− Bacteria Matching Antibiotics. SpringerPlus 2016, 5, 983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Shafi, S.; Osman, A.; Al-Mohammadi, A.-R.; Kamal, N.; Sitohy, M. Biochemical, Biological Characteristics and Antibacterial Activity of Glycoprotein Extracted from the Epidermal Mucus of African Catfish (Clarias Gariepinus). Int. J. Biol. Macromol. 2019, 138, 773–780. [Google Scholar] [CrossRef]
- Triveni, A.G.; Kumar, M.S.; Manjunath, C.; Shivnnavar, C.T.; Gaddad, S.M. Biofilm Formation by Clinically Isolated Staphylococcus Aureus from India. J. Infect. Dev. Ctries. 2018, 12, 1062–1066. [Google Scholar] [CrossRef] [Green Version]
- Gad, G.F.M.; El-Feky, M.A.; El-Rehewy, M.S.; Hassan, M.A.; Abolella, H.; Abd El-Baky, R.M. Detection of icaA, icaD Genes and Biofilm Production by Staphylococcus Aureus and Staphylococcus Epidermidis Isolated from Urinary Tract Catheterized Patients. J. Infect. Dev. Ctries. 2009, 3, 342–351. [Google Scholar]
- Nirwati, H.; Sinanjung, K.; Fahrunissa, F.; Wijaya, F.; Napitupulu, S.; Hati, V.P.; Nuryastuti, T. Biofilm formation and antibiotic Resistance of Klebsiella Pneumoniae Isolated from Clinical Samples in a Tertiary Care Hospital, Klaten, Indonesia. BMC 2019, 13, 20. [Google Scholar] [CrossRef]
- Fauzia, K.A.; Miftahussurur, M.; Syam, A.F.; Waskito, L.A.; Doohan, D.; Rezkitha, Y.A.A.; Yamaoka, Y. Biofilm Formation and Antibiotic Resistance Phenotype of Helicobacter Pylori Clinical Isolates. Toxins 2020, 12, 473. [Google Scholar] [CrossRef] [PubMed]
- Al-Mtory, H.K.Y.; Al-Shukri, M.S.; Al-Hassnawi, H.H. Detection of Intracellular Adhesion (ica) Genes and Biofilm Formation in Staphylococcus spp. Isolated from Different Clinical Samples. Glob. J. Biochem. Biotechnol. 2016, 5, 505–511. [Google Scholar]
- Diemond-Hernandez, B.; Solorzano- Santos, F.; Leanos-Miranda, B.; Peregrino-Bejarano, L.; Miranda- Novales, G. Production of icaADBC-Encoded Polysaccharide Intercellular Adhesin and Therapeutic Failure in Pediatric Patients with Staphylococcal Device-Related Infections. BMC Infect. Dis. 2010, 10, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazari, P.A.; Jahromy, S.H.; Karizi, S.Z. Phenotypic and Genotypic Characterization of Biofilm Formation among Staphylococcus Aureus Isolates from Clinical Specimens, an Atomic Force Microscopic (AFM) Study, Phenotypic and Genotypic Characterization of Biofilm Formation among Staphylococcus Aureus Isolates from Clinical Specimens, an Atomic Force Microscopic (AFM) Study. Microb. Pathog. 2017, 110, 533–539. [Google Scholar] [PubMed]
- Arciola, C.R.; Campoccia, D.; Speziale, P.; Montanaro, L.; Costerton, J.W. Biofilm Formation in Staphylococcus Implant Infections. A Review of Molecular Mechanisms and Implications for Biofilm-Resistant Materials. Biomaterials 2012, 33, 5967–5982. [Google Scholar] [CrossRef] [PubMed]
| Bacterial Type | Staphylococcus spp. | No. | % |
|---|---|---|---|
| CoPS | 46 | 69.7 | |
| S. aureus | 46 | 69.7 | |
| CoNS | 20 | 30.3 | |
| S. epidermidis | 6 | 9.1 | |
| S. hyicus | 4 | 6.1 | |
| S. hominis | 3 | 4.5 | |
| S. xylosus | 3 | 4.5 | |
| S. haemolyticus | 2 | 3.0 | |
| S. auricularies | 1 | 1.5 | |
| S. sciuri | 1 | 1.5 | |
| Total | 66 | 100 |
| Type of Antibiotics | CoPS | CoNS | p Value | |||
|---|---|---|---|---|---|---|
| Code | No | % | No | % | % | |
| Vancomycin | 1 | 11 | 23.9 | 3 | 15.0 | χ2 = 0.663 p = 0.416 |
| Amoxicillin–clavulanate | 2 | 20 | 43.5 | 19 | 95.0 | χ2 = 15.31 p < 0.001 * |
| Erythromycin | 3 | 28 | 60.9 | 15 | 75.0 | χ2 = 1.23 p = 0.268 |
| Trimethoprim–sulfamethoxazole | 4 | 21 | 45.7 | 8 | 40.0 | χ2 = 0.181 p = 0.671 |
| Imipenem | 5 | 15 | 32.6 | 18 | 90.0 | χ2 = 0.181 p < 0.001 * |
| Oxacillin | 6 | 43 | 93.5 | 18 | 90.0 | χ2 = 0.241 p = 0.624 |
| Ceftriaxone | 7 | 28 | 60.9 | 19 | 95.0 | χ2 = 7.92 p = 0.005 * |
| Clindamycin | 8 | 35 | 76.1 | 10 | 50.0 | χ2 = 4.37 p = 0.037 * |
| Biofilm Formation | CoPS | CoNS | p-Value | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Strong | 19 | 41.3 | 11 | 55 | 0.304 |
| Moderate | 15 | 32.6 | 8 | 40 | 0.562 |
| Weak | 12 | 26.1 | 1 | 5 | 0.047 * |
| Total | 46 | 100 | 20 | 100 | |
| No. of Isolates | Tested Isolates | icaA Gene | icaB Gene |
|---|---|---|---|
| 1 | S. aureus | + | + |
| 2 | S. aureus | − | + |
| 4 | S. aureus | − | − |
| 6 | S. aureus | + | + |
| 7 | S. aureus | − | + |
| 8 | S. aureus | − | − |
| 9 | S. aureus | + | + |
| 11 | S. aureus | + | − |
| 12 | S. aureus | − | + |
| 16 | S. aureus | + | + |
| 18 | S. aureus | + | + |
| 21 | S. aureus | + | + |
| 25 | S. aureus | + | − |
| 41 | S. aureus | − | + |
| 42 | S. aureus | + | − |
| 43 | S. aureus | − | − |
| 44 | S. aureus | − | − |
| 45 | S. aureus | − | − |
| 46 | S. aureus | − | − |
| 47 | S. sciuri | − | + |
| 48 | S. hominis | − | + |
| 52 | S. haemolyticus | + | − |
| 54 | S. hominis | − | − |
| 55 | S. xylosus | − | + |
| 56 | S. hyicus | − | + |
| 57 | S. hyicus | − | + |
| 58 | S. hyicus | − | − |
| 59 | S. xylosus | − | + |
| 60 | S. epidermidis | − | − |
| 63 | S. auricularies | − | − |
| Category | Isolate Code | Isolate Quantity | |||||
|---|---|---|---|---|---|---|---|
| No. | % | ||||||
| Two ica gene | 1, 6, 9, 16, 18, 21. | 6 | 20 | ||||
| One ica gene | 2, 7, 11, 12, 25, 41, 42, 47, 48,52, 55, 56, 57, 59. | 14 | 46.7 | ||||
| No ica gene | 4, 8, 43, 44, 45, 46, 54, 58, 60, 63. | 10 | 33.3 | ||||
| CoPS (n = 19) | CoNS (n = 11) | Total (n = 30) | p Value | ||||
| icaA | No. | % | No. | % | No. | % | |
| Positive | 9 | 47.4 | 1 | 9.1 | 10 | 33.3 | χ2 = 4.59 p = 0.032 * |
| Negative | 10 | 52.6 | 10 | 90.9 | 20 | 66.7 | |
| icaB | χ2 = 0.01 p = 0.919 | ||||||
| Positive | 10 | 52.60% | 6 | 54.50% | 16 | 53.30% | |
| Negative | 9 | 47.40% | 5 | 45.50% | 14 | 46.70% | |
| Antibiotic | Antibiotic Resistance | Biofilm Formation | % CoPS * | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strong | Medium | Weak | ||||||||||
| Quantity of Isolates | No. of Isolates | |||||||||||
| Total No | % | Total No | CoPS | CoNS | Total No | CoPS | CoNS | Total No | CoPS | CoNS | ||
| Vancomycin | 14 | 21 | 8 | 6 | 2 | 5 | 4 | 1 | 1 | 1 | 0 | 79 |
| Amox/clav | 39 | 59 | 19 | 8 | 11 | 14 | 7 | 7 | 6 | 5 | 1 | 51 |
| Erythpomycin | 43 | 65 | 17 | 11 | 6 | 17 | 9 | 8 | 9 | 8 | 1 | 65 |
| Trimeth/sulf | 29 | 44 | 10 | 6 | 4 | 12 | 8 | 4 | 7 | 7 | 0 | 72 |
| Imipenem | 33 | 50 | 17 | 7 | 10 | 14 | 7 | 7 | 2 | 1 | 1 | 45 |
| Oxacillin | 61 | 92 | 28 | 18 | 10 | 22 | 15 | 7 | 11 | 10 | 1 | 70 |
| Ceftriaxone | 47 | 71 | 20 | 13 | 5 | 18 | 15 | 4 | 9 | 8 | 0 | 77 |
| Clindamycin | 45 | 68 | 19 | 10 | 9 | 18 | 13 | 6 | 8 | 8 | 1 | 69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Shafi, S.; El-Serwy, H.; El-Zawahry, Y.; Zaki, M.; Sitohy, B.; Sitohy, M. The Association between icaA and icaB Genes, Antibiotic Resistance and Biofilm Formation in Clinical Isolates of Staphylococci spp. Antibiotics 2022, 11, 389. https://doi.org/10.3390/antibiotics11030389
Abdel-Shafi S, El-Serwy H, El-Zawahry Y, Zaki M, Sitohy B, Sitohy M. The Association between icaA and icaB Genes, Antibiotic Resistance and Biofilm Formation in Clinical Isolates of Staphylococci spp. Antibiotics. 2022; 11(3):389. https://doi.org/10.3390/antibiotics11030389
Chicago/Turabian StyleAbdel-Shafi, Seham, Heba El-Serwy, Yehia El-Zawahry, Maysaa Zaki, Basel Sitohy, and Mahmoud Sitohy. 2022. "The Association between icaA and icaB Genes, Antibiotic Resistance and Biofilm Formation in Clinical Isolates of Staphylococci spp." Antibiotics 11, no. 3: 389. https://doi.org/10.3390/antibiotics11030389
APA StyleAbdel-Shafi, S., El-Serwy, H., El-Zawahry, Y., Zaki, M., Sitohy, B., & Sitohy, M. (2022). The Association between icaA and icaB Genes, Antibiotic Resistance and Biofilm Formation in Clinical Isolates of Staphylococci spp. Antibiotics, 11(3), 389. https://doi.org/10.3390/antibiotics11030389








