Abstract
This review aims to summarize current progress in the management of critically ill, using biomarkers as guidance for antimicrobial treatment with a focus on antimicrobial stewardship. Accumulated evidence from randomized clinical trials (RCTs) and observational studies in adults for the biomarker-guided antimicrobial treatment of critically ill (mainly sepsis and COVID-19 patients) has been extensively searched and is provided. Procalcitonin (PCT) is the best studied biomarker; in the majority of randomized clinical trials an algorithm of discontinuation of antibiotics with decreasing PCT over serial measurements has been proven safe and effective to reduce length of antimicrobial treatment, antibiotic-associated adverse events and long-term infectious complications like infections by multidrug-resistant organisms and Clostridioides difficile. Other biomarkers, such as C-reactive protein and presepsin, are already being tested as guidance for shorter antimicrobial treatment, but more research is needed. Current evidence suggests that biomarkers, mainly procalcitonin, should be implemented in antimicrobial stewardship programs even in the COVID-19 era, when, although bacterial coinfection rate is low, antimicrobial overconsumption remains high.
1. Introduction
Early and appropriate antimicrobial treatment remains key for sepsis management [1]. It is, however, sometimes difficult even for the most experienced physician to rule-out an infection in the critically ill and withhold antibiotics. The appropriate duration of treatment for severe infections is also not fully described. Current sepsis guidelines recommend a shorter rather than longer duration of antimicrobial treatment, but the definite duration remains at the discretion of the treating physician [2]. Doubts and fear for relapse have led to injudicious broad-spectrum and unnecessarily long antimicrobial treatment adding up to the emergence of antimicrobial resistance. In 2019, about 5 million deaths have been associated with bacterial antimicrobial resistance, underlying the urgent need for tight infection control and robust antimicrobial stewardship programs [3].
A biomarker should be easily measured and interpreted as an indicator of biological or pharmacologic responses to a therapeutic intervention [4]. The optimal sepsis biomarker should be sensitive and specific enough to rule in/out diagnosis, predict unfavorable outcomes and evaluate the host’s response to treatment in order to encourage escalation or de-escalation; this is a strategy called “biomarker-guided treatment” [5]. More than one hundred biomarkers have been studied for sepsis management [6]. However, the only biomarker developed to guide antimicrobial treatment based on evidence coming from randomized clinical trials (RCTs) is procalcitonin (PCT). PCT is a precursor of the thyroid gland hormone calcitonin, and it is increased in the circulation during bacterial infection as a product of cells of mesenchymal origin. This review aims to present cumulative evidence from clinical trials, mainly RCTs, on the use of PCT-guidance in promoting antimicrobial stewardship for the critically-ill by restriction of injudicious antimicrobial treatment. Brief reference is also done to other biomarkers that are under consideration.
2. Results and Discussion
2.1. Antimicrobial Stewardship through PCT-Guidance for Lower Respiratory Tract Infections
PCT is the best studied biomarker to guide antimicrobial treatment in lower respiratory tract infections (LRTI). The majority of these RCTs shared a common design comparing an algorithm to start or discontinue antibiotics based on measurements of PCT, with standard-of-care (SOC); SOC was defined as start, continuation, or stop of antibiotics at the discretion of the treating physician and in accordance with local and international guidelines [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29]. Available trials of PCT-guidance versus SOC are summarized in Table 1. Participants have a wide spectrum of symptoms, ranging from acute exacerbation of asthma and chronic obstructive pulmonary disease admitted in the Emergency Department, to severe community- or hospital-acquired pneumonia necessitating admission in medical wards or in the Intensive Care Unit (ICU). The common finding of all studies is the reduction of antimicrobial treatment duration with PCT-guidance. This reduction of antimicrobial treatment did not generate any safety signal as far as infection relapse, new infection, adverse events, or mortality are concerned. The ProHOSP trial studied the efficacy of PCT guidance directed to both start and stop of antibiotics. More precisely, 671 patients with LRTI received PCT-guided treatment and 688 received SOC [12]. For the PCT group, physicians were advised to start antibiotics when serum PCT was more than 0.25 ng/mL. Measurements were repeated on days 3, 5 and, 7 and stop of treatment was encouraged when levels decreased to more than 80% from the baseline. PCT-guidance led to a shorter antimicrobial treatment compared to SOC (5.7 versus 8.7 days, p < 0.05). However, the ProACT trial conducted in the USA a decade later, failed to show a similar effect. In the ProACT trial, mean duration of treatment for the 826 patients randomized in the PCT group was 4.2 days compared to that of 4.3 days for the 830 patients allocated in the SOC group (p: 0.87) [24]. One explanation for this lack of effect is the already reduced SOC duration of treatment in patients following local guidelines which does not allow any further benefit from the intervention to be shown. The majority of the first trials evaluating PCT-guidance provided such promising results that led to a switch in the current guidelines to a shorter duration of antimicrobial treatment for pneumonia [30] and to the approval of PCT guidance by the US Food and Drug Administration [31].

Table 1.
Summary of randomized trials evaluating Procalcitonin (PCT)-guided antimicrobial treatment in patients with infections outside the Intensive Care Unit (ICU).
2.2. Antimicrobial Stewardship through PCT-Guidance in Sepsis
Critically ill and sepsis patients are the next most commonly studied population for benefit following PCT-guidance [32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51]. The efficacy of existing trials is summarized in Table 2. The majority of participants suffered from LRTI and intra-abdominal or urinary infections were less common. The majority of RCTs were conducted before Sepsis-3 implementation. One concern is that specific subgroups of patients, like pregnant and immunosuppressed, have been excluded from participation. Most of trials conclude that a PCT strategy reduces antimicrobial treatment duration without increase in adverse events and unfavorable outcomes.

Table 2.
Summary of randomized trials evaluating Procalcitonin (PCT)-guided antimicrobial treatment in critically ill patients with severe infection/sepsis in the Intensive Care Unit (ICU).
The PRORATA trial was the first large study evaluating PCT-guidance in ICU patients with suspicion of bacterial infection [36]. Three hundred and seven patients were randomized to PCT-guided treatment and 314 to SOC. For those in the PCT group, an algorithm of both initiation and cessation of antimicrobials was applied. When serum PCT was 0.5 ng/mL or more, physicians were encouraged to start antimicrobials and continue treatment until levels became less than 0.5 ng/mL in serial measurements or they decreased by at least 80% of the baseline value. The same algorithm was followed for every secondary infection episode until day 28 or discharge. The trial ended in a significant decrease in antimicrobial treatment duration from 14.3 days in SOC to 11.6 in PCT group (p < 0.0001). Mortality, relapse, and re-infection rate was similar between the two groups. There was however a trend for higher mortality with PCT-guidance after 60 days.
The largest SAPS trial so far incorporated this knowledge and was designed to evaluate a stopping algorithm based on serial PCT measurements [44]. In the PCT group, physicians were encouraged to stop antimicrobials when PCT was less than 0.5 ng/mL on two consecutive days or PCT decreased by at least 80% of the baseline value. Mean antimicrobial duration was 5 days for 761 patients allocated to PCT group compared to 7 days for 785 patients allocated to SOC (p < 0.0001). Surprisingly, SAPS investigators came across a novel, interesting finding; PCT-guidance reduced both 28-day (19.6% vs. 25%, p: 0.0122) and 1-year mortality (34.8% vs. 40.9%, p: 0.0158).
The recently published PROGRESS trial was the first trial conducted after the introduction of the Sepsis-3 definitions using the same stopping rule for antimicrobials as the SAPS trial [48]. PROGRESS was designed to provide an explanation of the findings of the SAPS trial on mortality. As a consequence, the primary endpoint of PROGRESS was the effect of long-term infectious complications in the critically ill, i.e., the incidence of new infection by multi-drug resistant organisms (MDRO) and Clostridioides difficile and mortality associated with baseline infection by MDRO or C. difficile. The incidence of these long-term complications after six months was 7.2% in the PCT and 15.3% in the SOC group (p: 0.045). Alongside this benefit, PCT guidance, decreased the length of antimicrobial treatment (5 vs. 7 days; p < 0.0001); and decreased 28-day mortality (15.2% vs. 28.2%; p: 0.02) among the 125 patients allocated in the PCT group compared to 131 patients allocated in the SOC group. The incidence of antibiotic-associated adverse events was strikingly decreased using PCT-guidance, in particular diarrhea and acute kidney injury (AKI); in the SOC arm, 36.6% of patients presented diarrhea and 17.6% AKI, compared to 19.2% (p: 0.002) and 7.2% (p: 0.01) in the PCT-guidance arm, respectively. Interestingly, the incidence of gut colonization by MDRO and C. difficile was similar between the two groups but the risk for clinical infection was significantly higher in colonized patients in the SOC but not in the PCT arm. These results indicate that long-term antibiotic exposure in the SOC arm could either affect the integrity of the mucosal barrier or modulate the composition of the gut microbiota resulting in the increased incidence of infections by MDRO and C. difficile.
Two trials similar in design to PROGRESS, are ongoing in France. The MultiCov trial (NCT04334850) is randomizing patients with severe COVID-19 into PCT-guided treatment or SOC. PCT-guidance is accompanied by sampling of respiratory secretions with multiplex PCR to identify bacterial pathogens [52]. The main aim of the study is to show a reduction in antibiotic exposure in the era of COVID-19 having as primary endpoint the number of antibiotic-free days until day 28 and among secondary outcomes the rate of colonization and/or infection by MDRO or C. difficile [53]. The MULTI-CAP trial randomizes patients with severe community-acquired pneumonia in the ICU to a combined PCT/multiplex respiratory PCR arm versus SOC; primary endpoint is antibiotic-free days until day 28.
The benefit disclosed by the larger ProHOSP and SAPS trial was further corroborated by smaller studies from developing countries [49,50,51] and meta-analyses [54,55,56,57,58,59,60,61,62]. A first meta-analysis was published in 2018 including a total of 4482 ICU patients and sub-analyzing patients meeting Sepsis-3 criteria [58]. PCT-guidance reduced 28-day mortality (OR 0.89; 95% CI: 0.80–0.99; p: 0.03) and mean duration of antibiotic treatment (−1.19 days; 95% CI: −1.73 to −0.66; p < 0.0001). Meta-analyses also confirmed reduction of antimicrobial treatment by PCT-guidance in special populations, such as patients with bacteremia [63], renal failure [64], or among the elderly [65]. Interestingly, some meta-analyses support that PCT-guidance is associated with decreased antimicrobial consumption and mortality only if cessation algorithms are applied [58]. A summary of published meta-analyses evaluating PCT-guidance is presented in Table 3 [54,55,56,57,58,59,60,61,62,63,64,65,66].

Table 3.
Summary of meta-analyses evaluating Procalcitonin (PCT)-guided antimicrobial treatment in critically ill patients.
2.3. Real-World Data
Evidence supporting PCT-guidance for antimicrobial stewardship in critically ill patients, as already discussed, is from RCTs with different degrees of compliance to the PCT rule applied in each RCT, ranging from 44% up to 97%. It has not yet been clear if low adherence to PCT algorithms interferes with results and affects antimicrobial duration and mortality. Results of RCTs may not be in alignment with real-world data. Treating physicians participating in a RCT are influenced in decision making as they may feel under observation from the Sponsor or trial coordinators; this is namely the “Hawthorne effect” [67]. With this in mind, real-world evidence is mandatory. Soon after ProHOSP trial has been published, real world data supported compliance of physicians with the suggested algorithm as high as 72.5% [68].
Several implementation trials have investigated the effect of PCT-guidance in antimicrobial stewardship programs [69,70,71,72,73,74,75,76,77]. Main conclusions of these trials include (i) reduction in antimicrobial consumption; (ii) reduction in length of stay; (iii) reduction in hospitalization cost; and iv) no difference in infection-relapse of rehospitalization rate. Best implementation of the biomarker in real-world settings requires constant education of treating physicians for rightful use [78,79].
2.4. Antimicrobial Stewardship through Other Biomarkers
Other biomarkers have been also tested in antibiotic stewardship programs like serum C-reactive protein (CRP), serum presepsin, and interleukin (IL)-1β/IL-18 in bronchoalveolar lavage (BAL).
In a former trial, CRP was compared to PCT for the early stop of antibiotics. Discontinuation of antibiotics in the PCT arm was advised by more than 90% baseline decreases (n = 49) and in the CRP arm by more than 50% baseline decreases or values less than 25 mg/L (n = 45) [41]. Both strategies were non-inferior in terms of length of treatment, relapse rate, and ICU length of stay. A recent trial compared in a 1:1:1 randomization pattern, the clinical effectiveness of CRP-guided stop of antibiotics with fixed 7- and 14-day antibiotic durations in 504 hospitalized patients with gram-negative bacteremia [80]. Median antibiotic duration in the CRP group was seven days; clinical failure between the three arms of treatment was non-inferior. In another open-label RCT, CRP-guided antimicrobial treatment was compared to SOC in 130 ICU patients with sepsis and/or septic shock [81]. In the CRP arm, the biomarker was measured after five days from start of antibiotics and antibiotics were stopped when CRP decrease more than 50% or when it was found less than 35 mg/L. This strategy did reduce antibiotic duration or 28-day mortality.
Presepsin is the soluble form of CD14 (sCD14), an anchored glycoprotein expressed on monocytes and macrophages, serving as a receptor for bacterial lipopolysaccharide (LPS) [82]. Compared to CRP and PCT, presepsin appears advantageous in sepsis diagnosis, as it rises early, already in the first two hours after an infection. Recently, Xiao et al., conducted a prospective, multicenter, not randomized trial in China, comparing presepsin-guidance to SOC in sepsis [83]. In the presepsin group, physicians were advised to stop the antibiotics by serum concentrations lower than 350 pg/mL or any baseline decrease more than 80%. Antibiotic adjustment was encouraged when the blood presepsin concentration did not decline. Although the primary outcome (days without antibiotics at day 28) was achieved, mortality did not differ between treatment arms.
In a recent trial conducted in the United Kingdom, 210 ICU patients with suspicion of ventilator-associated pneumonia (VAP) were allocated to a biomarker-guided approach (n: 104) or SOC (n: 106) [84]. In the biomarker-guided recommendation group measurements of IL-1β and IL-18 were performed in the bronchoalveolar lavage (BAL), and if concentrations were below a previously validated cutoff, clinicians were advised that VAP was unlikely and withheld antibiotics. The primary outcome was antibiotic-free days in the seven days following BAL; the trial did not achieve this endpoint.
2.5. Antimicrobial Stewardship Using Biomarkers in the COVID-19 Era
In December 2019, a novel coronavirus, namely SARS-CoV-2, spread rapidly around the globe causing millions of cases of pneumonia leading to a rapid increase in hospitalizations and deaths. Patients presenting with COVID-19 pneumonia share common features with bacterial pneumonia (fever, cough, dyspnea, infiltrates in chest X-ray, and elevated inflammation markers) making the differential diagnosis troublesome. In severe cases, COVID-19 may resemble bacterial sepsis leading to multiorgan failure and requiring organ support in the ICU [85]. Although data are very heterogenous, unlike other viral respiratory diseases, bacterial co-infection at the time of hospital admission is rare in COVID-19; this may occur during hospital and/or ICU stay. A recent systematic review reports a rate of 8% of COVID-19 bacterial coinfection; surprisingly, the proportion of patients receiving antimicrobials is as high as 72% [86]. In such case, biomarkers, mainly PCT, may be useful in reducing unnecessary antimicrobial consumption.
A number of small case-series support that PCT is not elevated in COVID-19 patients, in contrast to other inflammation markers like CRP and ferritin [87,88,89]. The largest of these observational studies, conducted in New York, reports that only 16.9% of patients have PCT levels 0.5 ng/mL or more at hospital admission [90] and such high levels are associated with development of critical disease, admission in the ICU and increased risk for death [91,92,93]. A recent meta-analysis of 10 cohort studies including a total of 7716 patients estimated a pooled risk of 1.77 (95% CI, 1.38 to 2.29) for severe and critical COVID-19 by elevated PCT levels at admission, although results are highly heterogenous (I2:85.6%) [94]. Similarly, rise in PCT is associated with secondary bacterial infections, such as VAP and bacteremia [91,95,96,97]. PCT levels less than 0.25 ng/mL have been suggested as an optimal cut-off to rule out bacterial co-infection (negative predictive value 81%) and levels more than 1 ng/mL as optimal cutoff to rule in bacterial co-infection (positive predictive value 93%) [95].
It is questionable if pre-treatment with dexamethasone and tocilizumab in these patients is limiting the diagnostic performance of biomarkers. Kooistra et al. studied 190 ICU patients with COVID-19 having received different immunomodulatory agents and concluded that after treatment with dexamethasone and/or tocilizumab, CRP levels remain suppressed in case of a secondary bacterial infection but that the kinetics of PCT were not affected [98]. Thus, it is reasonable that CRP, which is elevated by the COVID-19-driven hyperinflammation and is suppressed by the immunomodulatory treatment does not represent the optimal biomarker to screen for bacterial complications in critically ill COVID-19. In contrast, PCT may inform about the early diagnosis of bacterial superinfection.
Real world data of PCT-guidance in COVID-19 support its use for a judicious antimicrobial approach. In a small retrospective cohort of 48 patients, median duration of antimicrobials was shorter if at least one PCT measurement was performed [99]. Similar results were also reported by Calderon et al. [100]. Williams et al. implemented a PCT guideline in the first 48 h after hospital admission of COVID-19 patients to withhold antibiotics with PCT less than 25 ng/mL [101]. Adherence to the guideline was high (77%). This strategy ended in lower defined daily doses (DDDs) per day alive, lower 28-day mortality, lower intubation, and ICU-admission rate. Staub et al. reported an increase in the antimicrobial usage during the COVID-19 pandemic compared with the pre-COVID era, but this usage decreased again after implementation of a guidance team using biomarkers [102]. A summary of PCT trials in COVID-19 patients is provided in Table 4.

Table 4.
Summary of trials evaluating Procalcitonin (PCT) in COVID-19 patients.
In contrast with a plethora of RCTs evaluating PCT-guidance in sepsis, such high-quality data are missing for COVID-19. MultiCov is an ongoing RCT in France, evaluating PCT-guided treatment in combination with FilmArray syndromic diagnostics compared to SOC to prove a benefit in the number of antibiotic-free days, mortality, rate of bacterial superinfection and rate of colonization/infection by MDRO and/or C. difficile (NCT04334850) [52]. Results of the trial will be of great interest to guide appropriate antimicrobial administration in the COVID-19 era.
3. Materials and Methods
To address the aim of this review and to present recent evidence in biomarker-guidance in the critically ill with emphasis on antimicrobial stewardship, the authors searched independently “Pubmed” and the database “clinicaltrials.gov” under the terms: “sepsis”, “COVID-19”, “infection”, “critically ill”, “intensive care unit”, “biomarker guidance”, “guided treatment”, “procalcitonin”, and “c-reactive protein” about randomized clinical trials and observational studies conducted in humans aged equal to or older than 18 years old, published in English, with emphasis on trials published in the last decade (2012–2022). The literature search yielded 11,791 records; after removal of duplicates and records with irrelevant titles, 611 were screened in full-text by the reviewers. After applying exclusion criteria, 102 studies were finally analyzed (Figure 1).
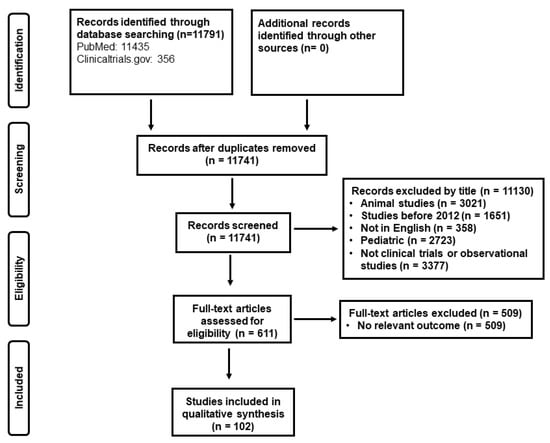
Figure 1.
Study selection.
4. Conclusions
Biomarkers, mainly procalcitonin, may guide antimicrobial treatment with safety in two directions; (i) improve patient outcomes by reduction in antibiotic-associated adverse events and (ii) globally reduce the high burden of antimicrobial resistance. Procalcitonin-guidance of antimicrobial treatment for the critically ill decreases the length of antimicrobial treatment, the length of stay (Hospital/ICU), and the cost of hospitalization and in parallel, the strategy improves both short- and long-term outcomes including mortality and rate of secondary infections by MDRO and C. difficile. In the COVID-19 era, data suggest a crucial role of the biomarker to reduce unnecessary antimicrobial overuse. Thus, biomarkers should be incorporated in antimicrobial stewardship programs and physicians’ education is key for their appropriate application in every day clinical practice.
Author Contributions
E.K. drafted the first version of the manuscript and E.J.G.-B. critically revised for intellectual content. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Hellenic Institute for the Study of Sepsis.
Data Availability Statement
All data are presented throughout the manuscript.
Conflicts of Interest
E.J.G.-B. has received honoraria from Abbott CH, bioMérieux, Brahms GmbH, GSK, InflaRx GmbH, Sobi and XBiotech Inc; independent educational grants from Abbott CH, AxisShield, bioMérieux Inc, InflaRx GmbH, Johnson & Johnson, MSD, Sobi and XBiotech Inc.; and funding from the Horizon2020 Marie-Curie Project European Sepsis Academy (granted to the National and Kapodistrian University of Athens), and the Horizon 2020 European Grants ImmunoSep and RISCinCOVID (granted to the Hellenic Institute for the Study of Sepsis). E.K. reports no conflicts of interest.
References
- Kumar, A.; Roberts, D.; Wood, K.E.; Light, B.; Parrillo, J.E.; Sharma, S.; Suppes, R.; Feinstein, D.; Zanotti, S.; Taiberg, L.; et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit. Care Med. 2006, 34, 1589–1596. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- The Biomarker Definitions Working Group. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar] [CrossRef]
- Sankar, V.; Webster, N.R. Clinical application of sepsis biomarkers. J. Anesth. 2013, 27, 269–283. [Google Scholar] [CrossRef]
- Pierrakos, C.; Vincent, J.L. Sepsis biomarkers: A review. Crit. Care 2010, 14, R15. [Google Scholar] [CrossRef]
- Christ-Crain, M.; Jaccard-Stolz, D.; Bingisser, R.; Gencay, M.M.; Huber, P.R.; Tamm, M.; Müller, B. Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: Cluster-randomised, single-blinded intervention trial. Lancet 2004, 363, 600–607. [Google Scholar] [CrossRef]
- Christ-Crain, M.; Stolz, D.; Bingisser, R.; Muller, C.; Miedinger, D.; Huber, P.R.; Zimmerli, W.; Harbarth, S.; Tamm, M.; Muller, B. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: A randomized trial. Am. J. Respir. Crit. Care Med. 2006, 174, 84–93. [Google Scholar] [CrossRef]
- Stolz, D.; Christ-Crain, M.; Bingisser, R.; Leuppi, J.; Miedinger, D.; Müller, C.; Huber, P.; Müller, B.; Tamm, M. Antibiotic treatment of exacerbations of COPD: A randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest 2007, 131, 9–19. [Google Scholar] [CrossRef]
- Briel, M.; Schuetz, P.; Mueller, B.; Young, J.; Schild, U.; Nusbaumer, C.; Périat, P.; Bucher, H.C.; Christ-Crain, M. Procalcitonin-guided antibiotic use vs a standard approach for acute respiratory tract infections in primary care. Arch. Intern. Med. 2008, 168, 2000–2008. [Google Scholar] [CrossRef]
- Kristoffersen, K.; Søgaard, O.; Wejse, C.; Black, F.; Greve, T.; Tarp, B.; Storgaard, M.; Sodemann, M. Antibiotic treatment interruption of suspected lower respiratory tract infections based on a single procalcitonin measurement at hospital admission—A randomized trial. Clin. Microbiol. Infect. 2009, 15, 481–487. [Google Scholar] [CrossRef]
- Schuetz, P.; Christ-Crain, M.; Thomann, R.; Falconnier, C.; Wolbers, M.; Widmer, I.; Neidert, S.; Fricker, T.; Blum, C.; Schild, U.; et al. Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: The ProHOSP randomized controlled trial. JAMA 2009, 302, 1059–1066. [Google Scholar] [CrossRef]
- Burkhardt, O.; Ewig, S.; Haagen, U.; Giersdorf, S.; Hartmann, O.; Wegscheider, K.; Hummers-Pradier, E.; Welte, T. Procalcitonin guidance and reduction of antibiotic use in acute respiratory tract infection. Eur. Respir. J. 2010, 36, 601–607. [Google Scholar] [CrossRef]
- Long, W.; Deng, X.; Zhang, Y.; Lu, G.; Xie, J.; Tang, J. Procalcitonin guidance for reduction of antibiotic use in low-risk outpatients with community-acquired pneumonia. Respirology 2011, 16, 819–824. [Google Scholar] [CrossRef]
- Tang, J.; Long, W.; Yan, L.; Zhang, Y.; Xie, J.; Lu, G.; Yang, C. Procalcitonin guided antibiotic therapy of acute exacerbations of asthma: A randomized controlled trial. BMC Infect. Dis. 2013, 13, 596. [Google Scholar] [CrossRef]
- Ogasawara, T.; Umezawa, H.; Naito, Y.; Takeuchi, T.; Kato, S.; Yano, T.; Kasamatsu, N.; Hashizume, I. Procalcitonin-guided antibiotic therapy in aspiration pneumonia and an assessment of the continuation of oral intake. Respir. Investig. 2014, 52, 107–113. [Google Scholar] [CrossRef]
- Long, W.; Li, L.-J.; Huang, G.-Z.; Zhang, X.-M.; Zhang, Y.-C.; Tang, J.-G.; Zhang, Y.; Lu, G. Procalcitonin guidance for reduction of antibiotic use in patients hospitalized with severe acute exacerbations of asthma: A randomized controlled study with 12-month follow-up. Crit. Care 2014, 18, 471. [Google Scholar] [CrossRef][Green Version]
- Verduri, A.; Luppi, F.; D’amico, R.; Balduzzi, S.; Vicini, R.; Liverani, A.; Ruggieri, V.; Plebani, M.; Barbaro, M.P.F.; Spanevello, A.; et al. Antibiotic treatment of severe exacerbations of chronic obstructive pulmonary disease with procalcitonin: A randomized noninferiority trial. PLoS ONE 2015, 10, e0118241. [Google Scholar] [CrossRef]
- Branche, A.R.; Walsh, E.E.; Vargas, R.; Hulbert, B.; Formica, M.A.; Baran, A.; Peterson, D.R.; Falsey, A.R. Serum procalcitonin measurement and viral testing to guide antibiotic use for respiratory infections in hospitalized adults: A randomized controlled trial. J. Infect. Dis. 2015, 212, 1692–1700. [Google Scholar] [CrossRef]
- Corti, C.; Fally, M.; Fabricius-Bjerre, A.; Mortensen, K.; Jensen, B.N.; Andreassen, H.; Porsbjerg, C.; Knudsen, J.D.; Jensen, J.-U. Point-of-care procalcitonin test to reduce antibiotic exposure in patients hospitalized with acute exacerbation of COPD. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 1381–1389. [Google Scholar] [CrossRef]
- Ulm, L.; Hoffmann, S.; Nabavi, D.; Hermans, M.; Mackert, B.-M.; Hamilton, F.; Schmehl, I.; Jungehuelsing, G.-J.; Montaner, J.; Bustamante, A.; et al. The randomized controlled STRAWINSKI trial: Procalcitonin-guided antibiotic therapy after stroke. Front. Neurol. 2017, 8, 153. [Google Scholar] [CrossRef]
- Bremmer, D.N.; DiSilvio, B.E.; Hammer, C.; Beg, M.; Vishwanathan, S.; Speredelozzi, D.; Moffa, M.A.; Hu, K.; Abdulmassih, R.; Makadia, J.T.; et al. Impact of procalcitonin guidance on management of adults hospitalized with chronic obstructive pulmonary disease exacerbations. J. Gen. Intern. Med. 2018, 33, 692–697. [Google Scholar] [CrossRef]
- Daubin, C.; Valette, X.; Thiollière, F.; Mira, J.P.; Hazera, P.; Annane, D.; Labbe, V.; Floccard, B.; Fournel, F.; Terzi, N.; et al. Procalcitonin algorithm to guide initial antibiotic therapy in acute exacerbations of COPD admitted to the ICU: A randomized multicenter study. Intensive Care Med. 2018, 44, 428–437. [Google Scholar] [CrossRef]
- Huang, D.T.; Yealy, D.M.; Filbin, M.R.; Brown, A.M.; Chang, C.-C.H.; Doi, Y.; Donnino, M.W.; Fine, J.; Fine, M.J.; Fischer, M.A.; et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N. Engl. J. Med. 2018, 379, 236–249. [Google Scholar] [CrossRef]
- van der Does, Y.; Limper, M.; Jie, K.E.; Schuit, S.C.E.; Jansen, H.; Pernot, N.; van Rosmalen, J.; Poley, M.J.; Ramakers, C.; Patka, P.; et al. Procalcitonin-guided antibiotic therapy in patients with fever in a general emergency department population: A multicentre non-inferiority randomized clinical trial (HiTEMP study). Clin. Microbiol. Infect. 2018, 24, 1282–1289. [Google Scholar] [CrossRef]
- Townsend, J.; Adams, V.; Galiatsatos, P.; Pearse, D.; Pantle, H.; Masterson, M.; Kisuule, F.; Jacob, E.; Kiruthi, C.; Ortiz, P.; et al. Procalcitonin-guided antibiotic therapy reduces antibiotic use for lower respiratory tract infections in a united states medical center: Results of a clinical trial. Open Forum Infect. Dis. 2018, 5, ofy327. [Google Scholar] [CrossRef]
- Montassier, E.; Javaudin, F.; Moustafa, F.; Nandjou, D.; Maignan, M.; Hardouin, J.-B.; Annoot, C.; Ogielska, M.; Orer, P.-L.; Schotté, T.; et al. Guideline-based clinical assessment versus procalcitonin-guided antibiotic use in pneumonia: A pragmatic randomized trial. Ann. Emerg. Med. 2019, 74, 580–591. [Google Scholar] [CrossRef]
- Akagi, T.; Nagata, N.; Wakamatsu, K.; Harada, T.; Miyazaki, H.; Takeda, S.; Ushijima, S.; Aoyama, T.; Yoshida, Y.; Yatsugi, H.; et al. Procalcitonin-guided antibiotic discontinuation might shorten the duration of antibiotic treatment without increasing pneumonia recurrence. Am. J. Med. Sci. 2019, 358, 33–44. [Google Scholar] [CrossRef]
- Möckel, M.; De Boer, R.A.; Slagman, A.; Von Haehling, S.; Schou, M.; Vollert, J.O.; Wiemer, J.C.; Ebmeyer, S.; Martín-Sánchez, F.J.; Maisel, A.S.; et al. Improve management of acute heart failure with ProcAlCiTonin in EUrope: Results of the randomized clinical trial IMPACT EU Biomarkers in Cardiology (BIC)18. Eur. J. Heart Fail. 2020, 22, 267–275. [Google Scholar] [CrossRef]
- Metlay, J.P.; Waterer, G.W.; Long, A.C.; Anzueto, A.; Brozek, J.; Crothers, K.; Cooley, L.A.; Dean, N.C.; Fine, M.J.; Flanders, S.A.; et al. Diagnosis and treatment of adults with community-acquired pneumonia, an official clinical practice guideline of the American thoracic society and infectious diseases society of America. Am. J. Respir. Crit. Care Med. 2019, 200, e45–e67. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. FDA News Release: FDA Clears Test to Help Manage Antibiotic Treatment for Lower Respiratory Tract Infections and Sepsis. 2017. Available online: https://www.fda.gov/news-events/press-announcements/fda-clears-test-help-manage-antibiotic-treatment-lower-respiratory-tract-infections-and-sepsis (accessed on 29 January 2022).
- Nobre, V.; Harbarth, S.; Graf, J.D.; Rohner, P.; Pugin, J. Use of procalcitonin to shorten antibiotic treatment duration in septic patients: A randomized trial. Am. J. Respir. Crit. Care Med. 2008, 177, 498–505. [Google Scholar] [CrossRef]
- Schroeder, S.; Hochreiter, M.; Koehler, T.; Schweiger, A.-M.; Bein, B.; Keck, F.S.; Von Spiegel, T. Procalcitonin (PCT)-guided algorithm reduces length of antibiotic treatment in surgical intensive care patients with severe sepsis: Results of a prospective randomized study. Langenbecks Arch. Surg. 2009, 394, 221–226. [Google Scholar] [CrossRef]
- Hochreiter, M.; Köhler, T.; Schweiger, A.M.; Keck, F.S.; Bein, B.; Von Spiegel, T.; Schroeder, S. Procalcitonin to guide duration of antibiotic therapy in intensive care patients: A randomized prospective controlled trial. Crit. Care 2009, 13, R83. [Google Scholar] [CrossRef]
- Stolz, D.; Smyrnios, N.; Eggimann, P.; Pargger, H.; Thakkar, N.; Siegemund, M.; Marsch, S.; Azzola, A.; Rakic, J.; Mueller, B.; et al. Procalcitonin for reduced antibiotic exposure in ventilator-associated pneumonia: A randomised study. Eur. Respir. J. 2009, 34, 1364–1375. [Google Scholar] [CrossRef]
- Bouadma, L.; Luyt, C.-E.; Tubach, F.; Cracco, C.; Alvarez, A.; Schwebel, C.; Schortgen, F.; Lasocki, S.; Veber, B.; Dehoux, M.; et al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): A multicentre randomised controlled trial. Lancet 2010, 375, 463–474. [Google Scholar] [CrossRef]
- Jensen, J.U.; Hein, L.; Lundgren, B.; Bestle, M.H.; Mohr, T.T.; Andersen, M.H.; Thornberg, K.J.; Løken, J.; Steensen, M.; Fox, Z.; et al. Procalcitonin-guided interventions against infections to increase early appropriate antibiotics and improve survival in the intensive care unit: A randomized trial. Crit. Care Med. 2011, 39, 2048–2058. [Google Scholar] [CrossRef]
- Layios, N.; Lambermont, B.; Canivet, J.-L.; Morimont, P.; Preiser, J.-C.; Garweg, C.; LeDoux, D.; Frippiat, F.; Piret, S.; Giot, J.-B.; et al. Procalcitonin usefulness for the initiation of antibiotic treatment in intensive care unit patients. Crit. Care Med. 2012, 40, 2304–2309. [Google Scholar] [CrossRef]
- Qu, R.; Ji, Y.; Ling, Y.; Ye, C.Y.; Yang, S.M.; Liu, Y.Y.; Yang, R.Y.; Luo, Y.F.; Guo, Z. Procalcitonin is a good tool to guide duration of antibiotic therapy in patients with severe acute pancreatitis. A randomized prospective single-center controlled trial. Saudi Med. J. 2012, 33, 382–387. [Google Scholar]
- Deliberato, R.O.; Marra, A.R.; Sanches, P.R.; Martino, M.D.V.; Ferreira, C.E.D.S.; Pasternak, J.; Paes, A.T.; Pinto, L.M.; dos Santos, O.F.P.; Edmond, M. Clinical and economic impact of procalcitonin to shorten antimicrobial therapy in septic patients with proven bacterial infection in an intensive care setting. Diagn. Microbiol. Infect. Dis. 2013, 76, 266–271. [Google Scholar] [CrossRef]
- Oliveira, C.F.; Botoni, F.A.; Oliveira, C.R.; Silva, C.B.; Pereira, H.A.; Serufo, J.C.; Nobre, V. Procalcitonin versus C-reactive protein for guiding antibiotic therapy in sepsis: A randomized trial. Crit. Care Med. 2013, 41, 2336–2343. [Google Scholar] [CrossRef]
- Annane, D.; Maxime, V.; Faller, J.P.; Mezher, C.; Clec’h, C.; Martel, P.; Gonzales, H.; Feissel, M.; Cohen, Y.; Capellier, G.; et al. Procalcitonin levels to guide antibiotic therapy in adults with non-microbiologically proven apparent severe sepsis: A randomised controlled trial. BMJ Open 2013, 3, e002186. [Google Scholar] [CrossRef]
- Shehabi, Y.; Sterba, M.; Garrett, P.M.; Rachakonda, K.S.; Stephens, D.; Harrigan, P.; Walker, A.; Bailey, M.; Johnson, B.; Millis, D.; et al. Procalcitonin algorithm in critically ill adults with undifferentiated infection or suspected sepsis. A randomized controlled trial. Am. J. Respir. Crit. Care Med. 2014, 190, 1102–1110. [Google Scholar] [CrossRef]
- de Jong, E.; van Oers, J.A.; Beishuizen, A.; Vos, P.; Vermeijden, W.J.; Haas, L.E.; Loef, B.G.; Dormans, T.; van Melsen, G.C.; Kluiters, Y.C.; et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: A randomised, controlled, open-label trial. Lancet Infect. Dis. 2016, 16, 819–827. [Google Scholar] [CrossRef]
- Bloos, F.; Trips, E.; Nierhaus, A.; Briegel, J.; Heyland, D.K.; Jaschinski, U.; Moerer, O.; Weyland, A.; Marx, G.; Gründling, M.; et al. Effect of sodium selenite administration and procalcitonin-guided therapy on mortality in patients with severe sepsis or septic shock: A randomized clinical trial. JAMA Intern. Med. 2016, 176, 1266–1276. [Google Scholar] [CrossRef]
- Jeon, K.; Suh, J.K.; Jang, E.J.; Cho, S.; Ryu, H.G.; Na, S.; Hong, S.B.; Lee, H.J.; Kim, J.Y.; Lee, S.M. Procalcitonin-guided treatment on duration of antibiotic therapy and cost in septic patients (PRODA): A multi-center randomized controlled trial. J. Korean Med. Sci. 2019, 34, e110. [Google Scholar] [CrossRef]
- Beye, F.; Vigneron, C.; Dargent, A.; Prin, S.; Andreu, P.; Large, A.; Quenot, J.P.; Bador, J.; Bruyere, R.; Charles, P.E. Adhering to the procalcitonin algorithm allows antibiotic therapy to be shortened in patients with ventilator-associated pneumonia. J. Crit. Care 2019, 53, 125–131. [Google Scholar] [CrossRef]
- Kyriazopoulou, E.; Liaskou-Antoniou, L.; Adamis, G.; Panagaki, A.; Melachroinopoulos, N.; Drakou, E.; Marousis, K.; Chrysos, G.; Spyrou, A.; Alexiou, N.; et al. Procalcitonin to reduce long-term infection-associated adverse events in sepsis. A randomized trial. Am. J. Respir. Crit. Care Med. 2021, 203, 202–210. [Google Scholar] [CrossRef]
- Chomba, R.N.; Moeng, M.S.; Lowman, W. Procalcitonin-guided antibiotic therapy for suspected and confirmed sepsis of patients in a surgical trauma ICU: A prospective, two period cross-over, interventional study. S. Afr. J. Surg. 2020, 5, 143–149. [Google Scholar] [CrossRef]
- Mazlan, M.Z.; Ismail, M.A.; Ali, S.; Salmuna, Z.N.; Shukeri, W.F.W.M.; Omar, M. Efficacy and safety of the point-of-care procalcitonin test for determining the antibiotic treatment duration in patients with ventilator-associated pneumonia in the intensive care unit: A randomised controlled trial. Anaesthesiol. Intensive Ther. 2021, 53, 207–214. [Google Scholar] [CrossRef]
- Vishalashi, S.G.; Gupta, P.; Verma, P.K. Serum procalcitonin as a biomarker to determine the duration of antibiotic therapy in adult patients with sepsis and septic shock in intensive care units: A prospective study. Indian J. Crit. Care Med. 2021, 25, 507–511. [Google Scholar]
- Use of a Respiratory Multiplex PCR and Procalcitonin to Reduce Antibiotics Exposure in Patients with Severe Confirmed COVID-19 Pneumonia (MultiCov). Available online: https://clinicaltrials.gov/ct2/show/NCT04334850 (accessed on 29 January 2022).
- Voiriot, G.; Fartoukh, M.; Durand-Zaleski, I.; Berard, L.; Rousseau, A.; Armand-Lefevre, L.; Verdet, C.; Argaud, L.; Klouche, K.; Megarbane, B.; et al. Combined use of a broad-panel respiratory multiplex PCR and procalcitonin to reduce duration of antibiotics exposure in patients with severe community-acquired pneumonia (MULTI-CAP): A multicentre, parallel-group, open-label, individual randomised trial conducted in French intensive care units. BMJ Open 2021, 11, e048187. [Google Scholar]
- Andriolo, B.N.; Andriolo, R.B.; Salomão, R.; Atallah, Á.N. Effectiveness and safety of procalcitonin evaluation for reducing mortality in adults with sepsis, severe sepsis or septic shock. Cochrane Database Syst. Rev. 2017, 1, CD010959. [Google Scholar] [CrossRef]
- Huang, H.B.; Peng, J.M.; Weng, L.; Wang, C.Y.; Jiang, W.; Du, B. Procalcitonin-guided antibiotic therapy in intensive care unit patients: A systematic review and meta-analysis. Ann. Intensive Care 2017, 7, 114. [Google Scholar] [CrossRef]
- Schuetz, P.; Wirz, Y.; Sager, R.; Christ-Crain, M.; Stolz, D.; Tamm, M.; Bouadma, L.; Luyt, C.E.; Wolff, M.; Chastre, J.; et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: A patient level meta-analysis. Lancet Infect. Dis. 2018, 18, 95–107. [Google Scholar] [CrossRef]
- Wirz, Y.; Meier, M.A.; Bouadma, L.; Luyt, C.E.; Wolff, M.; Chastre, J.; Tubach, F.; Schroeder, S.; Nobre, V.; Annane, D.; et al. Effect of procalcitonin-guided antibiotic treatment on clinical outcomes in intensive care unit patients with infection and sepsis patients: A patient-level meta-analysis of randomized trials. Crit. Care 2018, 22, 191. [Google Scholar] [CrossRef]
- Lam, S.W.; Bauer, S.R.; Fowler, R.; Duggal, A. Systematic review and meta-analysis of procalcitonin-guidance versus usual care for antimicrobial management in critically ill patients: Focus on subgroups based on antibiotic initiation, cessation, or mixed strategies. Crit. Care Med. 2018, 46, 684–690. [Google Scholar] [CrossRef]
- Iankova, I.; Thompson-Leduc, P.; Kirson, N.Y.; Rice, B.; Hey, J.; Krause, A.; Schonfeld, S.A.; DeBrase, C.R.; Bozzette, S.; Schuetz, P. Efficacy and safety of procalcitonin guidance in patients with suspected or confirmed sepsis: A systematic review and meta-analysis. Crit. Care Med. 2018, 46, 691–698. [Google Scholar] [CrossRef]
- Pepper, D.J.; Sun, J.; Rhee, C.; Welsh, J.; Powers, J.H., III; Danner, R.L.; Kadri, S.S. Procalcitonin-guided antibiotic discontinuation and mortality in critically ill adults: A systematic review and meta-analysis. Chest 2019, 155, 1109–1118. [Google Scholar] [CrossRef]
- Peng, F.; Chang, W.; Xie, J.F.; Sun, Q.; Qiu, H.B.; Yang, Y. Ineffectiveness of procalcitonin-guided antibiotic therapy in severely critically ill patients: A meta-analysis. Int. J. Infect. Dis. 2019, 85, 158–166. [Google Scholar] [CrossRef]
- Arulkumaran, N.; Khpal, M.; Tam, K.; Baheerathan, A.; Corredor, C.; Singer, M. Effect of antibiotic discontinuation strategies on mortality and infectious complications in critically ill septic patients: A meta-analysis and trial sequential analysis. Crit. Care Med. 2020, 48, 757–764. [Google Scholar] [CrossRef]
- Meier, M.A.; Branche, A.; Neeser, O.L.; Wirz, Y.; Haubitz, S.; Bouadma, L.; Wolff, M.; Luyt, C.E.; Chastre, J.; Tubach, F.; et al. Procalcitonin-guided antibiotic treatment in patients with positive blood cultures: A patient-level meta-analysis of randomized trials. Clin. Infect. Dis. 2019, 69, 388–396. [Google Scholar] [CrossRef]
- Heilmann, E.; Gregoriano, C.; Wirz, Y.; Luyt, C.E.; Wolff, M.; Chastre, J.; Tubach, F.; Christ-Crain, M.; Bouadma, L.; Annane, D.; et al. Association of kidney function with effectiveness of procalcitonin-guided antibiotic treatment: A patient-level meta-analysis from randomized controlled trials. Clin. Chem. Lab. Med. 2020, 59, 441–453. [Google Scholar] [CrossRef]
- Heilmann, E.; Gregoriano, C.; Annane, D.; Reinhart, K.; Bouadma, L.; Wolff, M.; Chastre, J.; Luyt, C.-E.; Tubach, F.; Branche, A.R.; et al. Duration of antibiotic treatment using procalcitonin-guided treatment algorithms in older patients: A patient-level meta-analysis from randomized controlled trials. Age Ageing 2021, 50, 1546–1556. [Google Scholar] [CrossRef]
- Gutiérrez-Pizarraya, A.; León-García, M.D.C.; De Juan-Idígoras, R.; Garnacho-Montero, J. Clinical impact of procalcitonin-based algorithms for duration of antibiotic treatment in critically ill adult patients with sepsis: A meta-analysis of randomized clinical trials. Expert Rev. Anti-Infect. Ther. 2022, 20, 103–112. [Google Scholar] [CrossRef]
- Schuetz, P.; Wahl, P.M. Additional real-world evidence supporting procalcitonin as an effective tool to improve antibiotic management and cost of the critically ill patient. Chest 2017, 151, 6–8. [Google Scholar] [CrossRef]
- Schuetz, P.; Batschwaroff, M.; Dusemund, F.; Albrich, W.; Bürgi, U.; Maurer, M.; Brutsche, M.; Huber, A.R.; Müller, B. Effectiveness of a procalcitonin algorithm to guide antibiotic therapy in respiratory tract infections outside of study conditions: A post-study survey. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 269–277. [Google Scholar] [CrossRef]
- Hohn, A.; Heising, B.; Hertel, S.; Baumgarten, G.; Hochreiter, M.; Schroeder, S. Antibiotic consumption after implementation of a procalcitonin-guided antimicrobial stewardship programme in surgical patients admitted to an intensive care unit: A retrospective before-and-after analysis. Infection 2015, 43, 405–412. [Google Scholar] [CrossRef]
- Walsh, T.L.; DiSilvio, B.E.; Hammer, C.; Beg, M.; Vishwanathan, S.; Speredelozzi, D.; Moffa, M.A.; Hu, K.; Abdulmassih, R.; Makadia, J.T.; et al. Impact of procalcitonin guidance with an educational program on management of adults hospitalized with pneumonia. Am. J. Med. 2018, 131, 201.e1–201.e8. [Google Scholar] [CrossRef]
- Balk, R.A.; Kadri, S.S.; Cao, Z.; Robinson, S.B.; Lipkin, C.; Bozzette, S.A. Effect of procalcitonin testing on health-care utilization and costs in critically ill patients in the United States. Chest 2017, 151, 23–33. [Google Scholar] [CrossRef]
- Newton, J.A.; Robinson, S.; Ling, C.L.L.; Zimmer, L.; Kuper, K.; Trivedi, K.K. Impact of procalcitonin levels combined with active intervention on antimicrobial stewardship in a community hospital. Open Forum Infect. Dis. 2019, 6, ofz355. [Google Scholar] [CrossRef]
- Collins, C.D.; Brockhaus, K.; Sim, T.; Suneja, A.; Malani, A.N. Analysis to determine cost-effectiveness of procalcitonin-guided antibiotic use in adult patients with suspected bacterial infection and sepsis. Am. J. Health Syst. Pharm. 2019, 76, 1219–1225. [Google Scholar] [CrossRef]
- Westwood, M.; Ramaekers, B.; Whiting, P.; Tomini, F.; Joore, M.; Armstrong, N.; Ryder, S.; Stirk, L.; Severens, H.; Kleijnen, J. Procalcitonin testing to guide antibiotic therapy for the treatment of sepsis in intensive care settings and for suspected bacterial infection in emergency department settings: A systematic review and cost-effectiveness analysis. Health Technol. Assess. 2015, 19, 3–236. [Google Scholar] [CrossRef]
- Langford, B.J.; Beriault, D.; Schwartz, K.L.; Seah, J.; Pasic, M.D.; Cirone, R.; Chan, A.; Downing, M. A real-world assessment of procalcitonin combined with antimicrobial stewardship in a community ICU. J. Crit. Care 2020, 57, 130–133. [Google Scholar] [CrossRef]
- Broyles, M.R. Impact of procalcitonin-guided antibiotic management on antibiotic exposure and outcomes: Real-world evidence. Open Forum Infect. Dis. 2017, 4, ofx213. [Google Scholar] [CrossRef]
- Gluck, E.; Nguyen, H.B.; Yalamanchili, K.; McCusker, M.; Madala, J.; Corvino, F.A.; Zhu, X.; Balk, R. Real-world use of procalcitonin and other biomarkers among sepsis hospitalizations in the United States: A retrospective, observational study. PLoS ONE 2018, 13, e0205924. [Google Scholar] [CrossRef]
- Chambliss, A.B. Embracing procalcitonin for antimicrobial stewardship. J. Appl. Lab. Med. 2019, 3, 712–715. [Google Scholar] [CrossRef]
- Christensen, I.; Haug, J.B.; Berild, D.; Bjørnholt, J.V.; Jelsness-Jørgensen, L.P. Hospital physicians’ experiences with procalcitonin—Implications for antimicrobial stewardship; a qualitative study. BMC Infect. Dis. 2020, 20, 515. [Google Scholar] [CrossRef]
- von Dach, E.; Albrich, W.C.; Brunel, A.S.; Prendki, V.; Cuvelier, C.; Flury, D.; Gayet-Ageron, A.; Huttner, B.; Kohler, P.; Lemmenmeier, E.; et al. Effect of C-reactive protein-guided antibiotic treatment duration, 7-day treatment, or 14-day treatment on 30-day clinical failure rate in patients with uncomplicated gram-negative bacteremia: A randomized clinical trial. JAMA 2020, 323, 2160–2169. [Google Scholar] [CrossRef]
- Borges, I.; Carneiro, R.; Bergo, R.; Martins, L.; Colosimo, E.; Oliveira, C.; Saturnino, S.; Andrade, M.V.; Ravetti, C.; Nobre, V. Duration of antibiotic therapy in critically ill patients: A randomized controlled trial of a clinical and C-reactive protein-based protocol versus an evidence-based best practice strategy without biomarkers. Crit. Care 2020, 24, 281. [Google Scholar] [CrossRef]
- Shozushima, T.; Takahashi, G.; Matsumoto, N.; Kojika, M.; Okamura, Y.; Endo, S. Usefulness of presepsin (sCD14-ST) measurements as a marker for the diagnosis and severity of sepsis that satisfied diagnostic criteria of systemic inflammatory response syndrome. J. Infect. Chemother. 2011, 17, 764–769. [Google Scholar] [CrossRef]
- Xiao, H.; Wang, G.; Wang, Y.; Tan, Z.; Sun, X.; Zhou, J.; Duan, M.; Zhi, D.; Tang, Z.; Hang, C.; et al. Potential value of presepsin guidance in shortening antibiotic therapy in septic patients: A multicenter, prospective cohort trial. Shock 2022, 57, 63–71. [Google Scholar] [CrossRef]
- Hellyer, T.P.; McAuley, D.F.; Walsh, T.S.; Anderson, N.; Morris, A.C.; Singh, S.; Dark, P.; Roy, A.I.; Perkins, G.D.; McMullan, R.; et al. Biomarker-guided antibiotic stewardship in suspected ventilator-associated pneumonia (VAPrapid2): A randomised controlled trial and process evaluation. Lancet Respir. Med. 2020, 8, 182–191. [Google Scholar] [CrossRef]
- Karakike, E.; Giamarellos-Bourboulis, E.J.; Kyprianou, M.; Fleischmann-Struzek, C.; Pletz, M.W.; Netea, M.G.; Reinhart, K.; Kyriazopoulou, E. Coronavirus disease 2019 as cause of viral sepsis: A systematic review and meta-analysis. Crit. Care Med. 2021, 49, 2042–2057. [Google Scholar] [CrossRef]
- Rawson, T.M.; Moore, L.S.P.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A.H. Bacterial and fungal coinfection in individuals with coronavirus: A rapid review to support COVID-19 antimicrobial prescribing. Clin. Infect. Dis. 2020, 71, 2459–2468. [Google Scholar] [CrossRef]
- Arentz, M.; Yim, E.; Klaff, L.; Lokhandwala, S.; Riedo, F.X.; Chong, M.; Lee, M. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA 2020, 323, 1612–1614. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical characteristics of 138 hospitalized patients with 2019 Novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Goyal, P.; Choi, J.J.; Pinheiro, L.C.; Schenck, E.J.; Chen, R.; Jabri, A.; Satlin, M.J.; Campion, T.R., Jr.; Nahid, M.; Ringel, J.B.; et al. Clinical characteristics of Covid-19 in New York city. N. Engl. J. Med. 2020, 382, 2372–2374. [Google Scholar] [CrossRef]
- Atallah, N.J.; Warren, H.M.; Roberts, M.B.; Elshaboury, R.H.; Bidell, M.R.; Gandhi, R.G.; Adamsick, M.; Ibrahim, M.K.; Sood, R.; Eddine, S.B.Z.; et al. Baseline procalcitonin as a predictor of bacterial infection and clinical outcomes in COVID-19: A case-control study. PLoS ONE 2022, 17, e0262342. [Google Scholar] [CrossRef]
- Houghton, R.; Moore, N.; Williams, R.; El-Bakri, F.; Peters, J.; Mori, M.; Vernet, G.; Lynch, J.; Lewis, H.; Tavener, M.; et al. C-reactive protein-guided use of procalcitonin in COVID-19. JAC Antimicrob. Resist. 2021, 3, dlab180. [Google Scholar] [CrossRef]
- Zattera, L.; Veliziotis, I.; Benitez-Cano, A.; Ramos, I.; Larrañaga, L.; Nuñez, M.; Román, L.; Adalid, I.; Ferrando, C.; Muñoz, G.; et al. Early procalcitonin to predict mortality in critically ill COVID-19 patients: A multicentric cohort study. Minerva Anestesiol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Cheng, C.; Zheng, X.; Jin, Y.; Duan, G.; Chen, M.; Chen, S. Elevated procalcitonin is positively associated with the severity of COVID-19: A meta-analysis based on 10 cohort studies. Medicina 2021, 57, 594. [Google Scholar] [CrossRef] [PubMed]
- Van Berkel, M.; Kox, M.; Frenzel, T.; Pickkers, P.; Schouten, J. Biomarkers for antimicrobial stewardship: A reappraisal in COVID-19 times? Crit. Care 2020, 24, 600. [Google Scholar] [CrossRef] [PubMed]
- Richards, O.; Pallmann, P.; King, C.; Cheema, Y.; Killick, C.; Thomas-Jones, E.; Harris, J.; Bailey, C.; Szakmany, T. Procalcitonin increase is associated with the development of critical care-acquired infections in COVID-19 ARDS. Antibiotics 2021, 10, 1425. [Google Scholar] [CrossRef] [PubMed]
- Pink, I.; Raupach, D.; Fuge, J.; Vonberg, R.-P.; Hoeper, M.M.; Welte, T.; Rademacher, J. C-reactive protein and procalcitonin for antimicrobial stewardship in COVID-19. Infection 2021, 49, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Kooistra, E.J.; van Berkel, M.; van Kempen, N.F.; van Latum, C.R.; Bruse, N.; Frenzel, T.; van den Berg, M.J.; Schouten, J.A.; Kox, M.; Pickkers, P. Dexamethasone and tocilizumab treatment considerably reduces the value of C-reactive protein and procalcitonin to detect secondary bacterial infections in COVID-19 patients. Crit. Care 2021, 25, 281. [Google Scholar] [CrossRef]
- Moseley, P.; Jackson, N.; Omar, A.; Eldoadoa, M.; Samaras, C.; Birk, R.; Ahmed, F.; Chakrabarti, P. Single-centre experience of using procalcitonin to guide antibiotic therapy in COVID-19 intensive care patients. J. Hosp. Infect. 2022, 119, 194–195. [Google Scholar] [CrossRef]
- Calderon, M.; Li, A.; Bazo-Alvarez, J.C.; Dennis, J.; Baker, K.F.; van der Loeff, I.S.; Hanrath, A.T.; Capstick, R.; Payne, B.A.I.; Weiand, D.; et al. Evaluation of procalcitonin-guided antimicrobial stewardship in patients admitted to hospital with COVID-19 pneumonia. JAC Antimicrob. Resist. 2021, 3, dlab133. [Google Scholar] [CrossRef]
- Williams, E.J.; Mair, L.; de Silva, T.I.; Green, D.J.; House, P.; Cawthron, K.; Gillies, C.; Wigfull, J.; Parsons, H.; Partridge, D.G. Evaluation of procalcitonin as a contribution to antimicrobial stewardship in SARS-CoV-2 infection: A retrospective cohort study. J. Hosp. Infect. 2021, 110, 103–107. [Google Scholar] [CrossRef]
- Staub, M.B.; Ouedraogo, Y.; Evans, C.D.; Katz, S.E.; Talley, P.P.; Kainer, M.A.; Nelson, G.E. Analysis of a high-prescribing state’s 2016 outpatient antibiotic prescriptions: Implications for outpatient antimicrobial stewardship interventions. Infect. Control Hosp. Epidemiol. 2020, 41, 135–142. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).