Elastase-Activated Antimicrobial Peptide for a Safer Pulmonary Treatment of Cystic Fibrosis Infections
Abstract
:1. Introduction
2. Results
2.1. Design of the Pro-Peptide Pro-D-BMAP18 Activated by the Proteases in the Site of Infection
2.2. Antimicrobial Activity of Pro-D-BMAP18 against Bacterial and Fungal Strains
2.3. Proteolytic Activation of Pro-D-BMAP18
2.4. Antimicrobial Activity of Pro-D-BMAP18 in the Presence of Elastase and in the Supernatant of HL-60 Cells
2.5. Biocompatibility of the Pro-D-BMAP18 in the Presence of Elastase
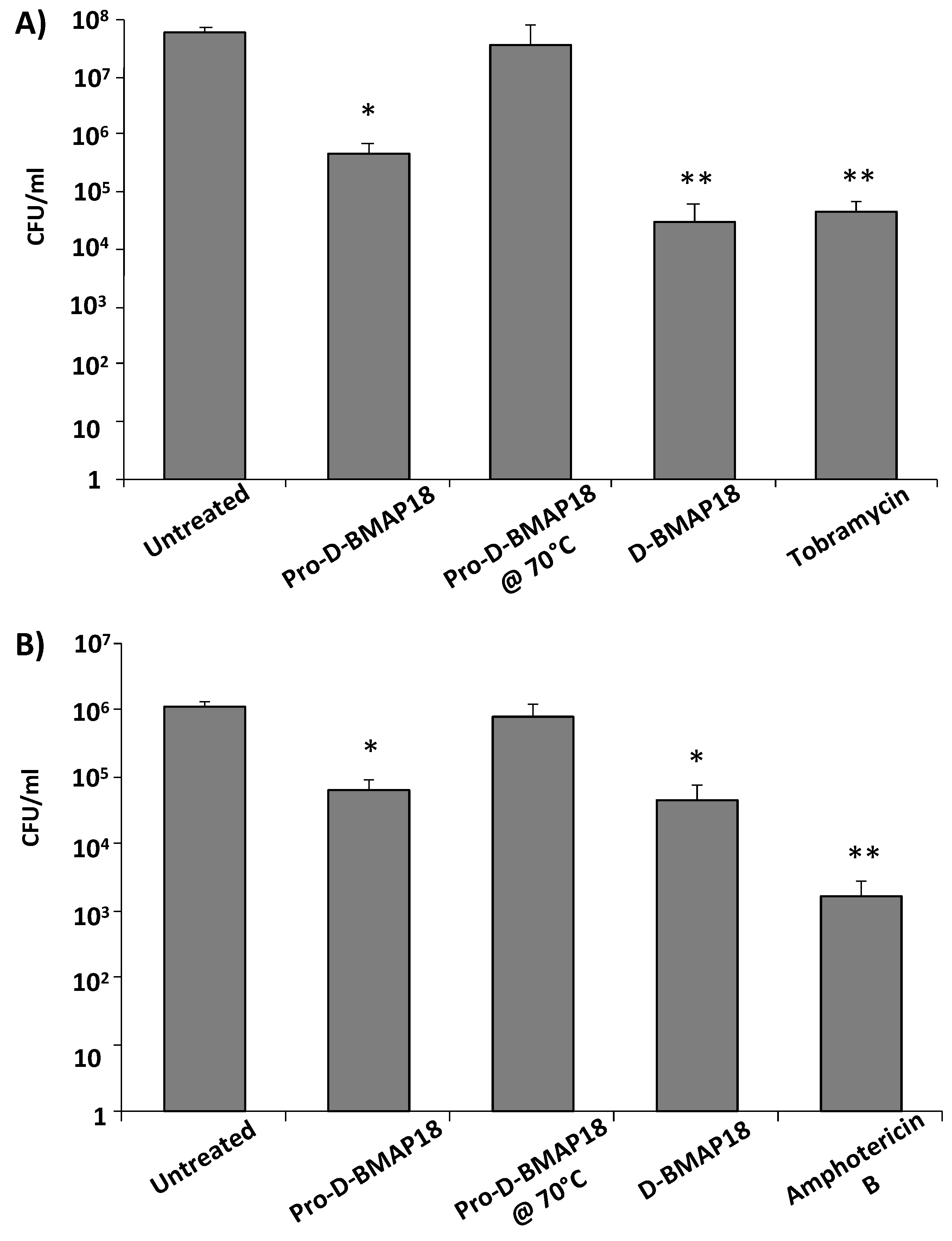
2.6. Antimicrobial Activity of Pro-D-BMAP18 in CF Sputum against P. aeruginosa RP73 or C. albicans SC 5314
3. Discussion
4. Materials and Methods
4.1. Bacterial and Fungal Strains
4.2. Antimicrobial Compounds and Synthesis of Pro-D-BMAP18
4.3. Cleavage of Pro-D-BMAP18 by Human Elastase in HEPES and by HL-60 Cells
4.4. Preparation of Conditioned Medium from Neutrophil-like Cells
4.5. Evaluation of Antimicrobial Activity
4.6. In Vitro Cytoxicity Assays
4.7. Bacterial Killing Assay in CF Sputum
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ratjen, F. Cystic fibrosis: Pathogenesis and future treatment strategies. Respir. Care 2009, 54, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Steinkamp, G.; Wiedemann, B.; Rietschel, E.; Krahl, A.; Gielen, J.; Bärmeier, H.; Ratjen, F. Prospective evaluation of emerging bacteria in cystic fibrosis. J. Cyst. Fibros. 2005, 4, 41–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Høiby, N.; Ciofu, O.; Johansen, H.K.; Song, Z.J.; Moser, C.; Jensen, P.Ø.; Molin, S.; Givskov, M.; Tolker-Nielsen, T.; Bjarnsholt, T. The clinical impact of bacterial biofilms. Int. J. Oral Sci. 2011, 3, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Ciofu, O.; Tolker-Nielsen, T.; Jensen, P.Ø.; Wang, H.; Høiby, N. Antimicrobial resistance, respiratory tract infections and role of biofilms in lung infections in cystic fibrosis patients. Adv. Drug Deliv. Rev. 2015, 85, 7–23. [Google Scholar] [CrossRef]
- Döring, G.; Conway, S.P.; Heijerman, H.G.M.; Hodson, M.E.; Høiby, N.; Smyth, A.; Touw, D.J. Antibiotic therapy against Pseudomonas aeruginosa in cystic fibrosis: A European consensus. Eur. Respir. J. 2000, 16, 749–767. [Google Scholar] [CrossRef] [PubMed]
- Haiko, J.; Saeedi, B.; Bagger, G.; Karpati, F.; Özenci, V. Coexistence of Candida species and bacteria in patients with cystic fibrosis. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1071–1077. [Google Scholar] [CrossRef] [Green Version]
- Hancock, R.E.; Brown, K.L.; Mookherjee, N. Host defence peptides from invertebrates—Emerging antimicrobial strategies. Immunobiology 2006, 211, 315–322. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.J.; Park, S.J.; Mishig-Ochir, T.; Lee, B.J. Antimicrobial peptides: Therapeutic potentials. Expert Rev. Anti. Infect. Ther. 2014, 12, 1477–1486. [Google Scholar] [CrossRef]
- Datta, S.; Roy, A. Antimicrobial Peptides as Potential Therapeutic Agents: A Review. Int. J. Pept. Res. Ther. 2020, 27, 555–577. [Google Scholar] [CrossRef]
- Pasupuleti, M.; Schmidtchen, A.; Malmsten, M. Antimicrobial peptides: Key components of the innate immune system. Crit. Rev. Biotechnol. 2012, 32, 143–171. [Google Scholar] [CrossRef] [Green Version]
- Gallo, R.L.; Murakami, M.; Ohtake, T.; Zaiou, M. Biology and clinical relevance of naturally occurring antimicrobial peptides. J. Allergy Clin. Immunol. 2002, 110, 823–831. [Google Scholar] [CrossRef]
- Haney, E.F.; Hancock, R.E. Peptide design for antimicrobial and immunomodulatory applications. Biopolymers 2013, 100, 572–583. [Google Scholar] [CrossRef]
- Brogden, K. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Raheem, N.; Straus, S.K. Mechanisms of Action for Antimicrobial Peptides with Antibacterial and Antibiofilm Functions. Font. Microbiol. 2019, 10, 2866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollmann, A.; Martinez, M.; Maturana, P.; Semorile, L.C.; Maffia, P.C. Antimicrobial Peptides: Interaction with Model and Biological Membranes and Synergism with Chemical Antibiotics. Font. Chem. 2018, 6, 204. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Parente, J.; Harris, S.M.; Woods, D.E.; Hancock, R.E.; Falla, T.J. Antimicrobial Peptide Therapeutics for Cystic Fibrosis Antimicrobial Peptide Therapeutics for Cystic Fibrosis. Society 2005, 49, 2921–2927. [Google Scholar]
- Water, V.; Smyth, A. Cystic fibrosis microbiology: Advances in antimicrobial therapy. J. Cyst. Fibros. 2015, 14, 551–560. [Google Scholar] [CrossRef]
- Schwab, U.; Gilligan, P.; Jaynes, J.; Henke, D. In Vitro Activities of Designed Antimicrobial Peptides against Multidrug-Resistant Cystic Fibrosis Pathogens. Antimicrob. Agents Chemother. 1999, 43, 1435–1440. [Google Scholar] [CrossRef] [Green Version]
- Forde, É.; Kelly, G.; Sweeney, L.; Fitzgerald-Hughes, D.; MacLoughlin, R.; Devocelle, M. Vibrating Mesh Nebulisation of Pro-Antimicrobial Peptides for Use in Cystic Fibrosis. Pharmaceutics 2019, 11, 239. [Google Scholar] [CrossRef] [Green Version]
- Pompilio, A.; Scocchi, M.; Pomponio, S.; Guida, F.; Di Primio, A.; Fiscarelli, E.; Gennaro, R.; Di Bonaventura, G. Antibacterial and anti-biofilm effects of cathelicidin peptides against pathogens isolated from cystic fibrosis patients. Peptides 2011, 32, 1807–1814. [Google Scholar] [CrossRef] [PubMed]
- Pompilio, A.; Crocetta, V.; Scocchi, M.; Pomponio, S.; Di Vincenzo, V.; Mardirossian, M.; Gherardi, G.; Fiscarelli, E.; Dicuonzo, G.; Gennaro, R.; et al. Potential novel therapeutic strategies in cystic fibrosis: Antimicrobial and anti-biofilm activity of natural and designed -helical peptides against Staphylococcus aureus, Pseudomonas aeruginosa, and Stenotrophomonas maltophilia. BMC Microbiol. 2012, 12, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skerlavaj, B.; Gennaro, R.; Bagella, L.; Merluzzi, L.; Risso, A.; Zanetti, M. Biological characterization of two novel cathelicidin-derived peptides and identification of structural requirements for their antimicrobial and cell lytic activities. J. Biol. Chem. 1996, 271, 28375–28381. [Google Scholar] [CrossRef] [Green Version]
- Mardirossian, M.; Pompilio, A.; Crocetta, V.; De Nicola, S.; Guida, F.; Degasperi, M.; Gennaro, R.; Di Bonaventura, G.; Scocchi, M. In Vitro and in vivo evaluation of BMAP-derived peptides for the treatment of cystic fibrosis-related pulmonary infections. Amino Acids 2016, 48, 2253–2260. [Google Scholar] [CrossRef] [PubMed]
- Degasperi, M.; Agostinis, C.; Mardirossian, M.; Maschio, M.; Taddio, A.; Bulla, R.; Scocchi, M. The Anti-Pseudomonal Peptide D-BMAP18 Is Active in Cystic Fibrosis Sputum and Displays Anti-Inflammatory In Vitro Activity. Microorganisms 2020, 8, 1407. [Google Scholar] [CrossRef]
- Mardirossian, M.; Pompilio, A.; Degasperi, M.; Runti, G.; Pacor, S.; Di Bonaventura, G.; Scocchi, M. D-BMAP18 Antimicrobial Peptide Is Active In vitro, Resists to Pulmonary Proteases but Loses Its Activity in a Murine Model of Pseudomonas aeruginosa Lung Infection. Front. Chem. 2017, 5, 40. [Google Scholar] [CrossRef]
- Forde, É.; Humphreys, H.; Greene, C.M.; Fitzgerald-Hughes, D.; Devocelle, M. Potential of host defense peptide prodrugs as neutrophil elastase-dependent anti-infective agents for cystic fibrosis. Antimicrob. Agents Chemother. 2014, 58, 978–985. [Google Scholar] [CrossRef] [Green Version]
- Forde, É.; Schütte, A.; Reeves, E.; Greene, C.; Humphreys, H.; Mall, M.; Fitzgerald-Hughes, D.; Devocelle, M. Differential In Vitro and In Vivo Toxicities of Antimicrobial Peptide Prodrugs for Potential Use in Cystic Fibrosis. Antimicrob. Agents Chemother. 2016, 60, 2813–2821. [Google Scholar] [CrossRef] [Green Version]
- Scocchi, M.; Skerlavaj, B.; Romeo, D.; Gennaro, R. Proteolytic cleavage by neutrophil elastase converts inactive storage proforms to antibacterial bactenecins. Eur. J. Biochem. 1992, 209, 589–595. [Google Scholar] [CrossRef]
- Panyutich, A.; Shi, J.; Boutz, P.L.; Zhao, C.; Ganz, T. Porcine polymorphonuclear leukocytes generate extracellular microbicidal activity by elastase-mediated activation of secreted proprotegrins. Infect. Immun. 1997, 65, 978–985. [Google Scholar] [CrossRef] [Green Version]
- Jubeh, B.; Breijyeh, Z.; Karaman, R. Antibacterial Prodrugs to Overcome Bacterial Resistance. Molecules 2020, 25, 1543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rautio, J.; Kumpulainen, H.; Heimbach, T.; Oliyai, R.; Oh, D.; Järvinen, T.; Savolainen, J. Prodrugs: Design and clinical applications. Nat. Rev. Drug Discov. 2008, 7, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Laval, J.; Ralhan, A.; Hartl, D. Neutrophils in cystic fibrosis. Biol. Chem. 2016, 397, 485–496. [Google Scholar] [CrossRef]
- Reeves, E.P.; Molloy, K.; Pohl, K.; McElvaney, N.G. Hypertonic saline in treatment of pulmonary disease in cystic fibrosis. Sci. World J. 2012, 2012, 465230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Döring, G. The role of neutrophil elastase in chronic inflammation. Am. J. Respir. Crit. Care Med. 1994, 150 Pt 2, S114–S117. [Google Scholar] [CrossRef]
- Hancock, R.E.; Sahl, H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef]
- Chen, C.H.; Lu, T.K. Development and Challenges of Antimicrobial Peptides for Therapeutic Applications. Antibiotics 2020, 9, 24. [Google Scholar] [CrossRef] [Green Version]
- Kuipers, B.J.; Gruppen, H. Prediction of Molar Extinction Coefficients of Proteins and Peptides Using UV Absorption of the Constituent Amino Acids at 214 nm To Enable Quantitative Reverse Phase High-Performance Liquid Chromatography−Mass Spectrometry Analysis. J. Agric. Food Chem. 2007, 55, 5445–5451. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Gao, F.; Wang, Q.; Wang, K.; Pan, S.; Pan, Z.; Xu, S.; Li, L.; Zhao, D. Differentiation of HL-60 cells in serum-free hematopoietic cell media enhances the production of neutrophil extracellular traps. Exp. Ther. Med. 2021, 21, 353. [Google Scholar] [CrossRef]
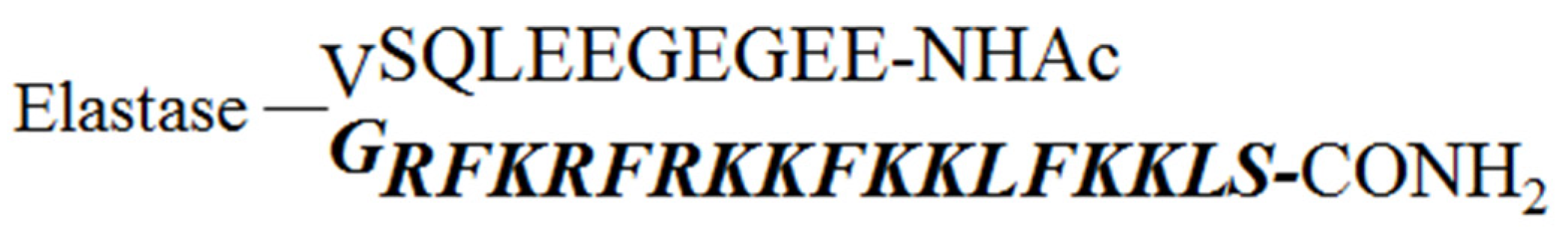

| Strains | Pro-D-BMAP18 | D-BMAP18 |
|---|---|---|
| PAO1 | 128 | 4 |
| RP 73 | 128 | 4 |
| PA 03 | 128 | 8 |
| PA 05 | 32 | 4 |
| PA07 | 64 | 8 |
| PA08 | 128 | 8 |
| PA 09 | 64 | 4 |
| PA 10 | 64 | 8 |
| PA 14 | 64 | 16 |
| PA 21 | 128 | 16 |
| PA 22 | 16 | 4 |
| PA 31 | 128 | 16 |
| Compound | P. aeruginosa | C. albicans | ||
|---|---|---|---|---|
| PAO1 | RP73 | ATCC 90029 | SC5314 | |
| D-BMAP18 | 16 | 8 | 2 | 16 |
| Pro-D-BMAP18 | 128 | 128 | 16 | >128 |
| Pro-D-BMAP18 + NE | 32 | 32 | 4 | 32 |
| Neutrophil elastase (NE) | >128 | >128 | >128 | >128 |
| Pro-D-BMAP18 + NE + 350 mM NaCl | 16 | 8 | 2 | 16 |
| Pro-D-BMAP18 + cell supernatant, 4 h | 64 | 64 | nd | nd |
| Pro-D-BMAP18 + cell supernatant, 18 h | 32 | 32 | nd | nd |
| Pro-D-BMAP18 + cell supernatant, 24 h | 32 | 32 | nd | nd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Degasperi, M.; Sgarra, R.; Mardirossian, M.; Pacor, S.; Maschio, M.; Scocchi, M. Elastase-Activated Antimicrobial Peptide for a Safer Pulmonary Treatment of Cystic Fibrosis Infections. Antibiotics 2022, 11, 319. https://doi.org/10.3390/antibiotics11030319
Degasperi M, Sgarra R, Mardirossian M, Pacor S, Maschio M, Scocchi M. Elastase-Activated Antimicrobial Peptide for a Safer Pulmonary Treatment of Cystic Fibrosis Infections. Antibiotics. 2022; 11(3):319. https://doi.org/10.3390/antibiotics11030319
Chicago/Turabian StyleDegasperi, Margherita, Riccardo Sgarra, Mario Mardirossian, Sabrina Pacor, Massimo Maschio, and Marco Scocchi. 2022. "Elastase-Activated Antimicrobial Peptide for a Safer Pulmonary Treatment of Cystic Fibrosis Infections" Antibiotics 11, no. 3: 319. https://doi.org/10.3390/antibiotics11030319
APA StyleDegasperi, M., Sgarra, R., Mardirossian, M., Pacor, S., Maschio, M., & Scocchi, M. (2022). Elastase-Activated Antimicrobial Peptide for a Safer Pulmonary Treatment of Cystic Fibrosis Infections. Antibiotics, 11(3), 319. https://doi.org/10.3390/antibiotics11030319







