Why d-Mannose May Be as Efficient as Antibiotics in the Treatment of Acute Uncomplicated Lower Urinary Tract Infections—Preliminary Considerations and Conclusions from a Non-Interventional Study
Abstract
:1. Epidemiology and Infectiology of Acute Uncomplicated Urinary Tract Infections
2. Antibiotics as Current Standard Therapy and Relevance of Resistance Development
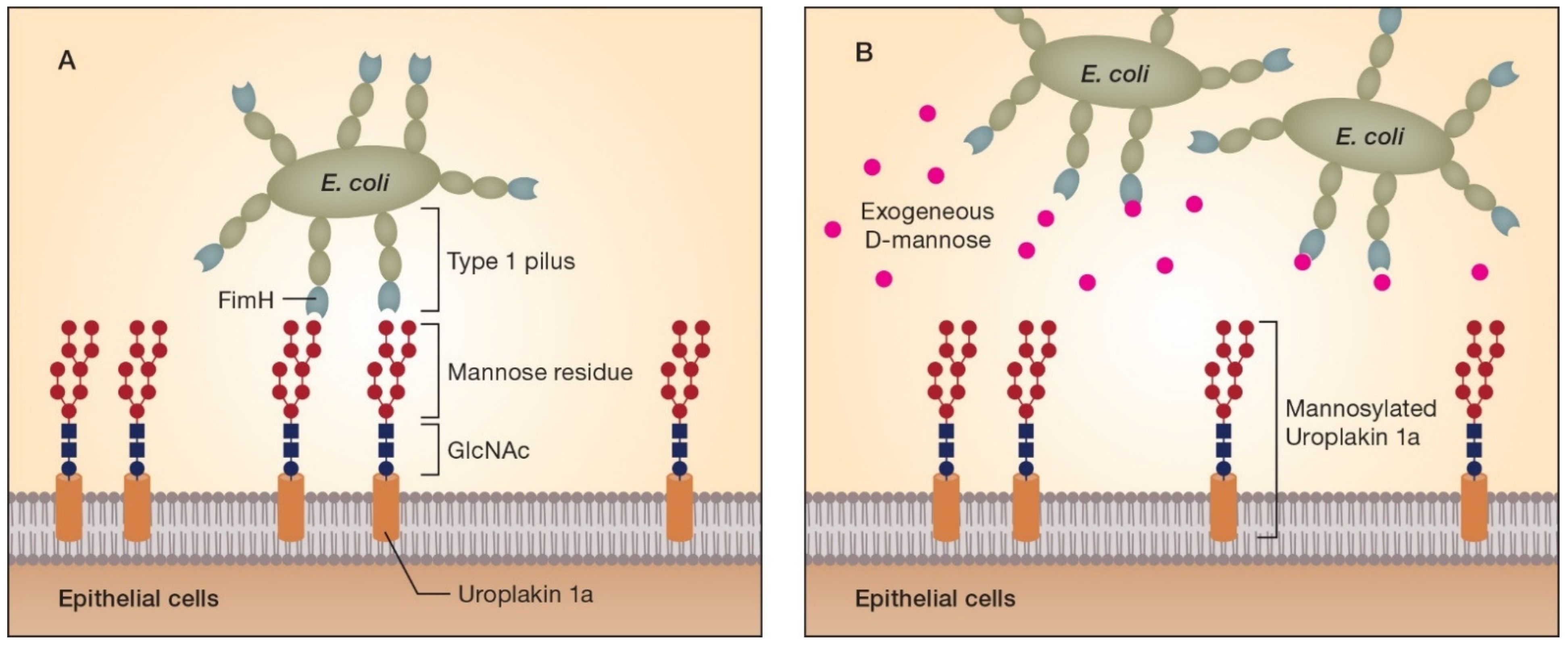
3. Fundamental Aspects of the d-Mannose Mode of Action
4. Clinical Data for d-Mannose in UTI
5. Clinical Diagnosis and Assessment of Treatment Efficacy in UTIs with a Validated Measuring Instrument
6. Post Hoc Analysis of the Potential Efficacy of d-Mannose in the Treatment of Acute Episodes of UTI
6.1. Clinical Efficacy of d-Mannose in the Treatment of Acute UTI—A Non-Interventional Study
6.2. Transfer of Clinical Data of the NIS to the ACSS as a Validated Instrument
6.3. Estimated Cure Rates from Reanalysis of the NIS with d-Mannose Treatment for Acute UTI
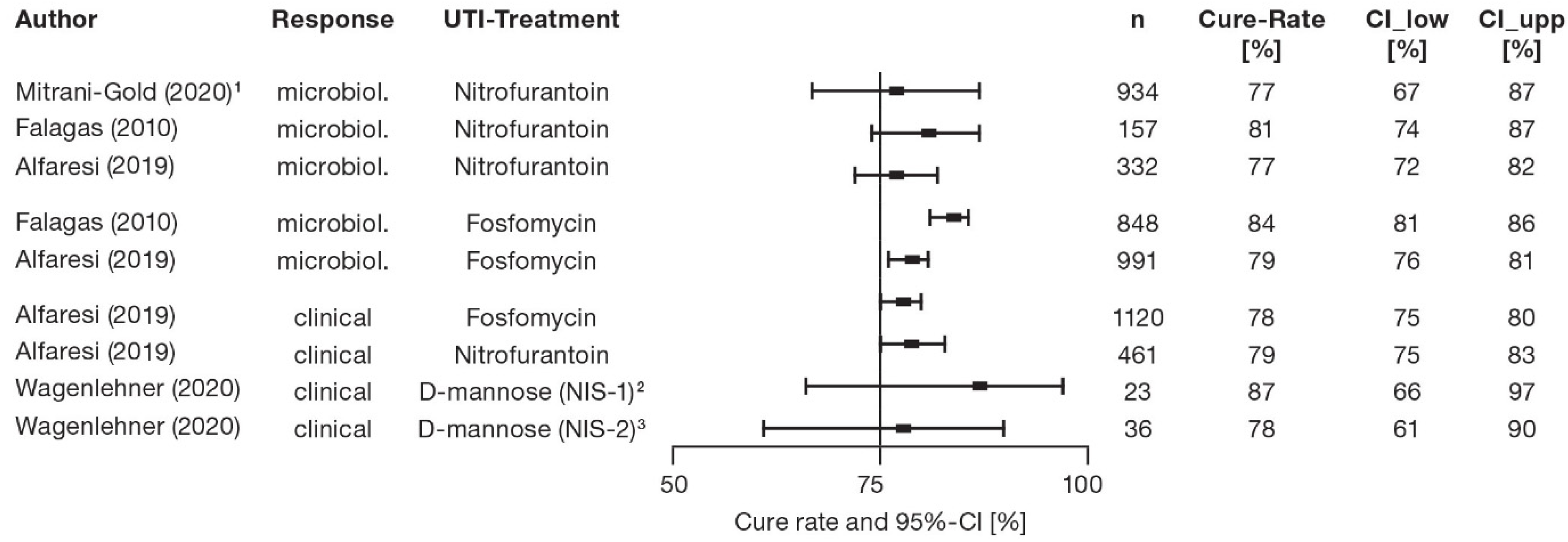
7. Cure Rates of Controlled Trials—Antibiotic Treatment of Acute UTI
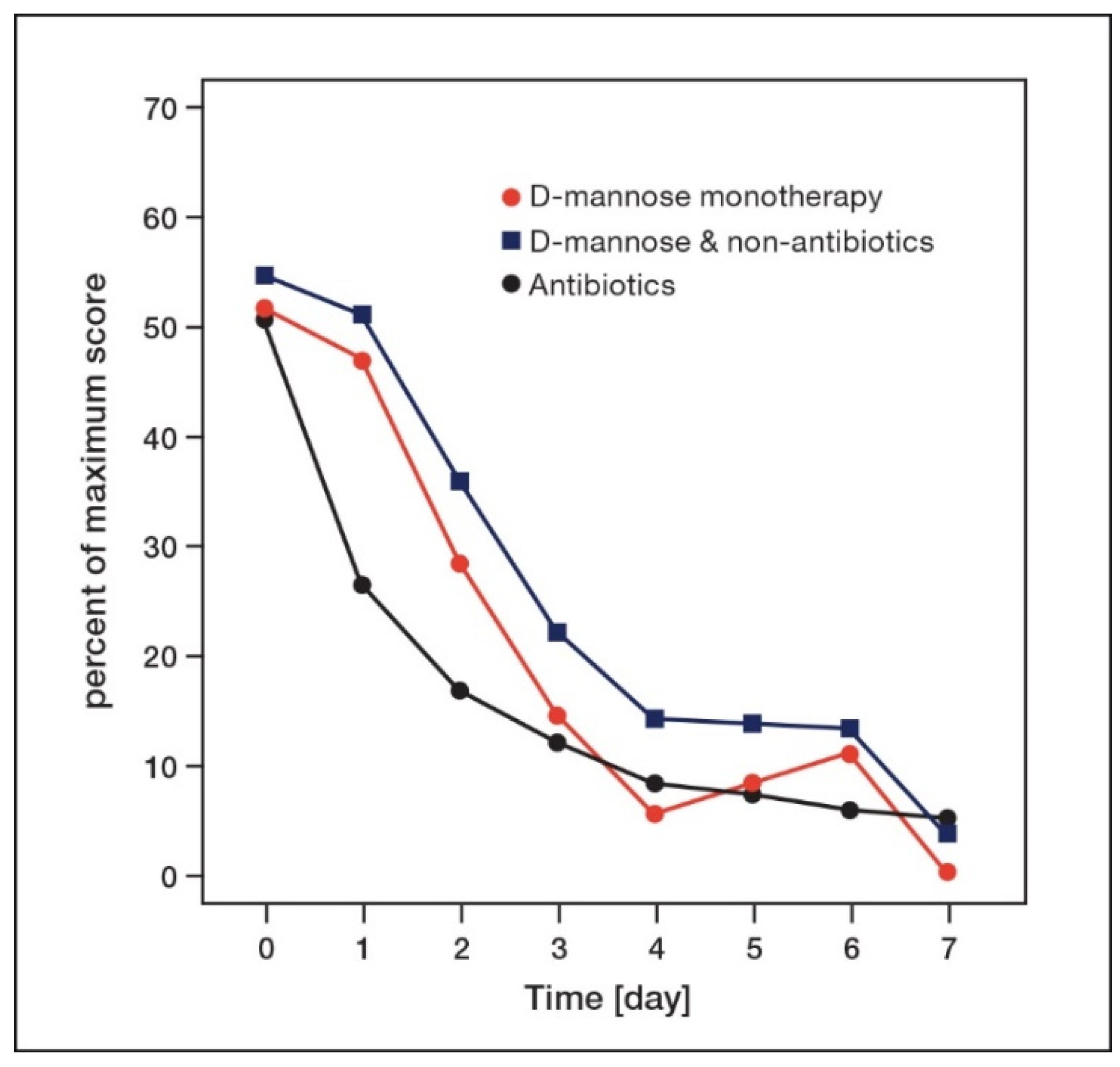
8. Time-Dependent Changes of Symptoms in AUC Patients Treated with d-Mannose or Antibiotics
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Öztürk, R.; Murt, A. Epidemiology of urological infections: A global burden. World J. Urol. 2020, 38, 2669–2679. [Google Scholar] [CrossRef]
- Leitlinienprogramm DGU: Interdisziplinäre S3 Leitlinie: Epidemiologie, Diagnostik, Therapie, Prävention und Management Unkomplizierter, Bakterieller, Ambulant Erworbener Harnwegsinfektionen Bei Erwachsenen Patienten. Langversion 1.1-2, 2017 AWMF Registernummer: 043/044. Available online: https://www.awmf.org/uploads/tx_szleitlinien/043-044l_S3_Harnwegsinfektionen_2017-05.pdf (accessed on 29 October 2021).
- Sarshar, M.; Behzadi, P.; Ambrosi, C.; Zagaglia, C.; Palamara, A.T.; Scribano, D. FimH and Anti-Adhesive Therapeutics: A Disarming Strategy Against Uropathogens. Antibiotics 2020, 9, 397. [Google Scholar] [CrossRef]
- Bonkat, G.; Bartoletti, R.; Bruyère, F.; Cai, T.; Geerlings, S.E.; Köves, B.; Schubert, S.; Wagenlehner, F.M. EAU Guidelines on Urological Infections; EAU Guidelines Office: Arnhem, The Netherlands, 2021; ISBN 978-94-92671-13-4. [Google Scholar]
- Foxman, B. Urinary tract infection syndromes: Occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect. Dis. Clin. N. Am. 2014, 28, 1–13. [Google Scholar] [CrossRef]
- Storme, O.; Tirán Saucedo, J.; Garcia-Mora, A.; Dehesa-Dávila, M.; Naber, K.G. Risk factors and predisposing conditions for urinary tract infection. Ther. Adv. Urol. 2019, 11, 1756287218814382. [Google Scholar] [CrossRef]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef]
- Naber, K.G.; Bonkat, G.; Wagenlehner, F.M. The EAU and AUA/CUA/SUFU Guidelines on Recurrent Urinary Tract Infections: What is the Difference? Eur. Urol. 2020, 78, 645–646. [Google Scholar] [CrossRef]
- Bischoff, S.; Walter, T.; Gerigk, M.; Ebert, M.; Vogelmann, R. Empiric antibiotic therapy in urinary tract infection in patients with risk factors for antibiotic resistance in a German emergency department. BMC Infect. Dis. 2018, 18, 56. [Google Scholar] [CrossRef]
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Negus, M.; Phillips, C.; Hindley, R. Recurrent urinary tract infections: A critical review of the currently available treatment options. Obstet. Gynaecol. 2020, 22, 115–121. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Antimicrobial Resistance. Available online: https://www.ecdc.europa.eu/en/antimicrobial-resistance (accessed on 16 November 2021).
- Foxman, B.; Gillespie, B.; Koopman, J.; Zhang, L.; Palin, K.; Tallman, P.; Marsh, J.V.; Spear, S.; Sobel, J.D.; Marty, M.J.; et al. Risk factors for second urinary tract infection among college women. Am. J. Epidemiol. 2000, 151, 1194–1205. [Google Scholar] [CrossRef] [Green Version]
- Wiedemann, B.; Heisig, A.; Heisig, P. Uncomplicated Urinary Tract Infections and Antibiotic Resistance-Epidemiological and Mechanistic Aspects. Antibiotics 2014, 3, 341–352. [Google Scholar] [CrossRef] [Green Version]
- European Centre for Disease Prevention and Control. ECDC/EMEA Joint Technical Report. The Bacterial Challenge: Time to React. Available online: https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/0909_TER_The_Bacterial_Challenge_Time_to_React.pdf (accessed on 16 November 2021).
- Wagenlehner, F.M.; Wullt, B.; Ballarini, S.; Zingg, D.; Naber, K.G. Social and economic burden of recurrent urinary tract infections and quality of life: A patient web-based study (GESPRIT). Expert Rev. Pharm. Outcomes Res. 2018, 18, 107–117. [Google Scholar] [CrossRef]
- Terlizzi, M.E.; Gribaudo, G.; Maffei, M.E. UroPathogenic Escherichia coli (UPEC) Infections: Virulence Factors, Bladder Responses, Antibiotic, and Non-antibiotic Antimicrobial Strategies. Front. Microbiol. 2017, 8, 1566. [Google Scholar] [CrossRef]
- Domenici, L.; Monti, M.; Bracchi, C.; Giorgini, M.; Colagiovanni, V.; Muzii, L.; Benedetti Panici, P. D-mannose: A promising support for acute urinary tract infections in women. A pilot study. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2920–2925. [Google Scholar]
- Fronzes, R.; Remaut, H.; Waksman, G. Architectures and biogenesis of non-flagellar protein appendages in Gram-negative bacteria. EMBO J. 2008, 27, 2271–2280. [Google Scholar] [CrossRef] [Green Version]
- Zhou, G.; Mo, W.J.; Sebbel, P.; Min, G.; Neubert, T.A.; Glockshuber, R.; Wu, X.R.; Sun, T.T.; Kong, X.P. Uroplakin Ia is the urothelial receptor for uropathogenic Escherichia coli: Evidence from in vitro FimH binding. J. Cell Sci. 2001, 114, 4095–4103. [Google Scholar] [CrossRef]
- Sauer, M.M.; Jakob, R.P.; Eras, J.; Baday, S.; Eriş, D.; Navarra, G.; Bernèche, S.; Ernst, B.; Maier, T.; Glockshuber, R. Catch-bond mechanism of the bacterial adhesin FimH. Nat. Commun. 2016, 7, 10738. [Google Scholar] [CrossRef] [Green Version]
- Abraham, S.N.; Sun, D.; Dale, J.B.; Beachey, E.H. Conservation of the D-mannose-adhesion protein among type 1 fimbriated members of the family Enterobacteriaceae. Nature 1988, 336, 682–684. [Google Scholar] [CrossRef]
- Wellens, A.; Garofalo, C.; Nguyen, H.; Van Gerven, N.; Slättegård, R.; Hernalsteens, J.P.; Wyns, L.; Oscarson, S.; De Greve, H.; Hultgren, S.; et al. Intervening with urinary tract infections using anti-adhesives based on the crystal structure of the FimH-oligomannose-3 complex. PLoS ONE 2008, 3, e2040. [Google Scholar] [CrossRef]
- Porru, D.; Parmigiani, A.; Tinelli, C.; Barletta, D.; Choussos, D.; Di Franco, C.; Bobbi, V.; Bassi, S.; Miller, O.; Gardella, B.; et al. Oral D-mannose in recurrent urinary tract infections in women: A pilot study. J. Clin. Urol. 2014, 7, 208–213. [Google Scholar] [CrossRef]
- Kranjčec, B.; Papeš, D.; Altarac, S. D-mannose powder for prophylaxis of recurrent urinary tract infections in women: A randomized clinical trial. World J. Urol. 2014, 32, 79–84. [Google Scholar] [CrossRef]
- Phé, V.; Pakzad, M.; Haslam, C.; Gonzales, G.; Curtis, C.; Porter, B.; Chataway, J.; Panicker, J.N. Open label feasibility study evaluating D-mannose combined with home-based monitoring of suspected urinary tract infections in patients with multiple sclerosis. Neurourol. Urodyn. 2017, 36, 1770–1775. [Google Scholar] [CrossRef] [Green Version]
- Lenger, S.M.; Bradley, M.S.; Thomas, D.A.; Bertolet, M.H.; Lowder, J.L.; Sutcliffe, S. D-mannose vs other agents for recurrent urinary tract infection prevention in adult women: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2020, 223, 265.e1–265.e13. [Google Scholar] [CrossRef]
- Bent, S.; Nallamothu, B.K.; Simel, D.L.; Fihn, S.D.; Saint, S. Does this woman have an acute uncomplicated urinary tract infection? JAMA 2002, 287, 2701–2710. [Google Scholar] [CrossRef]
- Alidjanov, J.F.; Pilatz, A.; Abdufattaev, U.A.; Wiltink, J.; Weidner, W.; Naber, K.G.; Wagenlehner, F.M. German validation of the Acute Cystitis Symptom Score. Urologe A 2015, 54, 1269–1276. [Google Scholar] [CrossRef]
- Alidjanov, J.F.; Abdufattaev, U.A.; Makhsudov, S.A.; Pilatz, A.; Akilov, F.A.; Naber, K.G.; Wagenlehner, F.M. New self-reporting questionnaire to assess urinary tract infections and differential diagnosis: Acute cystitis symptom score. Urol. Int. 2014, 92, 230–236. [Google Scholar] [CrossRef]
- Alidjanov, J.F.; Abdufattaev, U.A.; Makhsudov, S.A.; Pilatz, A.; Akilov, F.A.; Naber, K.G.; Wagenlehner, F.M. The Acute Cystitis Symptom Score for Patient-Reported Outcome Assessment. Urol. Int. 2016, 97, 402–409. [Google Scholar] [CrossRef]
- Alidjanov, J.F.; Naber, K.G.; Abdufattaev, U.A.; Pilatz, A.; Wagenlehner, F.M. Reevaluation of the Acute Cystitis Symptom Score, a Self-Reporting Questionnaire. Part II. Patient-Reported Outcome Assessment. Antibiotics 2018, 7, 43. [Google Scholar] [CrossRef] [Green Version]
- Alidjanov, J.F.; Naber, K.G.; Abdufattaev, U.A.; Pilatz, A.; Wagenlehner, F.M. Reliability of Symptom-Based Diagnosis of Uncomplicated Cystitis. Urol. Int. 2019, 102, 83–95. [Google Scholar] [CrossRef]
- Alidjanov, J.F.; Naber, K.G.; Abdufattaev, U.A.; Pilatz, A.; Wagenlehner, F.M. Reevaluation of the Acute Cystitis Symptom Score, a Self-Reporting Questionnaire. Part I. Development, Diagnosis and Differential Diagnosis. Antibiotics 2018, 7, 6. [Google Scholar] [CrossRef] [Green Version]
- Alidjanov, J.F.; Naber, K.G.; Pilatz, A.; Radzhabov, A.; Zamuddinov, M.; Magyar, A.; Tenke, P.; Wagenlehner, F.M. Evaluation of the draft guidelines proposed by EMA and FDA for the clinical diagnosis of acute uncomplicated cystitis in women. World J. Urol. 2020, 38, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Alidjanov, J.F.; Naber, K.G.; Pilatz, A.; Radzhabov, A.; Zamuddinov, M.; Magyar, A.; Tenke, P.; Wagenlehner, F.M. Additional assessment of Acute Cystitis Symptom Score questionnaire for patient-reported outcome measure in female patients with acute uncomplicated cystitis: Part II. World J. Urol. 2020, 38, 1977–1988. [Google Scholar] [CrossRef] [Green Version]
- Alidjanov, J.F.; Overesch, A.; Abramov-Sommariva, D.; Hoeller, M.; Steindl, H.; Wagenlehner, F.M.; Naber, K.G. Acute Cystitis Symptom Score questionnaire for measuring patient-reported outcomes in women with acute uncomplicated cystitis: Clinical validation as part of a phase III trial comparing antibiotic and nonantibiotic therapy. Investig. Clin. Urol. 2020, 61, 498–507. [Google Scholar] [CrossRef]
- Wagenlehner, F.M.; Abramov-Sommariva, D.; Höller, M.; Steindl, H.; Naber, K.G. Non-Antibiotic Herbal Therapy (BNO 1045) versus Antibiotic Therapy (Fosfomycin Trometamol) for the Treatment of Acute Lower Uncomplicated Urinary Tract Infections in Women: A Double-Blind, Parallel-Group, Randomized, Multicentre, Non-Inferiority Phase III Trial. Urol. Int. 2018, 101, 327–336. [Google Scholar] [CrossRef]
- Alidjanov, J.F.; Pilatz, A.; Abdufattaev, U.A.; Wiltink, J.; Weidner, W.; Naber, K.G.; Wagenlehner, F.M. New questionnaire for the German validation of the Acute Cystitis Symptom Score. Urol. A 2017, 56, 364–366. [Google Scholar] [CrossRef] [Green Version]
- Food and Drug Administration (FDA). Uncomplicated Urinary Tract Infections: Developing Drugs for Treatment. In Guidance for Industry.; 2019. Available online: https://www.fda.gov/media/129531/download (accessed on 16 November 2021).
- European Medicines Agency (EMA). Guideline on the Evaluation of Medicinal Products Indicated for Treatment of Bacterial Infections. Rev 3. 2019. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-evaluation-medicinal-products-indicated-treatment-bacterial-infections-revision-3_en.pdf (accessed on 16 November 2021).
- Wagenlehner, F.M.; Baumgartner, L.N.; Schopf, B.; Milde, J. Nicht interventionelle Studie mit Femannose® N zur Untersuchung von Verträglichkeit, Lebensqualität und Symptomverlauf bei akuter unkomplizierter Harnweginfektion [Non-interventional study with Femannose® N to investigate tolerance, quality of life and course of symptoms in acute uncomplicated urinary tract infection]. J. Pharmakol Ther. 2020, 29, 4–9. (In German) [Google Scholar]
- Agresti, A.; Caffo, B. Simple and Effective Confidence Intervals for Proportions and Differences of Proportions Result from Adding Two Successes and Two Failures. Am. Stat. 2000, 54, 280–288. [Google Scholar] [CrossRef]
- Falagas, M.E.; Vouloumanou, E.K.; Togias, A.G.; Karadima, M.; Kapaskelis, A.M.; Rafailidis, P.I.; Athanasiou, S. Fosfomycin versus other antibiotics for the treatment of cystitis: A meta-analysis of randomized controlled trials. J. Antimicrob. Chemother. 2010, 65, 1862–1877. [Google Scholar] [CrossRef] [Green Version]
- Mitrani-Gold, F.S.; Raychaudhuri, A.; Rao, S. Systematic review and meta-analysis to estimate the antibacterial treatment effect of nitrofurantoin for a non-inferiority trial in uncomplicated urinary tract infection. J. Glob. Antimicrob. Resist. 2020, 22, 68–77. [Google Scholar] [CrossRef]
- Alfaresi, M.; Hassan, K.; Alnjadat, R.M.H. Single-Dose Fosfomycin Trometamol Versus Other Antimicrobial Regimens For Treatment of Uncomplicated Lower Urinary Tract Infection: A Systematic Review And Meta-Analysis. Open Microbiol. J. 2019, 13, 193–199. [Google Scholar] [CrossRef]
- Gágyor, I.; Bleidorn, J.; Kochen, M.M.; Schmiemann, G.; Wegscheider, K.; Hummers-Pradier, E. Ibuprofen versus fosfomycin for uncomplicated urinary tract infection in women: Randomised controlled trial. BMJ 2015, 351, h6544. [Google Scholar] [CrossRef] [Green Version]
- Kronenberg, A.; Bütikofer, L.; Odutayo, A.; Mühlemann, K.; da Costa, B.R.; Battaglia, M.; Meli, D.N.; Frey, P.; Limacher, A.; Reichenbach, S.; et al. Symptomatic treatment of uncomplicated lower urinary tract infections in the ambulatory setting: Randomised, double blind trial. BMJ 2017, 359, j4784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vik, I.; Bollestad, M.; Grude, N.; Bærheim, A.; Damsgaard, E.; Neumark, T.; Bjerrum, L.; Cordoba, G.; Olsen, I.C.; Lindbæk, M. Ibuprofen versus pivmecillinam for uncomplicated urinary tract infection in women-A double-blind, randomized non-inferiority trial. PLoS Med. 2018, 15, e1002569. [Google Scholar] [CrossRef] [PubMed]
- Bleidorn, J.; Gágyor, I.; Kochen, M.M.; Wegscheider, K.; Hummers-Pradier, E. Symptomatic treatment (ibuprofen) or antibiotics (ciprofloxacin) for uncomplicated urinary tract infection?--results of a randomized controlled pilot trial. BMC Med. 2010, 8, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.; Kim, Y.H.; Bae, J.H. Quality of life and changes in symptom relief in patients with acute uncomplicated cystitis treated with antibiotics: A prospective, open-label, multicenter, observational study. Eur. J. Clin. Microbiol. Infect. Dis 2015, 34, 1119–1124. [Google Scholar] [CrossRef]
- Jansåker, F.; Thønnings, S.; Hertz, F.B.; Kallemose, T.; Værnet, J.; Bjerrum, L.; Benfield, T.; Frimodt-Møller, N.; Knudsen, J.D. Three versus five days of pivmecillinam for community-acquired uncomplicated lower urinary tract infection: A randomised, double-blind, placebo-controlled superiority trial. EClinicalMedicine 2019, 12, 62–69. [Google Scholar] [CrossRef] [Green Version]
| Symptom | Original Assessment | Rules for Transfer to ACSS “Typical Domain” |
|---|---|---|
| Urination frequency | 0–3 | 0–3 (no adaptation necessary) |
| Urination urgency | No, yes | No ⇒ 0; yes ⇒ 2 |
| Urination burning/pain (rated twice) | 0–5 | 0 ⇒ 0; 1 ⇒ 1; 2, 3 ⇒ 2; 4, 5 ⇒ 3 |
| Incomplete bladder emptying | No, yes | No ⇒ 0; yes ⇒ 2 |
| Visible blood in urine | No, yes | No ⇒ 0; yes ⇒ 2 |
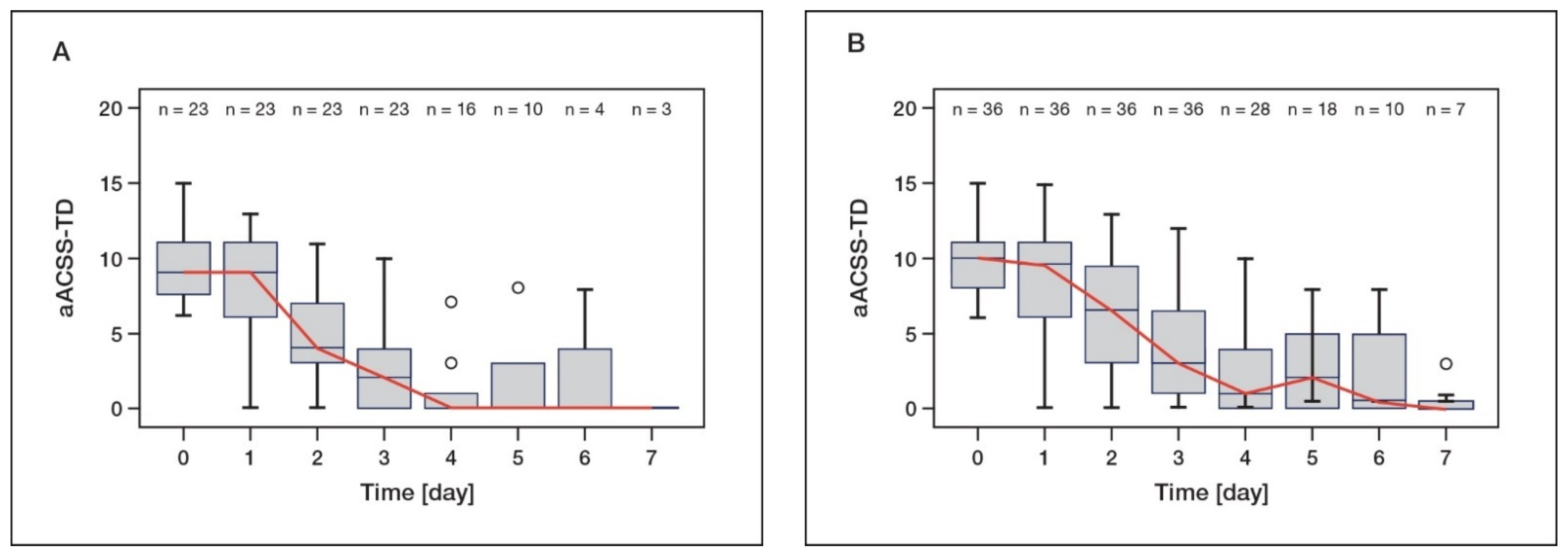
| Group 1: d-Mannose Monotherapy (n = 23) | Group 2: d-Mannose and Other Measures (n = 36) | |||||
|---|---|---|---|---|---|---|
| Day | aACSS-TD (Median) | Cure Rate 1 | Cure Rate 2 | aACSS-TD (Median) | Cure Rate 1 | Cure Rate 2 |
| 0 | 9.0 | - | - | 10.0 | - | - |
| 1 | 9.0 | 4.4% | 4.4% | 9.5 | 5.6% | 2.8% |
| 2 | 4.0 | 52.2% | 4.4% | 6.5 | 38.9% | 5.6% |
| 3 | 2.0 | 73.9% | 56.5% | 3.0 | 61.1% | 41.7% |
| 4 | 0.0 | 91.3% | 82.6% | 1.0 | 80.6% | 61.1% |
| 5 | 0.0 | 91.3% | 82.6% | 2.0 | 80.6% | 66.7% |
| 6 | 0.0 | 91.3% | 87.0% | 0.5 | 86.1% | 77.8% |
| 7 | 0.0 | 91.3% | 87.0% | 0.0 | 86.1% | 77.8% |
| Cure rate [95% CI] on day 7 | - | 91.3% [72–99%] | 87.0% [66–97%] | - | 86.1% [71–95%] | 77.8% [61–90%] |
| Treatment [Reference] | Baseline | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7–8 |
|---|---|---|---|---|---|---|---|---|
| d-mannose monotherapy 1 | 51.7 | 46.9 | 28.5 | 14.5 | 5.6 | 8.3 | 11.1 | 0 |
| d-mannose and other measures 1 | 54.5 | 50.9 | 35.9 | 21.9 | 13.9 | 13.6 | 13.3 | 3.2 |
| Fosfomycin (single dose) 1 [38] | 56.1 | - | - | 25.0 | - | - | - | 11.7 |
| Fosfomycin (single dose) 2 [47] | 50.8 | 26.7 | 16.7 | 10.0 | 8.3 | 7.5 | 5.8 | 4.2 |
| Pivmecillinam (5 days) 3 [52] | 42.6 | 26.7 | 14.0 | 12.0 | 8.0 | 7.3 | 7.3 | 6.7 |
| Pivmecillinam (3 days) 1 [49] | 68.3 | 41.7 | 22.2 | 13.9 | 5.6 | 5.0 | 3.9 | - |
| Ciprofloxacin (3 days) 4 [50] | 48.3 | - | - | - | 10.8 | - | - | 5.0 |
| Ciprofloxacin (5 days) 3 [51] | 50.7 | - | - | - | - | - | - | 10.0 |
| Norfloxacin (3 days) 5 [48] | 46.0 | - | - | 11.6 | - | - | - | 4.0% |
| Summary of antibiotic treatment (median) | 50.7 | 26.7 | 16.7 | 12.0 | 8.2 | 7.3 | 5.8 | 5.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagenlehner, F.; Lorenz, H.; Ewald, O.; Gerke, P. Why d-Mannose May Be as Efficient as Antibiotics in the Treatment of Acute Uncomplicated Lower Urinary Tract Infections—Preliminary Considerations and Conclusions from a Non-Interventional Study. Antibiotics 2022, 11, 314. https://doi.org/10.3390/antibiotics11030314
Wagenlehner F, Lorenz H, Ewald O, Gerke P. Why d-Mannose May Be as Efficient as Antibiotics in the Treatment of Acute Uncomplicated Lower Urinary Tract Infections—Preliminary Considerations and Conclusions from a Non-Interventional Study. Antibiotics. 2022; 11(3):314. https://doi.org/10.3390/antibiotics11030314
Chicago/Turabian StyleWagenlehner, Florian, Horst Lorenz, Oda Ewald, and Peter Gerke. 2022. "Why d-Mannose May Be as Efficient as Antibiotics in the Treatment of Acute Uncomplicated Lower Urinary Tract Infections—Preliminary Considerations and Conclusions from a Non-Interventional Study" Antibiotics 11, no. 3: 314. https://doi.org/10.3390/antibiotics11030314
APA StyleWagenlehner, F., Lorenz, H., Ewald, O., & Gerke, P. (2022). Why d-Mannose May Be as Efficient as Antibiotics in the Treatment of Acute Uncomplicated Lower Urinary Tract Infections—Preliminary Considerations and Conclusions from a Non-Interventional Study. Antibiotics, 11(3), 314. https://doi.org/10.3390/antibiotics11030314





