Applying Diagnostic Stewardship to Proactively Optimize the Management of Urinary Tract Infections
Abstract
1. Introduction
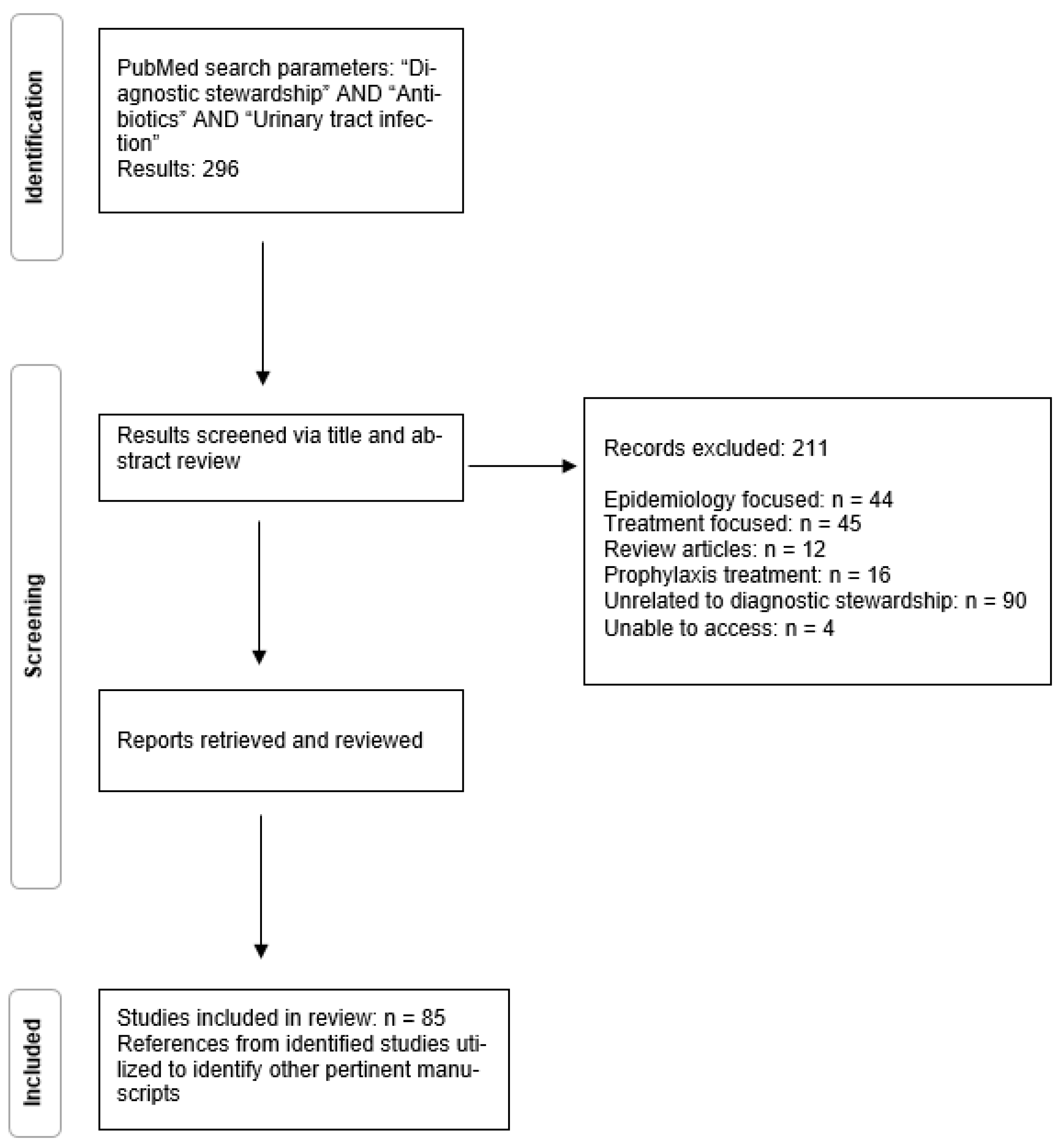
2. Methods
3. Results
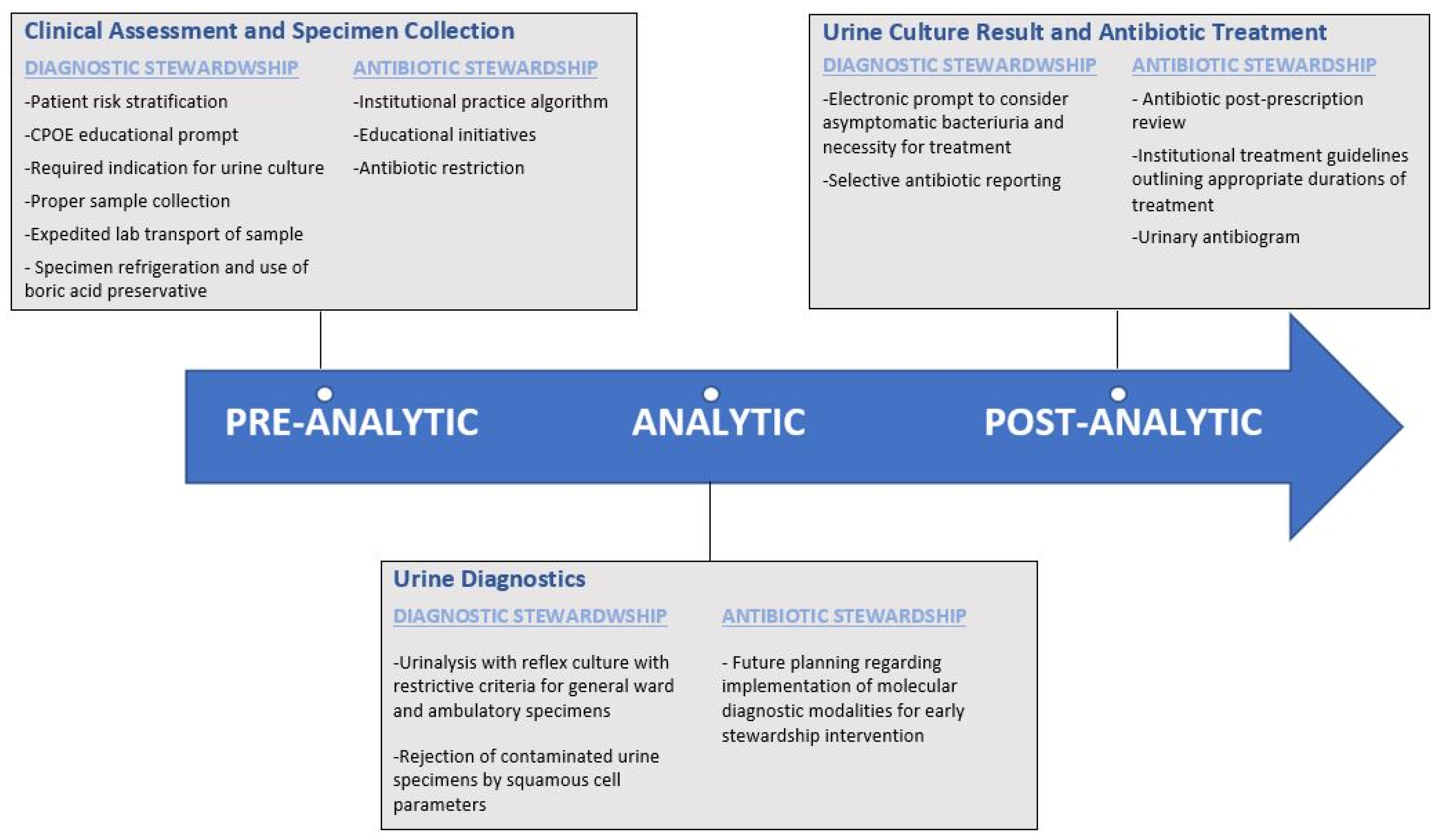
3.1. Pre-Analytical Urinary Stewardship Interventions
3.2. Analytical Urinary Stewardship Interventions
3.3. Post-Analytical Urinary Stewardship Interventions
3.4. Diagnostic Strategies for Urinary Tract Infections in Development
4. Discussion: Recommended Evidence-Based Strategies for the Implementation of Diagnostic Stewardship in Urinary Infections
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pollack, L.A.; Srinivasan, A. Core elements of hospital antibiotic stewardship programs from the Centers for Disease Control and Prevention. Clin. Infect. Dis. 2014, 59 (Suppl. 3), S97–S100. [Google Scholar] [CrossRef]
- CDC. Antibiotic Resistance Threats in the United States; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2019.
- Wong, D.W. Carriage of antibiotic resistant bacteria flora and its role in the guidance of clinical decision making. Pathog. Dis. 2020, 78, ftaa030. [Google Scholar] [CrossRef]
- Doron, S.; Davidson, L.E. Antimicrobial stewardship. Mayo Clin. Proc. 2011, 86, 1113–1123. [Google Scholar] [CrossRef]
- Carling, P.; Fung, T.; Killion, A.; Terrin, N.; Barza, M. Favorable impact of a multidisciplinary antibiotic management program conducted during 7 years. Infect. Control Hosp. Epidemiol. 2003, 24, 699–706. [Google Scholar] [CrossRef]
- Ansari, F.; Gray, K.; Nathwani, D.; Phillips, G.; Ogston, S.; Ramsay, C.; Davey, P. Outcomes of an intervention to improve hospital antibiotic prescribing: Interrupted time series with segmented regression analysis. J. Antimicrob. Chemother. 2003, 52, 842–848. [Google Scholar] [CrossRef]
- Malani, A.N.; Richards, P.G.; Kapila, S.; Otto, M.H.; Czerwinski, J.; Singal, B. Clinical and economic outcomes from a community hospital’s antimicrobial stewardship program. Am. J. Infect. Control 2013, 41, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Costelloe, C.; Metcalfe, C.; Lovering, A.; Mant, D.; Hay, A.D. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: Systematic review and meta-analysis. BMJ 2010, 340, c2096. [Google Scholar] [CrossRef]
- Yong, M.K.; Buising, K.L.; Cheng, A.C.; Thursky, K.A. Improved susceptibility of Gram-negative bacteria in an intensive care unit following implementation of a computerized antibiotic decision support system. J. Antimicrob. Chemother. 2010, 65, 1062–1069. [Google Scholar] [CrossRef]
- Standiford, H.C.; Chan, S.; Tripoli, M.; Weekes, E.; Forrest, G.N. Antimicrobial stewardship at a large tertiary care academic medical center: Cost analysis before, during, and after a 7-year program. Infect. Control Hosp. Epidemiol. 2012, 33, 338–345. [Google Scholar] [CrossRef]
- Nagel, J.L.; Stevenson, J.G.; Eiland, E.H.; Kaye, K.S. Demonstrating the value of antimicrobial stewardship programs to hospital administrators. Clin. Infect. Dis. 2014, 59 (Suppl. 3), S146–S153. [Google Scholar] [CrossRef]
- Wong, D.; Spellberg, B. Leveraging antimicrobial stewardship into improving rates of carbapenem-resistant enterobacteriaceae. Virulence 2016, 8, 383–390. [Google Scholar] [CrossRef]
- DHHS. Hospital and Critical Access Hospital (CAH) Changes to Promote Innovation, Flexibility, and Improvement in Patient Care; Department of Health and Human Services: Washington, DC, USA, 2016; pp. 39447–39480.
- Joint Commission. Approved: New Antimicrobial Stewardship Standard. Jt Comm Perspect 2016, 36, 1–3. [Google Scholar]
- Dellit, T.H.; Owens, R.C.; McGowan, J.E.; Gerding, D.N.; Weinstein, R.A.; Burke, J.P.; Huskins, W.C.; Paterson, D.L.; Fishman, N.O.; Carpenter, C.F.; et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin. Infect. Dis. 2007, 44, 159–177. [Google Scholar] [CrossRef]
- Roca, I.; Akova, M.; Baquero, F.; Carlet, J.; Cavaleri, M.; Coenen, S.; Cohen, J.; Findlay, D.; Gyssens, I.; Heuer, O.E.; et al. The global threat of antimicrobial resistance: Science for intervention. New Microbes New Infect. 2015, 6, 22–29. [Google Scholar] [CrossRef]
- Tornimbene, B.; Eremin, S.; Escher, M.; Griskeviciene, J.; Manglani, S.; Pessoa-Silva, C.L. WHO Global Antimicrobial Resistance Surveillance System early implementation 2016–2017. Lancet Infect. Dis. 2018, 18, 241–242. [Google Scholar] [CrossRef]
- Freedman, D.B. Towards Better Test Utilization—Strategies to Improve Physician Ordering and Their Impact on Patient Outcomes. EJIFCC 2015, 26, 15–30. [Google Scholar]
- Claeys, K.C. Diagnostic Stewardship: Beyond Managing Bloodstream Infections. Contagion Live. 2020, Volume 5. Available online: https://www.contagionlive.com/view/diagnostic-stewardship-beyond-managing-bloodstream-infections (accessed on 16 February 2022).
- Patel, R.; Fang, F.C. Diagnostic Stewardship: Opportunity for a Laboratory-Infectious Diseases Partnership. Clin. Infect. Dis. 2018, 67, 799–801. [Google Scholar] [CrossRef]
- Watson, K.J.; Trautner, B.; Russo, H.; Phe, K.; Lasco, T.; Pipkins, T.; Lembcke, B.; Al Mohajer, M. Using clinical decision support to improve urine culture diagnostic stewardship, antimicrobial stewardship, and financial cost: A multicenter experience. Infect. Control Hosp. Epidemiol. 2020, 41, 564–570. [Google Scholar] [CrossRef]
- Claeys, K.C.; Blanco, N.; Morgan, D.J.; Leekha, S.; Sullivan, K.V. Advances and Challenges in the Diagnosis and Treatment of Urinary Tract Infections: The Need for Diagnostic Stewardship. Curr. Infect. Dis. Rep. 2019, 21, 11. [Google Scholar] [CrossRef]
- Foxman, B. Epidemiology of urinary tract infections: Incidence, morbidity, and economic costs. Am. J. Med. 2002, 113 (Suppl. 1A), 5S–13S. [Google Scholar] [CrossRef]
- Garcia, R.; Spitzer, E.D. Promoting appropriate urine culture management to improve health care outcomes and the accuracy of catheter-associated urinary tract infections. Am. J. Infect. Control 2017, 45, 1143–1153. [Google Scholar] [CrossRef]
- Markowitz, M.A.; Monti, G.K.; Kim, J.H.; Haake, D.A. Rapid diagnostic testing in the management of urinary tract infection: Potentials and limitations. Diagn. Microbiol. Infect. Dis. 2019, 94, 371–377. [Google Scholar] [CrossRef]
- Cortes-Penfield, N.W.; Trautner, B.W.; Jump, R.L.P. Urinary Tract Infection and Asymptomatic Bacteriuria in Older Adults. Infect. Dis. Clin. N. Am. 2017, 31, 673–688. [Google Scholar] [CrossRef]
- Fakih, M.G.; Khatib, R. Improving the Culture of Culturing: Critical Asset to Antimicrobial Stewardship. Infect. Control Hosp. Epidemiol. 2017, 38, 377–379. [Google Scholar] [CrossRef][Green Version]
- Drekonja, D.M.; Abbo, L.M.; Kuskowski, M.A.; Gnadt, C.; Shukla, B.; Johnson, J.R. A survey of resident physicians’ knowledge regarding urine testing and subsequent antimicrobial treatment. Am. J. Infect. Control 2013, 41, 892–896. [Google Scholar] [CrossRef]
- Watson, J.R.; Sánchez, P.J.; Spencer, J.D.; Cohen, D.M.; Hains, D.S. Urinary Tract Infection and Antimicrobial Stewardship in the Emergency Department. Pediatr. Emerg. Care 2018, 34, 93–95. [Google Scholar] [CrossRef]
- Shallcross, L.J.; Rockenschaub, P.; McNulty, D.; Freemantle, N.; Hayward, A.; Gill, M.J. Diagnostic uncertainty and urinary tract infection in the emergency department: A cohort study from a UK hospital. BMC Emerg. Med. 2020, 20, 40. [Google Scholar] [CrossRef]
- Lee, M.J.; Kim, M.; Kim, N.H.; Kim, C.J.; Song, K.H.; Choe, P.G.; Park, W.B.; Bang, J.H.; Kim, E.S.; Park, S.W.; et al. Why is asymptomatic bacteriuria overtreated?: A tertiary care institutional survey of resident physicians. BMC Infect. Dis. 2015, 15, 289. [Google Scholar] [CrossRef]
- Nicolle, L.E.; Gupta, K.; Bradley, S.F.; Colgan, R.; DeMuri, G.P.; Drekonja, D.; Eckert, L.O.; Geerlings, S.E.; Köves, B.; Hooton, T.M.; et al. Clinical Practice Guideline for the Management of Asymptomatic Bacteriuria: 2019 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2019, 68, e83–e110. [Google Scholar] [CrossRef]
- Flokas, M.E.; Andreatos, N.; Alevizakos, M.; Kalbasi, A.; Onur, P.; Mylonakis, E. Inappropriate Management of Asymptomatic Patients With Positive Urine Cultures: A Systematic Review and Meta-analysis. Open Forum Infect. Dis. 2017, 4, ofx207. [Google Scholar] [CrossRef]
- Daley, P.; Penney, C.; Wakeham, S.; Compton, G.; McKim, A.; O’Keefe, J.; Barrett, B.; Nicolle, L. Urinary tract infection diagnosis and response to therapy in long-term care: A prospective observational study. Can. J. Infect. Dis. Med. Microbiol. 2015, 26, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Cope, M.; Cevallos, M.E.; Cadle, R.M.; Darouiche, R.O.; Musher, D.M.; Trautner, B.W. Inappropriate treatment of catheter-associated asymptomatic bacteriuria in a tertiary care hospital. Clin. Infect. Dis. 2009, 48, 1182–1188. [Google Scholar] [CrossRef] [PubMed]
- Bruxvoort, K.J.; Bider-Canfield, Z.; Casey, J.A.; Qian, L.; Pressman, A.; Liang, A.S.; Robinson, S.; Jacobsen, S.J.; Tartof, S.Y. Outpatient Urinary Tract Infections in an Era of Virtual Healthcare: Trends From 2008 to 2017. Clin. Infect. Dis. 2020, 71, 100–108. [Google Scholar] [CrossRef]
- Chardavoyne, P.C.; Kasmire, K.E. Appropriateness of Antibiotic Prescriptions for Urinary Tract Infections. West. J. Emerg. Med. 2020, 21, 633–639. [Google Scholar] [CrossRef]
- Keller, S.C.; Feldman, L.; Smith, J.; Pahwa, A.; Cosgrove, S.E.; Chida, N. The Use of Clinical Decision Support in Reducing Diagnosis of and Treatment of Asymptomatic Bacteriuria. J. Hosp. Med. 2018, 13, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.L.H.; Leung, E.C.M.; Lee, M.K.P.; Lai, R.W.M. Diagnostic stewardship programme for urine culture: Impact on antimicrobial prescription in a multi-centre cohort. J. Hosp. Infect. 2021, 108, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Demonchy, E.; Dufour, J.C.; Gaudart, J.; Cervetti, E.; Michelet, P.; Poussard, N.; Levraut, J.; Pulcini, C. Impact of a computerized decision support system on compliance with guidelines on antibiotics prescribed for urinary tract infections in emergency departments: A multicentre prospective before-and-after controlled interventional study. J. Antimicrob. Chemother. 2014, 69, 2857–2863. [Google Scholar] [CrossRef]
- Shirley, D.; Scholtz, H.; Osterby, K.; Musuuza, J.; Fox, B.; Safdar, N. Optimizing Inpatient Urine Culture Ordering Practices Using the Electronic Medical Record: A Pilot Study. Infect. Control Hosp. Epidemiol. 2017, 38, 486–488. [Google Scholar] [CrossRef]
- Page, S.; Hazen, D.; Kelley, K.; Singh, R.; Rodgers, R.B.; Brewer, B.; Sadowski, J.; Desai, A.; Beeler, C.; Webb, D.; et al. Changing the culture of urine culturing: Utilizing Agile Implementation to improve diagnostic stewardship in the ICU. Am. J. Infect. Control 2020, 48, 1375–1380. [Google Scholar] [CrossRef]
- Messacar, K.; Parker, S.K.; Todd, J.K.; Dominguez, S.R. Implementation of Rapid Molecular Infectious Disease Diagnostics: The Role of Diagnostic and Antimicrobial Stewardship. J. Clin. Microbiol. 2017, 55, 715–723. [Google Scholar] [CrossRef]
- Luu, A.; Dominguez, F.; Yeshoua, B.; Vo, C.; Nallapa, S.; Chung, D.; Wald-Dickler, N.; Butler-Wu, S.M.; Khaleel, H.; Chang, K.; et al. Reducing Catheter-associated Urinary Tract Infections via Cost-saving Diagnostic Stewardship. Clin. Infect. Dis. 2021, 72, e883–e886. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Balmer, F.; Friedli-Wüthrich, H.; Mühlemann, K. Reduction of urinary catheter use and prescription of antibiotics for asymptomatic bacteriuria in hospitalised patients in internal medicine: Before-and-after intervention study. Swiss Med. Wkly. 2013, 143, w13796. [Google Scholar] [CrossRef] [PubMed]
- Nace, D.A.; Hanlon, J.T.; Crnich, C.J.; Drinka, P.J.; Schweon, S.J.; Anderson, G.; Perera, S. A Multifaceted Antimicrobial Stewardship Program for the Treatment of Uncomplicated Cystitis in Nursing Home Residents. JAMA Intern. Med. 2020, 180, 944–951. [Google Scholar] [CrossRef] [PubMed]
- James, D.; Lopez, L. Impact of a pharmacist-driven education initiative on treatment of asymptomatic bacteriuria. Am. J. Health Syst. Pharm. 2019, 76, S41–S48. [Google Scholar] [CrossRef]
- Trautner, B.W.; Grigoryan, L.; Petersen, N.J.; Hysong, S.; Cadena, J.; Patterson, J.E.; Naik, A.D. Effectiveness of an Antimicrobial Stewardship Approach for Urinary Catheter-Associated Asymptomatic Bacteriuria. JAMA Intern. Med. 2015, 175, 1120–1127. [Google Scholar] [CrossRef]
- Choi, P.W.; Benzer, J.A.; Coon, J.; Egwuatu, N.E.; Dumkow, L.E. Impact of pharmacist-led selective audit and feedback on outpatient antibiotic prescribing for UTIs and SSTIs. Am. J. Health Syst. Pharm. 2021, 78, S62–S69. [Google Scholar] [CrossRef]
- Lee, C.; Phillips, C.; Vanstone, J.R. Educational intervention to reduce treatment of asymptomatic bacteriuria in long-term care. BMJ Open Qual. 2018, 7, e000483. [Google Scholar] [CrossRef]
- McMaughan, D.K.; Nwaiwu, O.; Zhao, H.; Frentzel, E.; Mehr, D.; Imanpour, S.; Garfinkel, S.; Phillips, C.D. Impact of a decision-making aid for suspected urinary tract infections on antibiotic overuse in nursing homes. BMC Geriatr. 2016, 16, 81. [Google Scholar] [CrossRef]
- Bekeris, L.G.; Jones, B.A.; Walsh, M.K.; Wagar, E.A. Urine culture contamination: A College of American Pathologists Q-Probes study of 127 laboratories. Arch. Pathol. Lab. Med. 2008, 132, 913–917. [Google Scholar] [CrossRef]
- LaRocco, M.T.; Franek, J.; Leibach, E.K.; Weissfeld, A.S.; Kraft, C.S.; Sautter, R.L.; Baselski, V.; Rodahl, D.; Peterson, E.J.; Cornish, N.E. Effectiveness of Preanalytic Practices on Contamination and Diagnostic Accuracy of Urine Cultures: A Laboratory Medicine Best Practices Systematic Review and Meta-analysis. Clin. Microbiol. Rev. 2016, 29, 105–147. [Google Scholar] [CrossRef]
- Lahanas, S.; Stathopoulos, G.; Chan, R.C.; van Hal, S.J. Evaluation of the Alfred 60/AST device as a screening test for urinary tract infections. J. Clin. Microbiol. 2013, 51, 3406–3408. [Google Scholar] [CrossRef][Green Version]
- Claeys, K.C.; Zhan, M.; Pineles, L.; Lydecker, A.; Clore, G.; Goto, M.; Leekha, S.; Linkin, D.; Evans, C.T.; Trautner, B.W.; et al. Conditional reflex to urine culture: Evaluation of a diagnostic stewardship intervention within the Veterans’ Affairs and Centers for Disease Control and Prevention Practice-Based Research Network. Infect. Control. Hosp. Epidemiol. 2021, 42, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.W.; Culbreath, K.D.; Mehrotra, A.; Gilligan, P.H. Reflect urine culture cancellation in the emergency department. J. Emerg. Med. 2014, 46, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.S.; Appleby-Sigler, A.; Bork, J.T.; Davé, R.; Agnes, K.; Sanikop, M.; Heath, D.; Clark, A.F.; Claeys, K.; Zhan, M.; et al. Effect of urine reflex culturing on rates of cultures and infections in acute and long-term care. Antimicrob. Resist. Infect. Control 2020, 9, 96. [Google Scholar] [CrossRef] [PubMed]
- Stagg, A.; Lutz, H.; Kirpalaney, S.; Matelski, J.J.; Kaufman, A.; Leis, J.; McCready, J.; Powis, J. Impact of two-step urine culture ordering in the emergency department: A time series analysis. BMJ Qual. Saf. 2018, 27, 140–147. [Google Scholar] [CrossRef]
- Richards, K.A.; Cesario, S.; Best, S.L.; Deeren, S.M.; Bushman, W.; Safdar, N. Reflex urine culture testing in an ambulatory urology clinic: Implications for antibiotic stewardship in urology. Int. J. Urol. 2019, 26, 69–74. [Google Scholar] [CrossRef]
- Sarg, M.; Waldrop, G.E.; Beier, M.A.; Heil, E.L.; Thom, K.A.; Preas, M.A.; Johnson, J.K.; Harris, A.D.; Leekha, S. Impact of Changes in Urine Culture Ordering Practice on Antimicrobial Utilization in Intensive Care Units at an Academic Medical Center. Infect. Control Hosp. Epidemiol. 2016, 37, 448–454. [Google Scholar] [CrossRef]
- Munigala, S.; Rojek, R.; Wood, H.; Yarbrough, M.L.; Jackups, R.R.; Burnham, C.D.; Warren, D.K. Effect of changing urine testing orderables and clinician order sets on inpatient urine culture testing: Analysis from a large academic medical center. Infect. Control Hosp. Epidemiol. 2019, 40, 281–286. [Google Scholar] [CrossRef]
- Sullivan, K.V.; Morgan, D.J.; Leekha, S. Use of diagnostic stewardship practices to improve urine culturing among SHEA Research Network hospitals. Infect. Control Hosp. Epidemiol. 2019, 40, 228–231. [Google Scholar] [CrossRef]
- Ling, D.; Seidelman, J.; Dodds-Ashley, E.; Lewis, S.; Moehring, R.W.; Anderson, D.J.; Advani, S. Navigating reflex urine culture practices in community hospitals: Need for a validated approach. Am. J. Infect. Control 2020, 48, 1549–1551. [Google Scholar] [CrossRef]
- Fok, C.; Fitzgerald, M.P.; Turk, T.; Mueller, E.; Dalaza, L.; Schreckenberger, P. Reflex testing of male urine specimens misses few positive cultures may reduce unnecessary testing of normal specimens. Urology 2010, 75, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Humphries, R.M.; Dien Bard, J. Point-Counterpoint: Reflex Cultures Reduce Laboratory Workload and Improve Antimicrobial Stewardship in Patients Suspected of Having Urinary Tract Infections. J. Clin. Microbiol. 2016, 54, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.L.; Gaido, L. Laboratory diagnosis of urinary tract infections in adult patients. Clin. Infect. Dis. 2004, 38, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Howard-Anderson, J.R.; Ashraf, S.; Overton, E.C.; Reif, L.; Murphy, D.J.; Jacob, J.T. Sustained decrease in urine culture utilization after implementing a reflex urine culture intervention: A multicenter quasi-experimental study. Infect. Control Hosp. Epidemiol. 2020, 41, 369–371. [Google Scholar] [CrossRef]
- Epstein, L.; Edwards, J.R.; Halpin, A.L.; Preas, M.A.; Blythe, D.; Harris, A.D.; Hunt, D.; Johnson, J.K.; Filippell, M.; Gould, C.V.; et al. Evaluation of a Novel Intervention to Reduce Unnecessary Urine Cultures in Intensive Care Units at a Tertiary Care Hospital in Maryland, 2011–2014. Infect. Control Hosp. Epidemiol. 2016, 37, 606–609. [Google Scholar] [CrossRef]
- Tambyah, P.A.; Maki, D.G. The relationship between pyuria and infection in patients with indwelling urinary catheters: A prospective study of 761 patients. Arch. Intern. Med. 2000, 160, 673–677. [Google Scholar] [CrossRef]
- Schwartz, D.S.; Barone, J.E. Correlation of urinalysis and dipstick results with catheter-associated urinary tract infections in surgical ICU patients. Intensive Care Med. 2006, 32, 1797–1801. [Google Scholar] [CrossRef]
- Stalenhoef, J.E.; van Nieuwkoop, C.; Wilson, D.C.; van der Starre, W.E.; van der Reijden, T.J.K.; Delfos, N.M.; Leyten, E.M.S.; Koster, T.; Ablij, H.C.; van’t Wout, J.J.W.; et al. Procalcitonin, mid-regional proadrenomedullin and C-reactive protein in predicting treatment outcome in community-acquired febrile urinary tract infection. BMC Infect. Dis. 2019, 19, 161. [Google Scholar] [CrossRef]
- Drozdov, D.; Schwarz, S.; Kutz, A.; Grolimund, E.; Rast, A.C.; Steiner, D.; Regez, K.; Schild, U.; Guglielmetti, M.; Conca, A.; et al. Procalcitonin and pyuria-based algorithm reduces antibiotic use in urinary tract infections: A randomized controlled trial. BMC Med. 2015, 13, 104. [Google Scholar] [CrossRef][Green Version]
- Covino, M.; Manno, A.; Merra, G.; Simeoni, B.; Piccioni, A.; Carbone, L.; Forte, E.; Ojetti, V.; Franceschi, F.; Murri, R. Reduced utility of early procalcitonin and blood culture determination in patients with febrile urinary tract infections in the emergency department. Intern. Emerg. Med. 2020, 15, 119–125. [Google Scholar] [CrossRef]
- Fritzenwanker, M.; Imirzalioglu, C.; Chakraborty, T.; Wagenlehner, F.M. Modern diagnostic methods for urinary tract infections. Expert Rev. Anti Infect. Ther. 2016, 14, 1047–1063. [Google Scholar] [CrossRef] [PubMed]
- Gill, K.; Horsley, H.; Kupelian, A.S.; Baio, G.; De Iorio, M.; Sathiananamoorthy, S.; Khasriya, R.; Rohn, J.L.; Wildman, S.S.; Malone-Lee, J. Urinary ATP as an indicator of infection and inflammation of the urinary tract in patients with lower urinary tract symptoms. BMC Urol. 2015, 15, 7. [Google Scholar] [CrossRef]
- Burillo, A.; Rodríguez-Sánchez, B.; Ramiro, A.; Cercenado, E.; Rodríguez-Créixems, M.; Bouza, E. Gram-stain plus MALDI-TOF MS (Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry) for a rapid diagnosis of urinary tract infection. PLoS ONE 2014, 9, e86915. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Zhang, G.; Fan, Y.Y.; Yang, X.; Sui, W.J.; Lu, X.X. Direct identification of bacteria causing urinary tract infections by combining matrix-assisted laser desorption ionization-time of flight mass spectrometry with UF-1000i urine flow cytometry. J. Microbiol. Methods 2013, 92, 231–235. [Google Scholar] [CrossRef]
- Zboromyrska, Y.; Rubio, E.; Alejo, I.; Vergara, A.; Mons, A.; Campo, I.; Bosch, J.; Marco, F.; Vila, J. Development of a new protocol for rapid bacterial identification and susceptibility testing directly from urine samples. Clin. Microbiol. Infect. 2016, 22, 561.e1–561.e6. [Google Scholar] [CrossRef] [PubMed]
- Institute, C.a.L.S. Analysis and Presentation or Cumulative Antimicrobial Susceptibility Test Data: Approved Guideline, 4th ed.; CLSO document M39-A4; CLSI: Wayne, PA, USA, 2014. [Google Scholar]
- Barlam, T.F.; Cosgrove, S.E.; Abbo, L.M.; MacDougall, C.; Schuetz, A.N.; Septimus, E.J.; Srinivasan, A.; Dellit, T.H.; Falck-Ytter, Y.T.; Fishman, N.O.; et al. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin. Infect. Dis. 2016, 62, e51–e77. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; Daneman, N.; Diong, C.; Marchand-Austin, A.; Adomako, K.; Saedi, A.; Schwartz, K.L.; Johnstone, J.; MacFadden, D.R.; Matukas, L.M.; et al. Antibiotic susceptibility reporting and association with antibiotic prescribing: A cohort study. Clin. Microbiol. Infect. 2021, 27, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Katchanov, J.; Kluge, S.; MacKenzie, C.R.; Kaasch, A.J. “Nudging” in microbiological reports: A strategy to improve prescribing. Infection 2017, 45, 123–127. [Google Scholar] [CrossRef]
- Coupat, C.; Pradier, C.; Degand, N.; Hofliger, P.; Pulcini, C. Selective reporting of antibiotic susceptibility data improves the appropriateness of intended antibiotic prescriptions in urinary tract infections: A case-vignette randomised study. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 627–636. [Google Scholar] [CrossRef]
- McNulty, C.A.; Lasseter, G.M.; Charlett, A.; Lovering, A.; Howell-Jones, R.; Macgowan, A.; Thomas, M. Does laboratory antibiotic susceptibility reporting influence primary care prescribing in urinary tract infection and other infections? J. Antimicrob. Chemother. 2011, 66, 1396–1404. [Google Scholar] [CrossRef]
- Leis, J.A.; Rebick, G.W.; Daneman, N.; Gold, W.L.; Poutanen, S.M.; Lo, P.; Larocque, M.; Shojania, K.G.; McGeer, A. Reducing antimicrobial therapy for asymptomatic bacteriuria among noncatheterized inpatients: A proof-of-concept study. Clin. Infect. Dis. 2014, 58, 980–983. [Google Scholar] [CrossRef] [PubMed]
- Vissichelli, N.C.; Orndahl, C.M.; Cecil, J.A.; Hill, E.M.; Hitchcock, M.M.; Sabo, R.T.; Stevens, M.P.; Tassone, D.; Vaughan, L.B.; Markley, J.D. Impact of cascade reporting of antimicrobial susceptibility on fluoroquinolone and meropenem consumption at a Veterans’ Affairs medical center. Infect. Control Hosp. Epidemiol. 2021, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; Seah, J.; Chan, A.; Downing, M.; Johnstone, J.; Matukas, L.M. Antimicrobial Stewardship in the Microbiology Laboratory: Impact of Selective Susceptibility Reporting on Ciprofloxacin Utilization and Susceptibility of Gram-Negative Isolates to Ciprofloxacin in a Hospital Setting. J. Clin. Microbiol. 2016, 54, 2343–2347. [Google Scholar] [CrossRef]
- Daley, P.; Garcia, D.; Inayatullah, R.; Penney, C.; Boyd, S. Modified Reporting of Positive Urine Cultures to Reduce Inappropriate Treatment of Asymptomatic Bacteriuria Among Nonpregnant, Noncatheterized Inpatients: A Randomized Controlled Trial. Infect. Control Hosp. Epidemiol. 2018, 39, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Klinker, K.P.; Hidayat, L.K.; DeRyke, C.A.; DePestel, D.D.; Motyl, M.; Bauer, K.A. Antimicrobial stewardship and antibiograms: Importance of moving beyond traditional antibiograms. Ther. Adv. Infect. Dis. 2021, 8, 20499361211011373. [Google Scholar] [CrossRef] [PubMed]
- Shoff, C.J.; Townsend, M.L.; Tillekeratne, L.G.; Schulteis, R.D.; Yarrington, M.E.; Turner, N.A.; Woods, C.W.; Hostler, C.J. Improved empiric antibiotic prescribing for acute cystitis with use of local urinary antibiogram and clinical decision support system. Infect. Control Hosp. Epidemiol. 2020, 41, 1351–1353. [Google Scholar] [CrossRef]
- Xu, R.; Deebel, N.; Casals, R.; Dutta, R.; Mirzazadeh, M. A New Gold Rush: A Review of Current and Developing Diagnostic Tools for Urinary Tract Infections. Diagnostics 2021, 11, 479. [Google Scholar] [CrossRef]
- Dixon, M.; Sha, S.; Stefil, M.; McDonald, M. Is it Time to Say Goodbye to Culture and Sensitivity? The Case for Culture-independent Urology. Urology 2020, 136, 112–118. [Google Scholar] [CrossRef]
- Altobelli, E.; Mohan, R.; Mach, K.E.; Sin, M.L.Y.; Anikst, V.; Buscarini, M.; Wong, P.K.; Gau, V.; Banaei, N.; Liao, J.C. Integrated Biosensor Assay for Rapid Uropathogen Identification and Phenotypic Antimicrobial Susceptibility Testing. Eur. Urol. Focus 2017, 3, 293–299. [Google Scholar] [CrossRef]
- Lehmann, L.E.; Hauser, S.; Malinka, T.; Klaschik, S.; Weber, S.U.; Schewe, J.C.; Stüber, F.; Book, M. Rapid qualitative urinary tract infection pathogen identification by SeptiFast real-time PCR. PLoS ONE 2011, 6, e17146. [Google Scholar] [CrossRef]
- Davenport, M.; Mach, K.E.; Shortliffe, L.M.D.; Banaei, N.; Wang, T.H.; Liao, J.C. New and developing diagnostic technologies for urinary tract infections. Nat. Rev. Urol. 2017, 14, 296–310. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morado, F.; Wong, D.W. Applying Diagnostic Stewardship to Proactively Optimize the Management of Urinary Tract Infections. Antibiotics 2022, 11, 308. https://doi.org/10.3390/antibiotics11030308
Morado F, Wong DW. Applying Diagnostic Stewardship to Proactively Optimize the Management of Urinary Tract Infections. Antibiotics. 2022; 11(3):308. https://doi.org/10.3390/antibiotics11030308
Chicago/Turabian StyleMorado, Faiza, and Darren W. Wong. 2022. "Applying Diagnostic Stewardship to Proactively Optimize the Management of Urinary Tract Infections" Antibiotics 11, no. 3: 308. https://doi.org/10.3390/antibiotics11030308
APA StyleMorado, F., & Wong, D. W. (2022). Applying Diagnostic Stewardship to Proactively Optimize the Management of Urinary Tract Infections. Antibiotics, 11(3), 308. https://doi.org/10.3390/antibiotics11030308





