Icariin in Combination with Amoxycillin-Clavulanate and Ampicillin, but Not Vancomycin, Increases Antibiotic Sensitivity and Growth Inhibition against Methicillin-Resistant Staphylococcus aureus
Abstract
:1. Introduction
2. Results
2.1. Evaluation of the Antimicrobial Effect of Icariin on Laboratory and Clinical Isolates of Methicillin-Resistant Staphylococcus aureus
2.2. Assessment of Cell Viability in the Presence of Antibiotics with Icariin
3. Discussion
4. Materials and Methods
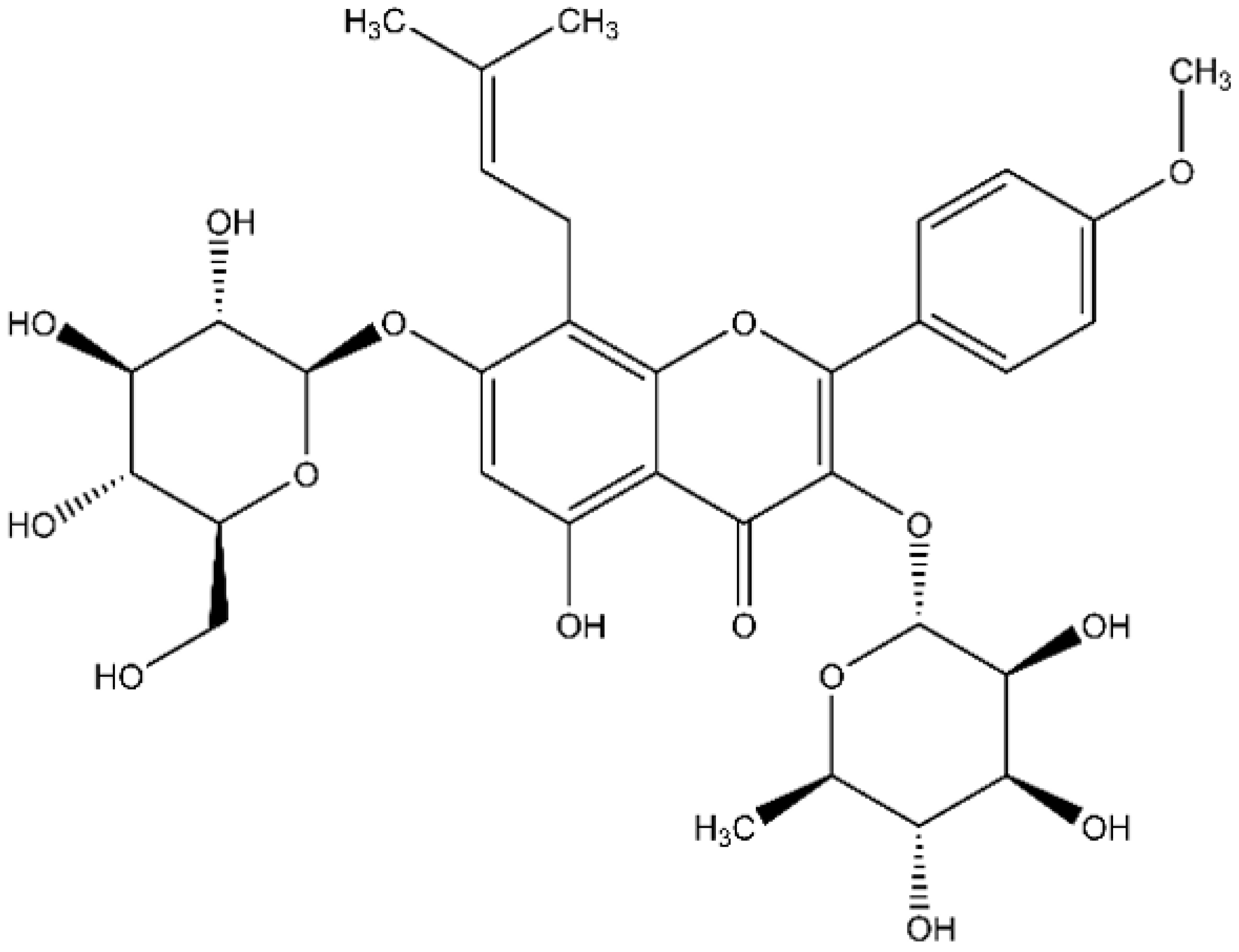
4.1. Icariin and Bacterial Strains
4.2. Antibiograms
4.3. Vital Staining
4.4. Calculation of Colony-Forming Units (CFU) to Determine Viability
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parrish, K.L.; Hogan, P.G.; Clemons, A.A.; Fritz, S.A. Spatial relationships among public places frequented by families plagued by methicillin-resistant Staphylococcus aureus. BMC Res. Notes 2018, 11, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Lazaris, A.; Coleman, D.; Kearns, A.M.; Pichon, B.; Kinnevey, P.; Earls, M.R.; Boyle, B.; O’Connell, B.; I Brennan, G.; Shore, A.C. Novel multiresistance cfr plasmids in linezolid-resistant methicillin-resistant Staphylococcus epidermidis and vancomycin-resistant Enterococcus faecium (VRE) from a hospital outbreak: Co-location of cfr and optrA in VRE. J. Antimicrob. Chemother. 2017, 72, 3252–3257. [Google Scholar] [CrossRef]
- Mun, S.-H.; Joung, D.-K.; Kim, Y.-S.; Kang, O.-H.; Kim, S.-B.; Seo, Y.-S.; Kim, Y.-C.; Lee, D.-S.; Shin, D.-W.; Kweon, K.-T.; et al. Synergistic antibacterial effect of curcumin against methicillin-resistant Staphylococcus aureus. Phytomedicine 2013, 20, 714–718. [Google Scholar] [CrossRef]
- Miklasińska-Majdanik, M.; Kępa, M.; Wojtyczka, R.D.; Idzik, D.; Wąsik, T.J. Phenolic Compounds Diminish Antibiotic Resistance of Staphylococcus Aureus Clinical Strains. Int. J. Environ. Res. Public Health 2018, 15, 2321. [Google Scholar] [CrossRef] [Green Version]
- European Centre for Disease Prevention and Control. Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report 2019; ECDC: Stockholm, Sweeden, 2020. [Google Scholar]
- Wang, A.; Xu, Y. Synthesis and antibacterial activity of novel icariin derivatives. Die Pharm. 2019, 74, 73–78. [Google Scholar]
- Perez-Vizcaino, F.; Fraga, C.G. Research trends in flavonoids and health. Arch. Biochem. Biophys. 2018, 646, 107–112. [Google Scholar] [CrossRef]
- Amin, M.U.; Khurram, M.; Khattak, B.; Khan, J. Antibiotic additive and synergistic action of rutin, morin and quercetin against methicillin resistant Staphylococcus aureus. BMC Complement. Altern. Med. 2015, 15, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Qin, R.; Xiao, K.; Li, B.; Jiang, W.; Peng, W.; Zheng, J.; Zhou, H. The combination of catechin and epicatechin callate from Fructus Crataegi potentiates beta-lactam antibiotics against methicillin-resistant staphylococcus au-reus (MRSA) in vitro and in vivo. Int. J. Mol. Sci. 2013, 14, 1802–1821. [Google Scholar] [CrossRef] [Green Version]
- Stapleton, P.D.; Shah, S.; Ehlert, K.; Hara, Y.; Taylor, P.W. The beta-lactam-resistance modifier (-)-epicatechin gallate alters the architecture of the cell wall of Staphylococcus aureus. Microbiology 2007, 153, 2093–2103. [Google Scholar] [CrossRef] [Green Version]
- Lin, R.-D.; Chin, Y.-P.; Hou, W.-C.; Lee, M.-H. The Effects of Antibiotics Combined with Natural Polyphenols against Clinical Methicillin-ResistantStaphylococcus aureus(MRSA). Planta Medica 2008, 74, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, R.; Coppo, E.; Marchese, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M. Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Microbiol. Res. 2017, 196, 44–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-H.; Otsuka, N.; Noyori, K.; Shiota, S.; Ogawa, W.; Kuroda, T.; Hatano, T.; Tsuchiya, T. Synergistic Effect of Kaempferol Glycosides Purified from Laurus nobilis and Fluoroquinolones on Methicillin-Resistant Staphylococcus aureus. Biol. Pharm. Bull. 2009, 32, 489–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, W.H.; Hu, Z.Q.; Okubo, S.; Hara, Y.; Shimamura, T. Mechanism of synergy between epigallocatechin gal-late and beta-lactams against methicillin-resistant S. aureus. Antimicrob. Agents Chemother. 2001, 45, 1737–1742. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.H.; Hu, Z.Q.; Okubo, S.; Hara, Y.; Shimamura, T. Inhibition of penicillinase by epigallocatechin gallate resulting in restoration of antibacterial activity of penicillin against penicillinase-producing S. aureus. Antimicrob. Agents Chemother. 2002, 46, 2266–2268. [Google Scholar] [CrossRef] [Green Version]
- Novy, P.; Rondevaldova, J.; Kouřimská, L.; Kokoska, L. Synergistic interactions of epigallocatechin gallate and oxytetracycline against various drug resistant Staphylococcus aureus strains in vitro. Phytomedicine 2013, 20, 432–435. [Google Scholar] [CrossRef]
- Hu, Z.Q.; Zhao, W.H.; Asano, N.; Yoda, Y.; Hara, Y.; Shimamura, T. Epigallocatechin gallate synergistically en-hances the activity of carbapenems against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2002, 46, 558–560. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.S.; Schiller, N.L.; Oh, K.H. Antibacterial effects of green tea polyphenols on clinical isolates of methicil-lin-resistant Staphylococcus aureus. Curr. Microbiol. 2008, 57, 542–546. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.Q.; Zhao, W.H.; Yoda, Y.; Asano, N.; Hara, Y.; Shimamura, T. Additive, indifferent and antagonistic ef-fects in combinations of epigallocatechin gallate with 12 non b-lactam antibiotics against methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 2002, 50, 1051–1054. [Google Scholar] [CrossRef] [Green Version]
- Roccaro, A.S.; Blanco, A.R.; Giuliano, F.; Rusciano, D.; Enea, V. Epigallocatechin-Gallate Enhances the Activity of Tetracycline in Staphylococci by Inhibiting Its Efflux from Bacterial Cells. Antimicrob. Agents Chemother. 2004, 48, 1968–1973. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.C.; Headley, C.; Stapleton, P.D.; Taylor, P.W. Synthesis and antibacterial activity of hydrolytically stable (-)-epicatechin gallate analogues for the modulation of b-lactam resistance in Staphylococcus aureus. Bioorg. Med. Chem. Lett. 2005, 15, 633–2635. [Google Scholar] [CrossRef] [Green Version]
- Coenye, T.; Brackman, G.; Rigole, P.; De Witte, E.; Honraet, K.; Rossel, B.; Nelis, H.J. Eradication of Propionibac-terium acnes biofilms by plant extracts and putative identification of icariin, resveratrol and salidroside as active compounds. Phytomedicine 2012, 19, 409–412. [Google Scholar] [CrossRef]
- Wang, N.; Fu, Q.; Xi, Z.; Che, X.; Li, N. Solubility of Icariin in a Binary Solvent System of Ethanol and Water. J. Solut. Chem. 2013, 42, 1837–1843. [Google Scholar] [CrossRef]
- Chung, B.H.; Kim, J.D.; Kim, C.K.; Kim, J.H.; Won, M.H.; Lee, H.S.; Dong, M.S.; Ha, K.S.; Kwon, Y.G.; Kim, Y.M. Icariin stimulates angiogenesis by activating the MEK/ERK- and PI3K/Akt/eNOS-dependent signal pathways in human endothelial cells. Biochem. Biophys. Res. Commun. 2008, 376, 404–408. [Google Scholar] [CrossRef]
- Li, S.; Dong, P.; Wang, J.; Zhang, J.; Gu, J.; Wu, X.; Wu, W.; Fei, X.; Zhang, Z.; Wang, Y.; et al. Icariin, a natural flavonol glycoside, induces apoptosis in human hepatoma SMMC-7721 cells via a ROS/JNK-dependent mitochondrial pathway. Cancer Lett. 2010, 298, 222–230. [Google Scholar] [CrossRef]
- Liu, M.H.; Sun, J.S.; Tsai, S.W.; Sheu, S.Y. Chen, M.H. Icariin protects murine chondrocytes from lipopolysac-charide-induced inflammatory responses and extracellular matrix degradation. Nutr. Res. 2010, 10, 57–65. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, H.; Xu, C.; Yang, G.; Tao, J.; Huang, J.; Wu, J.; Duan, X.; Cao, Y.; Dong, J. Neuroprotective effects of icariin on corticosterone-induced apoptosis in primary cultured rat hippocampal neurons. Brain Res. 2011, 1375, 59–67. [Google Scholar] [CrossRef]
- Hudzicki, J. Kirby-Bauer Disk Diffusion Susceptibility Test Protocol. [online] Microbelibrary.org. 2009. Available online: http://www.microbelibrary.org/component/resource/laboratory-test/3189-kirby-bauer-disk-diffusion-susceptibility-test-protocol (accessed on 10 February 2022).
- Lakhundi, S.; Zhang, K. Methicillin-Resistant Staphylococcus aureus: Molecular Characterization, Evolution, and Epidemiology. Clin. Microbiol. Rev. 2018, 31, e00020-18. [Google Scholar] [CrossRef] [Green Version]
- Lozano, C.; Fernández-Fernández, R.; Ruiz-Ripa, L.; Gómez, P.; Zarazaga, M.; Torres, C. Human mecC-Carrying MRSA: Clinical Implications and Risk Factors. Microorganisms 2020, 8, 1615. [Google Scholar] [CrossRef]
- Grua, E.M.; Pérez-Fuentes, S.; Muñoz-Silvestre, A.; Viana, D.; Fernández-Ros, A.B.; Sanz-Tejero, C.; Corpa, J.M.; Selva, L. Characterization of Livestock-Associated Methicillin-Resistant Staphylococcus aureus Isolates Obtained from Commercial Rabbitries Located in the Iberian Peninsula. Front. Microbiol. 2018, 9, 1812. [Google Scholar] [CrossRef] [Green Version]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 29th ed.; CLSI supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2019. [Google Scholar]
- Tyagi, P.; Singh, M.; Kumari, H.; Kumari, A.; Mukhopadhyay, K. Bactericidal activity of curcumin I is associated with damaging of bacterial membrane. PLoS ONE 2015, 10, e0121313. [Google Scholar] [CrossRef] [Green Version]
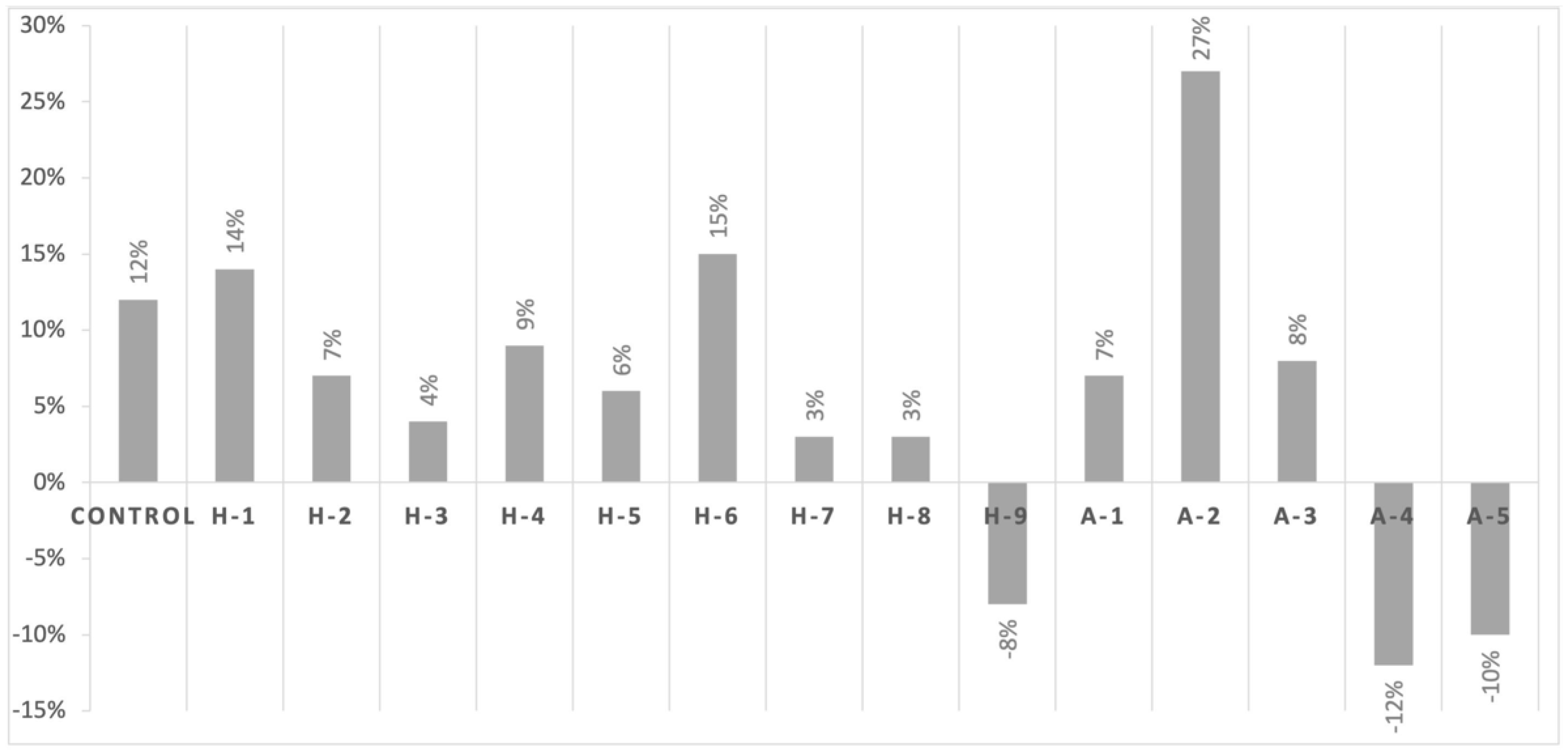

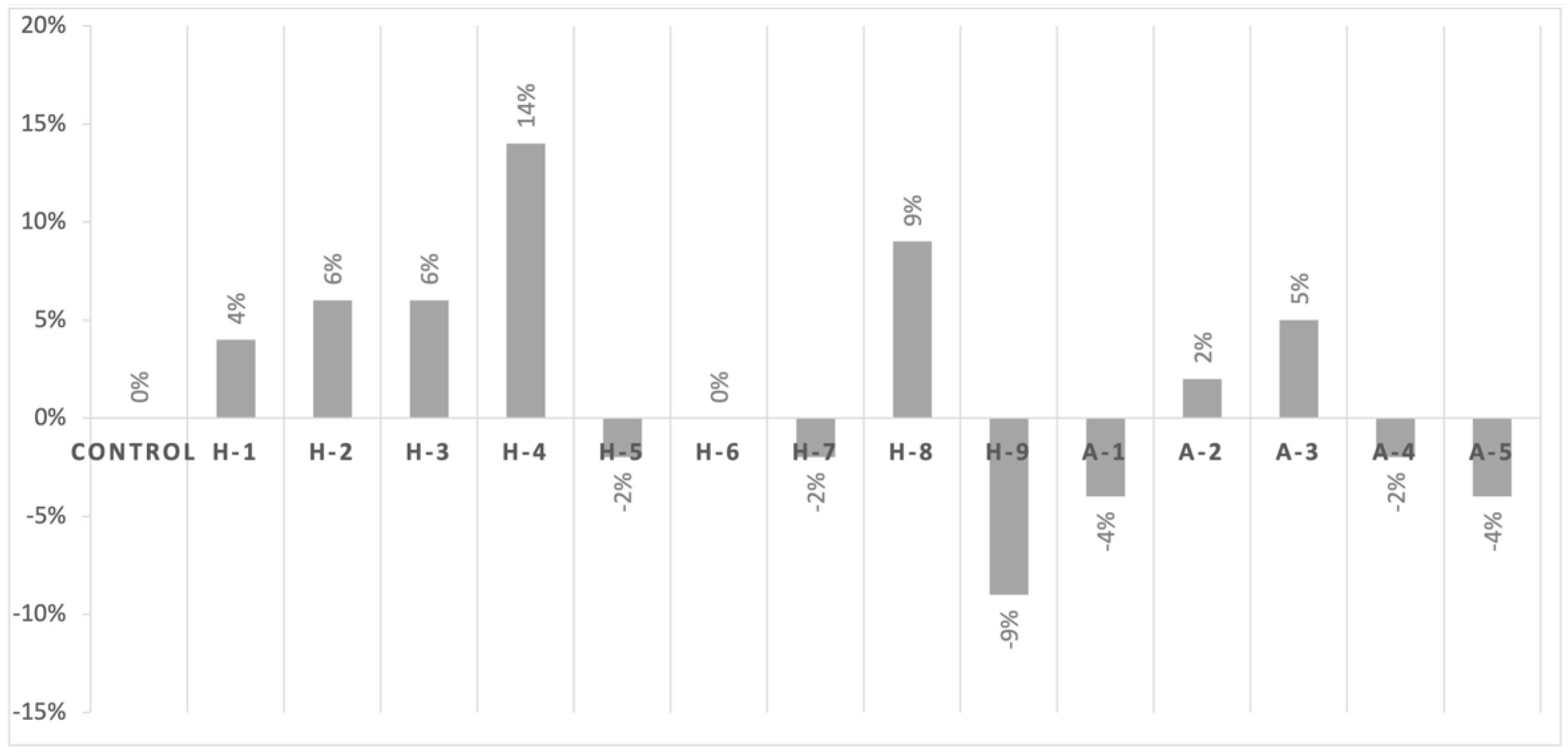
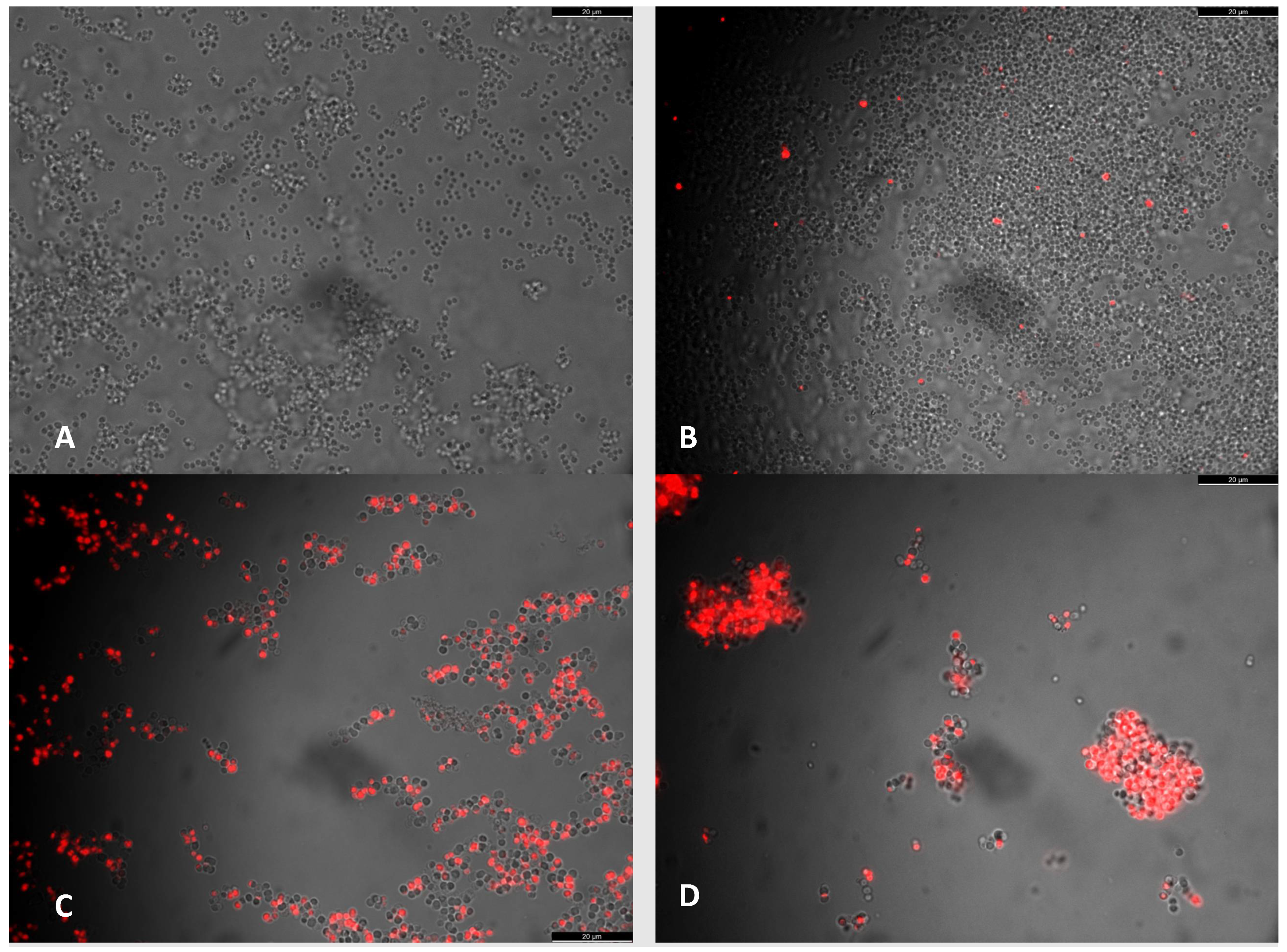
| Strain Name | Source |
|---|---|
| Control | Staphylococcus aureus Culture Collection Type Strain CECT 435 |
| H-1 | MRSA human isolate from La Fe University Hospital nº 24 |
| H-2 | MRSA human isolate from Guadalajara University Hospital nº 792943 |
| H-3 | MRSA human isolate from Guadalajara University Hospital nº 792945 |
| H-4 | MRSA human isolate from Guadalajara University Hospital nº 791426 |
| H-5 | MRSA human isolate from Guadalajara University Hospital nº 792765 |
| H-6 | MRSA human isolate from Guadalajara University Hospital nº 790623 |
| H-7 | MRSA human isolate from Guadalajara University Hospital nº 790473 |
| H-8 | MRSA human isolate from Guadalajara University Hospital nº 792362 |
| H-9 | MRSA human isolate from La Fe University Hospital nº 33 |
| A-1 | MRSA animal clinical isolate 1004 mecA+ genotype |
| A-2 | MRSA animal clinical isolate 1032 mecA+ genotype |
| A-3 | MRSA animal clinical isolate 990 mecA+ genotype |
| A-4 | MRSA animal clinical isolate 987 mecC+ genotype |
| A-5 | MRSA animal clinical isolate 999 mecC+ genotype |
| CFU/mL | BHI | BHI + Icariin | BHI + AmoxyClav | BHI+ AmoxyClav+ Icariin |
|---|---|---|---|---|
| Average of 3 replicates | 5.53 × 108 | 5.94 × 108 | 1.18 × 107 | 8.61 × 106 |
| Standard deviation | 3.87 × 108 | 4.74 × 108 | 7.77 × 106 | 5.85 × 106 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peris, M.C.; Martínez, A.; Ortíz, M.P.; Sheth, C.C.; Veses, V. Icariin in Combination with Amoxycillin-Clavulanate and Ampicillin, but Not Vancomycin, Increases Antibiotic Sensitivity and Growth Inhibition against Methicillin-Resistant Staphylococcus aureus. Antibiotics 2022, 11, 233. https://doi.org/10.3390/antibiotics11020233
Peris MC, Martínez A, Ortíz MP, Sheth CC, Veses V. Icariin in Combination with Amoxycillin-Clavulanate and Ampicillin, but Not Vancomycin, Increases Antibiotic Sensitivity and Growth Inhibition against Methicillin-Resistant Staphylococcus aureus. Antibiotics. 2022; 11(2):233. https://doi.org/10.3390/antibiotics11020233
Chicago/Turabian StylePeris, María Cardells, Alba Martínez, Marina Pascual Ortíz, Chirag C. Sheth, and Veronica Veses. 2022. "Icariin in Combination with Amoxycillin-Clavulanate and Ampicillin, but Not Vancomycin, Increases Antibiotic Sensitivity and Growth Inhibition against Methicillin-Resistant Staphylococcus aureus" Antibiotics 11, no. 2: 233. https://doi.org/10.3390/antibiotics11020233
APA StylePeris, M. C., Martínez, A., Ortíz, M. P., Sheth, C. C., & Veses, V. (2022). Icariin in Combination with Amoxycillin-Clavulanate and Ampicillin, but Not Vancomycin, Increases Antibiotic Sensitivity and Growth Inhibition against Methicillin-Resistant Staphylococcus aureus. Antibiotics, 11(2), 233. https://doi.org/10.3390/antibiotics11020233






