E-Learning versus Face-to-Face Methodology for Learning Antimicrobial Resistance and Prescription Practice in a Tertiary Hospital of a Middle-Income Country
Abstract
1. Introduction
2. Results
2.1. Assessment of Learning
2.2. Assessment of Antibiotic Appropriateness
2.3. Satisfaction Evaluation
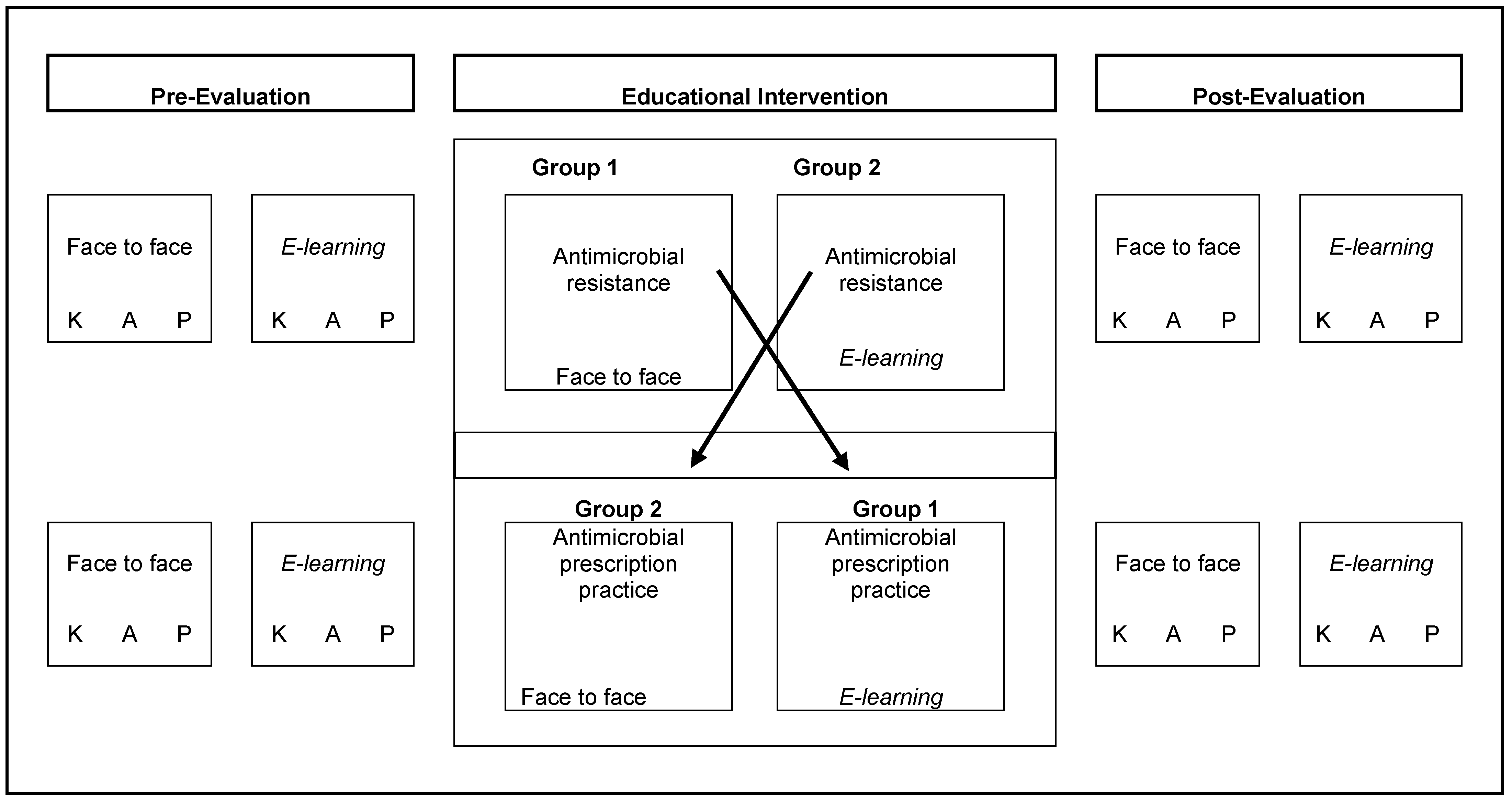
3. Methods
3.1. Study Design and Population
3.2. Data Description
3.3. Assessment of Methodology of Learning
3.4. Evaluation of Antibiotic Appropriateness
- Indication to start treatment: decision to give antibiotic treatment when the patient had an infection that justified it;
- Indication not to treat: clinical and laboratory justification of the decision not to treat a patient who did not present with an infection;
- Coverage of the microorganism: the antibiotic treatment covered the microorganism suspected by epidemiology in the empirical treatment or one identified by culture in the case of targeted treatment;
- Antibiotic spectrum: the used spectrum was sufficient for the suspected or reported microorganism;
- Greater spectrum than necessary: the antimicrobial spectrum was greater than necessary for the suspected or reported microorganism;
- Dose and duration of treatment: whether the dose and duration of the treatment administered were adequate or not;
- Rational use of antibiotics corresponds to the sum of the indication to give treatment and not to give it in case of clinical and laboratory justification, adequate coverage of the microorganism, the antibiotic spectrum used and the dose and duration of treatment.
3.5. Data Analysis and Sample Size
3.6. IRB Approval
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DDD | Defined daily dose |
| AMR | Antimicrobial resistance |
| AMS | Antimicrobial stewardship |
| APP | Antimicrobial prescription practice |
| CPD | Continuous professional development |
| ICU | Intensive care unit |
| IMW | Internal medicine ward |
| PAHO | Pan American Health Organization |
| UTI | Urinary tract infection |
| WHO | World Health Organization |
References
- World Health Organization. Recommendations for Implementing Antimicrobial Stewardship Programs in Latin America and the Caribbean: Manual for Public Health Decision-Makers; PAHO, FIU, Eds.; Pan American Health Organization and Florida International University: Washington, DC, USA, 2018.
- OMS. Plan de acción Mundial Sobre la Resistencia a los Antimicrobianos Proyecto de Resolución con Enmiendas Derivadas de las Consultas Oficiosas; Organización Mundial de la Salud: Geneva, Switzerland, 2015; 4p.
- Bbosa, G.S.; Wong, G.; Kyegombe, D.B.; Ogwal-Okeng, J. Effects of intervention measures on irrational antibiotics/antibacterial drug use in developing countries: A systematic review. Health 2014, 6, 171–187. [Google Scholar] [CrossRef]
- Davey, P.; Marwick, C.A.; Scott, C.L.; Charani, E.; Mcneil, K.; Brown, E.; Gould, I.M.; Ramsay, C.R.; Michie, S. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst. Rev. 2017, 2017, CD003543. [Google Scholar] [CrossRef] [PubMed]
- López-Medrano, F.; San Juan, R.; Serrano, O.; Chaves, F.; Lumbreras, C.; Lizasoaín, M.; Herreros de Tejada, A.; Aguado, J.M. PACTA: Efecto de un programa no impositivo de control y asesoramiento del tratamiento antibiótico sobre la disminución de los costes y el descenso de ciertas infecciones nosocomiales. Enferm. Infecc. Microbiol. Clin. 2005, 23, 186–190. [Google Scholar] [CrossRef]
- Sulis, G.; Adam, P.; Nafade, V.; Gore, G.; Daniels, B.; Daftary, A.; Das, J.; Gandra, S.; Pai, M. Antibiotic prescription practices in primary care in low- And middle-income countries: A systematic review and meta-analysis. PLoS Med. 2020, 17, e1003139. [Google Scholar] [CrossRef]
- Organización Mundial de la Salud. Programas de Optimización de los Antimicrobianos en Instituciones Sanitarias de los Paises de Ingresos bajos y Medianos. Manual Práctico de la OMS [Internet]; World Health Organization: Geneva, Switzerland, 2020; 51p.
- Alothman, A.; Algwizani, A.; Alsulaiman, M.; Alalwan, A.; Binsalih, S.; Bosaeed, M. Knowledge and Attitude of Physicians Toward Prescribing Antibiotics and the Risk of Resistance in Two Reference Hospitals. Infect. Dis. Res. Treat. 2016, 9, 33–38. [Google Scholar] [CrossRef]
- Baadani, A.M.; Baig, K.; Alfahad, W.A.; Aldalbahi, S.; Omrani, A.S. Physicians’ knowledge, perceptions, and attitudes toward antimicrobial prescribing in Riyadh, Saudi Arabia. Saudi Med. J. 2015, 36, 613–619. [Google Scholar] [CrossRef]
- García, C.; Llamocca, L.P.; García, K.; Jiménez, A.; Samalvides, F.; Gotuzzo, E.; Jacobs, J. Knowledge, attitudes and practice survey about antimicrobial resistance and prescribing among physicians in a hospital setting in Lima, Peru. BMC Clin. Pharmacol. 2011, 11, 18. [Google Scholar] [CrossRef]
- Salsgiver, E.; Bernstein, D.; Simon, M.S.; Eiras, D.P.; Greendyke, W.; Kubin, C.J.; Mehta, M.; Nelson, B.; Loo, A.; Ramos, L.G.; et al. Knowledge, attitudes, and practices regarding antimicrobial use and stewardship among prescribers at acute-care hospitals. Infect. Control Hosp. Epidemiol. 2018, 39, 316–322. [Google Scholar] [CrossRef]
- Gros Salvat, B. La evolución del e-learning: Del aula virtual a la red. RIED Rev. Iberoam. Educ. Distancia 2018, 21, 69. [Google Scholar] [CrossRef]
- Eley, C.V.; Young, V.L.; Hayes, C.V.; McNulty, C.A.M. Evaluation of an e-Learning platform for educators to improve education around infection prevention and antibiotics. Technol. Pedagog. Educ. 2019, 28, 485–501. [Google Scholar] [CrossRef]
- Bond, S.E.; Crowther, S.P.; Adhikari, S.; Chubaty, A.J.; Yu, P.; Borchard, J.P.; Boutlis, C.S.; Yeo, W.W.; Miyakis, S. Evaluating the effect of a web-based E-Learning tool for health professional education on clinical vancomycin use: Comparative study. JMIR Med. Educ. 2018, 4, e5. [Google Scholar] [CrossRef] [PubMed]
- Poss-Doering, R.; Kuehn, L.; Kamradt, M.; Glassen, K.; Wensing, M. Applying digital information delivery to convert habits of antibiotic use in primary care in Germany: Mixed-methods study. J. Med. Internet Res. 2020, 22, e18200. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Pereira, N.; Lafferty, N.; Nathwani, D. Educating healthcare professionals in antimicrobial stewardship: Can online-learning solutions help? J. Antimicrob. Chemother. 2015, 70, 3175–3177. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, P.M.; Kable, A.; Levett-Jones, T.; Booth, D. The effectiveness of Internet-based e-learning on clinician behaviour and patient outcomes: A systematic review. Int. J. Nurs. Stud. 2016, 57, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Richmond, H.; Copsey, B.; Hall, A.M.; Davies, D.; Lamb, S.E. A systematic review and meta-analysis of online versus alternative methods for training licensed health care professionals to deliver clinical interventions. BMC Med. Educ. 2017, 17, 227. [Google Scholar] [CrossRef]
- Poss-Doering, R.; Kühn, L.; Kamradt, M.; Stürmlinger, A.; Glassen, K.; Andres, E.; Kaufmann-Kolle, P.; Wambach, V.; Bader, L.; Szecsenyi, J.; et al. Fostering appropriate antibiotic use in a complex intervention: Mixed-methods process evaluation alongside the cluster-randomized trial arena. Antibiotics 2020, 9, 878. [Google Scholar] [CrossRef] [PubMed]
- Sudha, M.J.; Viveka, S.; Rassi, N.; Sharafudeen, S. The impact of educational intervention on knowledge and attitude regarding antibiotic resistance among medical doctors in a tertiary care hospital. Biomed. Pharmacol. J. 2021, 14, 351–361. [Google Scholar]
- World Health Organization. JAC—Antimicrobial Resistance Educational Resource Review: Antimicrobial Stewardship: A Competency-Based Approach [Internet]; WHO: Geneva, Switzerland, 2021; pp. 1–2.
- Organización Panamericana de la Salud. Tratamiento de las Enfermedades Infecciosas 2020–2022; Octava Edition; OPS: Washington, DC, USA, 2022; 396p.
- Nair, M.; Tripathi, S.; Mazumdar, S.; Mahajan, R.; Harshana, A.; Pereira, A.; Jimenez, C.; Halder, D.; Burza, S. Knowledge, attitudes, and practices related to antibiotic use in Paschim Bardhaman District: A survey of healthcare providers in West Bengal, India. PLoS ONE 2019, 14, e0217818. [Google Scholar] [CrossRef]
- Cuevas, C.; Batura, N.; Wulandari, L.P.L.; Khan, M.; Wiseman, V. Improving antibiotic use through behaviour change: A systematic review of interventions evaluated in low- And middle-income countries. Health Policy Plan. 2021, 36, 754–773. [Google Scholar] [CrossRef]
- Singh, S.; Charani, E.; Devi, S.; Sharma, A.; Edathadathil, F.; Kumar, A.; Warrier, A.; Shareek, P.S.; Jaykrishnan, A.V.; Ellangovan, K. A road-map for addressing antimicrobial resistance in low- and middle-income countries: Lessons learnt from the public private participation and co-designed antimicrobial stewardship programme in the State of Kerala, India. Antimicrob. Resist. Infect. Control 2021, 10, 32. [Google Scholar] [CrossRef]
- Ministerio de Salud Pública (MSP). Plan Nacional para la Prevención y Control de la Resistencia Antimicrobiana 2019–2023; Ministerio de Salud Pública: Quito, Ecuador, 2019; p. 34.
- Romo-Castillo, H. Uso de Antibióticos en un Hospital Ecuatoriano de Tercer Nivel, Ocho Años de Seguimiento. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2020. [Google Scholar]
- McMahon, C.J.; Tretter, J.T.; Faulkner, T.; Krishna Kumar, R.; Redington, A.N.; Windram, J.D. Are e-learning Webinars the future of medical education? An exploratory study of a disruptive innovation in the COVID-19 era. Cardiol. Young 2021, 31, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.R.; Westwood, D.; Daneman, N.; MacFadden, D.R. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Peng, W.; Zhang, F.; Hu, R.; Li, Y.; Yan, W. The effectiveness of blended learning in health professions: Systematic review and meta-analysis. J. Med. Internet Res. 2016, 18, e4807. [Google Scholar] [CrossRef] [PubMed]
- George, P.P.; Zhabenko, O.; Myint Kyaw, B.; Antoniou, P.; Posadzki, P.; Saxena, N.; Semwal, M.; Car, L.T.; Zary, N.; Lockwood, C.; et al. Online digital education for postregistration training of medical doctors: Systematic review by the digital health education collaboration. J. Med. Internet Res. 2019, 21, e13269. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, G.; Cossette, S.; Maheu-Cadotte, M.A.; Mailhot, T.; Deschênes, M.F.; Mathieu-Dupuis, G.; Côté1, J.; Gagnon, M.; Dubé, V. Efficacy of adaptive e-learning for health professionals and students: A systematic review and meta-analysis. BMJ Open 2019, 9, e025252. [Google Scholar] [CrossRef] [PubMed]
- Öcek, Z.; Sahin, H.; Baksi, G.; Apaydin, S. Development of a rational antibiotic usage course for dentists. Eur. J. Dent. Educ. 2008, 12, 41–47. [Google Scholar] [CrossRef]
- Chaves, J.; Lorca-Marín, A.A.; Delgado-Algarra, E.J. Methodology of specialist physicians training: From traditional to E-learning. Int. J. Environ. Res. Public Health 2020, 17, 7681. [Google Scholar] [CrossRef]
- Swamy, A.; Sood, R.; Kapil, A.; Vikram, N.K.; Ranjan, P.; Jadon, R.S.; Soneja, M.; Sreenivas, V. Antibiotic stewardship initiative in a Medicine unit of a tertiary care teaching hospital in India: A pilot stud. Indian J. Med. Res. 2019, 150, 175–185. [Google Scholar]
- Johnston, S.; Coyer, F.M.; Nash, R. Kirkpatrick’s evaluation of simulation and debriefing in health care education: A systematic review. J. Nurs. Educ. 2018, 57, 393–398. [Google Scholar] [CrossRef]
- Savul, S.; Ikram, A.; Khan, M.A.; Khan, M.A. Evaluation of Infection Prevention and Control Training Workshops Using Kirkpatrick’S Model. Int. J. Infect. Dis. 2021, 112, 76–80. [Google Scholar] [CrossRef]
| Module: Antimicrobial Resistance | Module: Antimicrobial Prescription Practice | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Questions | Face to Face | E-Learning | Questions | Face to Face | E-Learning | |||||||||||
| (Min–Max) | Median | (P25–P75) | Median | (P25–P75) | p * | (Min–Max) | Median | (P25–P75) | Median | (P25–P75) | p * | |||||
| Knowledge | 6 | 6 | ||||||||||||||
| Before training | (1–6) | 5 | (5–6) | 5 | (5–6) | 0.602 | (1–6) | 4 | (4–5) | 4 | (4–5) | 0.63 | ||||
| After training | (2–6) | 6 | (5–6) | 6 | (5–6) | 0.884 | (2–6) | 5 | (4–5) | 4 | (4–5) | 1.00 | ||||
| p ** | 0.097 | 0.259 | 0.205 | 0.603 | ||||||||||||
| Attitudes | 4 | 9 | ||||||||||||||
| Before training | (1–4) | 4 | (3–4) | 3 | (3–4) | 0.017 | (2–8) | 6 | (5–7) | 6 | (5–6) | 0.89 | ||||
| After training | (1–4) | 4 | (4–4) | 4 | (3–4) | 0.345 | (3–9) | 7 | (5–8) | 7 | (6–7) | 0.87 | ||||
| p ** | 0.94 | 0.005 | 0.001 | <0.001 | ||||||||||||
| Referred practices | 5 | 7 | ||||||||||||||
| Before training | (0–5) | 3 | (2–4) | 3 | (2–4) | 0.66 | (0–7) | 5 | (4–5) | 5 | (4–5) | 0.68 | ||||
| After training | (1–5) | 3 | (3–4) | 4 | (3–4) | 0.21 | (2–7) | 5 | (4–5) | 5 | (5–5) | 0.572 | ||||
| p ** | 0.59 | <0.001 | 0.521 | 0.037 | ||||||||||||
| Bacteremia | Pneumonia | Urinary Infection | Skin and Soft Tissue Infection | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | |||||||||||||||||
| n = 66 | n = 65 | n = 32 | n = 40 | n = 16 | n = 21 | n = 8 | n = 9 | |||||||||||||||||
| n | % | n | % | Chi2 (p) | OR (95% CI) | n | % | n | % | Chi2 (p) | OR (95% CI) | n | % | n | % | Chi2 (p) | OR (95% CI) | n | % | n | % | Chi2 (p) | OR (95% CI) | |
| Indication to treat | ||||||||||||||||||||||||
| No | 5 | 7.6 | 9 | 13.8 | 1.349 (0.245) | 1.96 (0.61–6.20) | 5 | 15.6 | 16 | 40 | 5.113 (0.024) | 0.27 (0.08–0.87) | 6 | 37.5 | 5 | 23.8 | 0.815 (0.367) | 1.92 (0.46–7.98) | 1 | 12.5 | 1 | 11.1 | 0.008 (0.929) | 1.14 (0.05–21.87) |
| Yes | 61 | 92.4 | 56 | 86.2 | 27 | 84.4 | 24 | 60 | 10 | 62.5 | 16 | 76.2 | 7 | 87.5 | 8 | 88.9 | ||||||||
| Coverage of the microorganism | ||||||||||||||||||||||||
| No | 17 | 25.8 | 2 | 3.1 | 13.999 (0.001) | 10.43 (2.28–47.61) | 4 | 12.5 | 1 | 2.5 | 6.756 (0.034) | 4.00 (0.41–38.57) | 1 | 6.3 | 1 | 4.8 | 0.932 (0.627) | 1.66 (0.09–30.06) | 0 | 0 | 0 | 0 | 1.195 (0.274) | |
| Yes | 44 | 66.7 | 54 | 83.1 | 23 | 71.9 | 23 | 57.5 | 9 | 56.3 | 15 | 71.4 | 7 | 87.5 | 9 | 100 | ||||||||
| NV | 5 | 7.6 | 9 | 13.8 | 5 | 15,56 | 16 | 40 | 6 | 37,5 | 5 | 23,8 | 1 | 12.5 | 0 | 0 | ||||||||
| Broader spectrum than necessary | ||||||||||||||||||||||||
| No | 19 | 28.8 | 9 | 13.8 | 4.988 (0.083) | 2.36 (0.96–5.78) | 11 | 34,4 | 18 | 45 | 11.247 (0.004) | 0.22 (0.06–0.76) | 4 | 25 | 11 | 52,4 | 2.824 (0.244) | 0.30 (0.05–1.57) | 3 | 37.5 | 8 | 88.9 | 5.031 (0.081) | 0.09 (0.007–1.21) |
| Yes | 42 | 63.6 | 47 | 72.3 | 16 | 50 | 6 | 15 | 6 | 37.5 | 5 | 23.8 | 4 | 50 | 1 | 11,1 | ||||||||
| NV | 5 | 7.6 | 9 | 13.8 | 5 | 15.6 | 16 | 40 | 6 | 37.5 | 5 | 23.8 | 1 | 12.5 | 0 | 0 | ||||||||
| Dosage and duration | ||||||||||||||||||||||||
| Inadequate | 27 | 40.9 | 6 | 9.2 | 17.548 (<0.001) | 6.61 (2.46–17.74) | 6 | 18.8 | 0 | 0 | 11.211 (0.004) | ----- | 2 | 12.5 | 0 | 0 | 4.158 (0.125) | --- | 1 | 12,5 | 0 | 0 | 2.550 (0.279) | ---- |
| Appropriate | 34 | 51.5 | 50 | 76.9 | 21 | 65.6 | 24 | 60 | 8 | 50 | 16 | 76.2 | 6 | 75 | 9 | 100 | ||||||||
| NV | 5 | 7.6 | 9 | 13.8 | 5 | 15.6 | 16 | 40 | 6 | 37.5 | 5 | 23.8 | 1 | 12,5 | 0 | 0 | ||||||||
| Rational use of antibiotics | ||||||||||||||||||||||||
| Inadequate | 30 | 45.5 | 5 | 7.7 | 23.851 (<0.001) | 10.0 (3.55–28.09) | 12 | 37.5 | 0 | 0 | 18.000 (<0.001) | ----- | 3 | 18.8 | 2 | 9.5 | 0.661 (0.416) | 2.19 (0.32–15.0) | 0 | 0 | 0 | 0 | — | ---- |
| Appropriate | 36 | 54.5 | 60 | 92.3 | 20 | 62.5 | 40 | 100 | 13 | 81.1 | 19 | 90.5 | 8 | 100 | 9 | 100 | ||||||||
| NV | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||
| NV = not valuable. | ||||||||||||||||||||||||
| Source: database | ||||||||||||||||||||||||
| Elaborated by the authors | ||||||||||||||||||||||||
| Bacteremia | Pneumonia | Urinary Infection | Skin and Soft Tissue Infection | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | |||||||||||||||||
| n = 66 | n = 65 | n = 32 | n = 40 | n = 16 | n = 21 | n = 8 | n = 9 | |||||||||||||||||
| n | % | n | % | Chi2 (p) | OR (95% CI) | n | % | n | % | Chi2 (p) | OR (95% CI) | n | % | n | % | Chi2 (p) | OR (95% CI) | n | % | n | % | Chi2 (p) | OR (95% CI) | |
| Indication to treat | ||||||||||||||||||||||||
| No | 12 | 18.2 | 21 | 32.3 | 3.468 (0.063) | 0.46 (0.20–1.05) | 8 | 25 | 7 | 17.5 | 0.0606 (0.436) | 1.57 (0.50–4.92) | 6 | 37.5 | 10 | 47.6 | 0.379 (0.538) | 0.66 (0.17–2.48) | 7 | 87.5 | 4 | 44.4 | 3.438 (0.064) | 8.75 (0.73–103.82) |
| Yes | 54 | 81.8 | 44 | 67.7 | 24 | 75 | 33 | 82.5 | 10 | 62.5 | 11 | 52.4 | 1 | 12.5 | 5 | 55.6 | ||||||||
| Coverage of the microorganism | ||||||||||||||||||||||||
| No | 7 | 10.6 | 2 | 3.1 | 3.894 (0.143) | 3.22 (0.62–16.57) | 5 | 15.6 | 0 | 0 | 8.046 (0.018) | ---- | 0 | 0 | 0 | 0 | 0.379 (0.538) | ---- | 0 | 0 | 0 | 0 | 3.438 (0.064) | ----- |
| Yes | 38 | 57.6 | 35 | 53.8 | 19 | 59.4 | 33 | 82.5 | 10 | 62.5 | 11 | 52.4 | 1 | 12.5 | 5 | 55.6 | ||||||||
| NV | 21 | 31.8 | 28 | 43.1 | 8 | 25 | 7 | 17.5 | 6 | 37.5 | 10 | 47.6 | 7 | 87.5 | 4 | 44.4 | ||||||||
| Broader spectrum than Necessary | ||||||||||||||||||||||||
| No | 8 | 12.1 | 6 | 9.2 | 1.808 (0.405) | 1.11 (0.34–3.56) | 8 | 25 | 23 | 57.5 | 7.918 (0.019) | 0.21 (0.07–0.67) | 3 | 18.8 | 9 | 42.9 | 6.216 (0.045) | 0.09 (0.01–0.73) | 0 | 0 | 4 | 44.4 | 4.776 (0.092) | ----- |
| Yes | 37 | 56.1 | 31 | 47.7 | 16 | 50 | 10 | 25 | 7 | 43.8 | 2 | 9.5 | 1 | 12.5 | 1 | 11.1 | ||||||||
| NV | 21 | 31.8 | 28 | 43.1 | 8 | 25 | 7 | 17.5 | 6 | 37.5 | 10 | 47.6 | 7 | 87.5 | 4 | 44.4 | ||||||||
| Dosage and duration | ||||||||||||||||||||||||
| Inadequate | 18 | 27.3 | 7 | 10.8 | 5.991 (0.050) | 2.85 (1.03–7.89) | 3 | 9.4 | 0 | 0 | 4.905 (0.086) | --- | 0 | 0 | 0 | 0 | 0.379 (0.538) | --- | 0 | 0 | 0 | 0 | 3.438 (0.064) | ----- |
| Appropriate | 27 | 40.9 | 30 | 46.2 | 21 | 65.6 | 33 | 82.5 | 10 | 62.5 | 11 | 52.4 | 1 | 12.5 | 5 | 55.6 | ||||||||
| NV | 21 | 31.8 | 28 | 43.1 | 8 | 25 | 7 | 17.5 | 6 | 37.5 | 10 | 47.6 | 7 | 87.5 | 4 | 44.4 | ||||||||
| Rational use of antibiotics | ||||||||||||||||||||||||
| Inadequate | 23 | 34.8 | 6 | 9.2 | 13.976 (0.001) | 5.86 (2.16–15.89) | 16 | 50 | 2 | 5 | 24.891 (<0.001) | 26.18 (5.19–131.99) | 3 | 18.8 | 0 | 0 | 4.285 (0.038) | ---- | 1 | 12.5 | 0 | 0 | 2.015 (0.365) | ----- |
| Appropriate | 34 | 51.5 | 52 | 80 | 11 | 34.4 | 36 | 90 | 13 | 81.3 | 21 | 100 | 7 | 87.5 | 8 | 88.9 | ||||||||
| NV | 9 | 13.6 | 7 | 10.8 | 5 | 15.6 | 2 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 11.1 | ||||||||
| Empirical Antibiotic Treatment | Targeted Antibiotic Treatment | |||||
|---|---|---|---|---|---|---|
| Before | After | Student’s T (p) | Before | After | Student’s T (p) | |
| Bacteremia | ||||||
| Mean (SD) | 2.07 (1.16) | 1.81 (1.18) | 0.207 | 2.19 (1.50) | 1.53 (1.37) | 0.01 |
| Pneumonia | ||||||
| Mean (SD) | 1.87 (1.09) | 1.05 (1.13) | 0.003 | 2.15 (0.98) | 2.05 (1.18) | 0.618 |
| Urinary Infections | ||||||
| Mean (SD) | 1.06 (0.99) | 1.28 (1.00) | 0.507 | 1.18 (1.27) | 1.00 (1.14) | 0.641 |
| Skin and Soft Tissue Infection | ||||||
| Mean (SD) | 1.75 (0.88) | 1.77 (0.66) | 0.942 | 0.25 (0.70) | 1.22 (1.20) | 0.064 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armas Freire, P.I.; Gaspar, G.G.; Zurita, J.; Salazar, G.; Velez, J.W.; Bollela, V.R. E-Learning versus Face-to-Face Methodology for Learning Antimicrobial Resistance and Prescription Practice in a Tertiary Hospital of a Middle-Income Country. Antibiotics 2022, 11, 1829. https://doi.org/10.3390/antibiotics11121829
Armas Freire PI, Gaspar GG, Zurita J, Salazar G, Velez JW, Bollela VR. E-Learning versus Face-to-Face Methodology for Learning Antimicrobial Resistance and Prescription Practice in a Tertiary Hospital of a Middle-Income Country. Antibiotics. 2022; 11(12):1829. https://doi.org/10.3390/antibiotics11121829
Chicago/Turabian StyleArmas Freire, Paulina Isabel, Gilberto Gambero Gaspar, Jeannete Zurita, Grace Salazar, Jorge Washington Velez, and Valdes Roberto Bollela. 2022. "E-Learning versus Face-to-Face Methodology for Learning Antimicrobial Resistance and Prescription Practice in a Tertiary Hospital of a Middle-Income Country" Antibiotics 11, no. 12: 1829. https://doi.org/10.3390/antibiotics11121829
APA StyleArmas Freire, P. I., Gaspar, G. G., Zurita, J., Salazar, G., Velez, J. W., & Bollela, V. R. (2022). E-Learning versus Face-to-Face Methodology for Learning Antimicrobial Resistance and Prescription Practice in a Tertiary Hospital of a Middle-Income Country. Antibiotics, 11(12), 1829. https://doi.org/10.3390/antibiotics11121829






