Ongoing Efforts to Improve Antimicrobial Utilization in Hospitals among African Countries and Implications for the Future
Abstract
1. Introduction
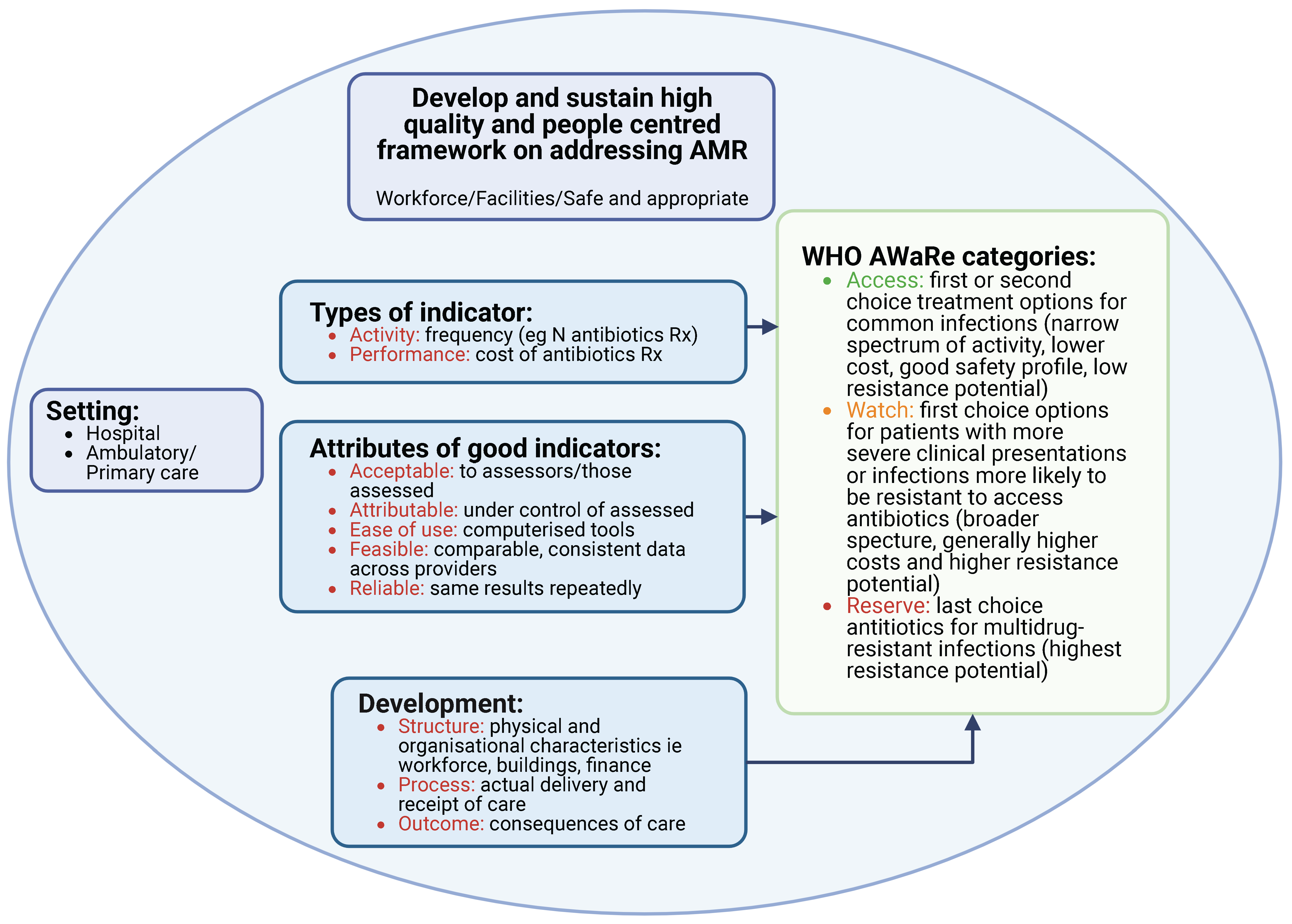
2. Results
2.1. Current Antimicrobial Utilization Patterns in Hospitals across Africa
2.2. Antibiotic Prophylaxis to Prevent Surgical Site Infections
| Country | Year (and Reference) | Findings |
|---|---|---|
| Low Income * | ||
| Burkina Faso | 2019 [154] |
|
| Ethiopia | 2018 [155] |
|
| 2018 [156] |
| |
| 2022 [157] |
| |
| Rwanda | 2019 [158] |
|
| Tanzania | 2020 [159] |
|
| 2020 [160] |
| |
| 2021 [81] |
| |
| Uganda | 2020 [161] |
|
| 2021 [81] |
| |
| 2022 [13] |
| |
| Low-Middle Income * | ||
| Congo | 2020 [162] |
|
| Ghana | 2019 [163] |
|
| 2020 [76] |
| |
| 2021 [81] |
| |
| 2021 [164] |
| |
| 2022 [141] |
| |
| Kenya | 2017 [165] |
|
| 2018 [78] |
| |
| 2018 [166] |
| |
| 2019 [79] |
| |
| Nigeria | 2016 [167] |
|
| 2017 [168] |
| |
| 2020 [82,169] |
| |
| 2020 [114] |
| |
| 2021 [16] |
| |
| 2022 [14] |
| |
| Zambia | 2021 [81] |
|
| Upper-Middle Income * | ||
| Botswana | 2018 [170] |
|
| 2019 [19] |
| |
| South Africa | 2021 [171] |
|
| 2022 [61] |
| |
2.3. Prescribing Indicators Currently Being Used in Hospitals to Improve Antimicrobial Prescribing
| Indicator | References |
|---|---|
| Activity/Performance Indicators | |
| % of in-patients prescribed antibiotics in a single PPS/ over specific time periods, e.g., successive waves of COVID-19 | [19,61,112,172] |
| % of antibiotics prescribed by defined daily doses (DDDs), e.g., DDDs/1000 patient-days in a PPS or over a specified time | [171,173,174,175] |
| % of a course of antibiotics prescribed (duration) in accordance with agreed guidance/ Days of antibiotic therapy per 1000 patient-days | [166,176] |
| % of antibiotics administered to in-patients within the first hour of prescribing within a designated period of time | [177] |
| % of patients where the indication for prescribing and/ or stop and review dates are included in patients’ notes | [15,19,76,81,114,168,169,178,179] |
| % oral vs. IV antibiotics (including as part of de-escalation policies) | [15,76,82,114,166,168,171,178,179,180,181] |
| % of missed doses documented in patients’ notes, e.g., as part of a PPS | [19,148] |
| % of antibiotics prescribed by their international non-proprietary name, e.g., as part of a PPS | [182,183] |
| % compliance to agreed process measures surrounding AMS | [184] |
| % of patients prescribed antibiotics within the country’s essential medicine list over an agreed period of time | [61,171,180,182,183] |
| Process quality indicators | |
| % of in-patients prescribed antibiotics in adherence to agreed guidelines within a specified time period/part of a PPS | [81,112,134,168,184,185,186,187,188,189,190,191] |
| % of patients prescribed a course of antibiotics in accordance with guideline duration recommendations within a specified time period/ part of a PPS | [166,176] |
| % of patients where cultures are taken and sent for analysis to guide antibiotic prescribing/ targeted therapy within a specified time period/ part of a PPS | [76,114,169,192] |
| % of antibiotics prescribed based on the AWaRe classification/% reduction in the prescribing of target antibiotics, e.g., ‘Watch’ cephalosporins to potential ‘Access’ antibiotics (current target is 60% of current prescribing should be ‘Access’ antibiotics) | [60,76,81,193] |
| % of patients prescribed antibiotics post-operatively to prevent SSIs/% appropriate use of antibiotics to prevent SSIs during an agreed time period | [194,195] |
| % of key antibiotics available for prescribing/ Whether there are agreed therapeutic interchange policies in the hospital when there are likely to be shortages of standard antibiotics for the condition (over a specific time period) | [183,196] |
| % of all admitted patients with pneumonia to the hospital correctly classified and treated to agreed guidelines (over a specified time period) | [187,190] |
| Outcome Indicators | |
| % SSIs following operations (over an agreed time period) | [160,194,197] |
| % Mortality rates (post-intervention versus pre-intervention) following changes in antimicrobial prescribing, e.g., reducing extensive antimicrobial prescribing post-surgery for SAP or reducing extensive prescribing of ‘Watch’ antibiotics | [175,176,193] |
2.4. Antimicrobial Stewardship Programs
- Has your hospital management formally identified AMS as a priority objective and included it as a key performance indicator?
- Does your hospital have a formalized structure and group responsible for AMS activities including researching and promoting appropriate antibiotic use as part of agreed ASPs?
- Is this currently a multidisciplinary AMS group available in your hospital to implement agreed ASPs, and does this group include a designated leader?
- Is there access to HCPs in infection management and stewardship in the hospital willing to be part of AMS teams?
- Does your hospital currently offer educational resources to support training of HCPs regarding antimicrobial prescribing and its monitoring to improve future care?
- Is there dedicated and sufficient budget to support AMS activities
- Do you have access to laboratory/imaging services to support improved antibiotic use and away from untargeted and unnecessary prescribing, and are the results available in a timely manner to support diagnosis and appropriate antibiotic prescribing?
- Does your ASP currently monitor compliance with one or more agreed interventions, e.g., improved compliance to national or local guidelines, and report back the findings to improve future care including any changes in the quality/ appropriateness of antimicrobial prescribing in agreed areas?
- Has your hospital conducted a PPS in the past year and used the findings to improve future antimicrobial prescribing?
- Does your hospital have available and up-to-date recommendations for infection management, and are these readily available to prescribers?
- Does your hospital currently have any published AMS protocols such as a restricted antimicrobial list especially surrounding ‘Watch’ and ‘Reserve’ antibiotics and IV to oral switching policies
- Does your hospital currently have any published Infection Prevention and Control protocols, and are these regularly monitored, e.g., surrounding hand hygiene protocols?
| Author, Country and Year | Intervention and Aim | Impact of the Intervention |
|---|---|---|
| Low Income * | ||
| Gebretekle et al., Ethiopia, 2020 [176] |
|
|
| Alabi et al., Liberia, 2022 [134] |
| Improvements were seen in all QIs:
|
| Lester et al., Malawi, 2020 [193] |
|
|
| Suliman et al., Sudan, 2020 [188] |
|
|
| Gentilotti et al., Tanzania, 2020 [160] |
|
|
| Ashiru-Oredope et al., 2022 [135] |
|
|
| Ngonzi et al., Uganda, 2021 [197] |
|
|
| Low-Middle Income * | ||
| Aitken et al., Kenya, 2013 [152] |
|
|
| Amdany et al., Kenya, 2014 [181] |
|
|
| Ntumba et al., Kenya, 2015 [194] |
|
|
| Ayieko et al., Kenya, 2019 [187] |
|
|
| Allegranzi et al., Kenya, Uganda, Zambia, and Zimbabwe, 2018 [195] |
|
|
| Abubakar et al., Nigeria, 2019 [200] |
|
|
| Upper-Middle Income * | ||
| Messina et al., South Africa, 2015 [177] |
|
|
| Brink et al., South Africa, 2016 [174] |
|
|
| Boyles et al., South Africa, 2017 [175] |
|
|
| Brink et al., South Africa, 2017 [189] |
|
|
| Junaid et al., South Africa, 2018 [192] |
|
|
| van den Bergh et al., South Africa, 2020 [184] |
|
|
| Bashar et al., South Africa, 2021 [173] |
|
|
2.5. Suggested Activities to Improve Future Antimicrobial Prescribing in Hospitals
3. Discussion
4. Materials and Methods
4.1. Antimicrobial Utilization Patterns in Hospitals across Africa
4.2. Antibiotic Prophylaxis to Prevent Surgical Site Infections
4.3. Prescribing Indicators
4.4. Antimicrobial Stewardship Programs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hofer, U. The cost of antimicrobial resistance. Nat. Rev. Microbiol. 2019, 17, 3. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Michele Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Founou, R.C.; Founou, L.L.; Essack, S.Y. Clinical and economic impact of antibiotic resistance in developing countries: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0189621. [Google Scholar] [CrossRef] [PubMed]
- Nkengasong, J.N.; Tessema, S.K. Africa Needs a New Public Health Order to Tackle Infectious Disease Threats. Cell 2020, 183, 296–300. [Google Scholar] [CrossRef]
- Bell, D.; Schultz Hansen, K. Relative Burdens of the COVID-19, Malaria, Tuberculosis, and HIV/AIDS Epidemics in Sub-Saharan Africa. Am. J. Trop. Med. Hyg. 2021, 105, 1510–1515. [Google Scholar] [CrossRef]
- Dwyer-Lindgren, L.; Cork, M.A.; Sligar, A.; Steuben, K.M.; Wilson, K.F.; Provost, N.R.; Mayala, B.K.; VanderHeide, J.D.; Collison, M.L.; Hall, J.B.; et al. Mapping HIV prevalence in sub-Saharan Africa between 2000 and 2017. Nature 2019, 570, 189–193. [Google Scholar] [CrossRef]
- Williams, P.C.M.; Isaacs, D.; Berkley, J.A. Antimicrobial resistance among children in sub-Saharan Africa. Lancet Infect. Dis. 2018, 18, e33–e44. [Google Scholar] [CrossRef]
- Godman, B.; Egwuenu, A.; Haque, M.; Malande, O.; Schellack, N.; Kumar, S.; Saleem, Z.; Sneddon, J.; Hoxha, I.; Islam, S.; et al. Strategies to Improve Antimicrobial Utilization with a Special Focus on Developing Countries. Life 2021, 11, 528. [Google Scholar] [CrossRef]
- Prevention of Opportunistic Infections in HIV/AIDS. In StatPearls; StatPearls Publishing Copyright © 2022; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022.
- Belachew, S.A.; Hall, L.; Selvey, L.A. Non-prescription dispensing of antibiotic agents among community drug retail outlets in Sub-Saharan African countries: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control 2021, 10, 13. [Google Scholar] [CrossRef]
- Godman, B.; Haque, M.; McKimm, J.; Abu Bakar, M.; Sneddon, J.; Wale, J.; Campbell, S.; Martin, A.P.; Hoxha, I.; Abilova, V.; et al. Ongoing strategies to improve the management of upper respiratory tract infections and reduce inappropriate antibiotic use particularly among lower and middle-income countries: Findings and implications for the future. Curr. Med. Res. Opin. 2020, 36, 301–327. [Google Scholar] [CrossRef]
- Kiggundu, R.; Wittenauer, R.; Waswa, J.; Nakambale, H.N.; Kitutu, F.E.; Murungi, M.; Okuna, N.; Morries, S.; Lawry, L.L.; Joshi, M.P.; et al. Point Prevalence Survey of Antibiotic Use across 13 Hospitals in Uganda. Antibiotics 2022, 11, 199. [Google Scholar] [CrossRef]
- Ogunleye, O.O.; Oyawole, M.R.; Odunuga, P.T.; Kalejaye, F.; Yinka-Ogunleye, A.F.; Olalekan, A.; Ogundele, S.O.; Ebruke, B.E.; Richard, A.K.; Paramadhas, B.D.A.; et al. A multicentre point prevalence study of antibiotics utilization in hospitalized patients in an urban secondary and a tertiary healthcare facilities in Nigeria: Findings and implications. Expert Rev. Anti-Infect. Ther. 2022, 20, 297–306. [Google Scholar] [CrossRef]
- Gwebu, P.C.; Meyer, J.C.; Schellack, N.; Matsebula-Myeni, Z.C.; Godman, B. A web-based point prevalence survey of antimicrobial use and quality indicators at Raleigh Fitkin Memorial Hospital in the Kingdom of Eswatini and the implications. Hosp. Pract. 2022, 50, 214–221. [Google Scholar] [CrossRef]
- Aboderin, A.O.; Adeyemo, A.T.; Olayinka, A.A.; Oginni, A.S.; Adeyemo, A.T.; Oni, A.A.; Olabisi, O.F.; Fayomi, O.D.; Anuforo, A.C.; Egwuenu, A.; et al. Antimicrobial use among hospitalized patients: A multi-center, point prevalence survey across public healthcare facilities, Osun State, Nigeria. Germs 2021, 11, 523–535. [Google Scholar] [CrossRef]
- Sulis, G.; Adam, P.; Nafade, V.; Gore, G.; Daniels, B.; Daftary, A.; Das, J.; Gandra, S.; Pai, M. Antibiotic prescription practices in primary care in low- and middle-income countries: A systematic review and meta-analysis. PLoS Med. 2020, 17, e1003139. [Google Scholar] [CrossRef]
- Gasson, J.; Blockman, M.; Willems, B. Antibiotic prescribing practice and adherence to guidelines in primary care in the Cape Town Metro District, South Africa. S. Afr. Med. J. 2018, 108, 304–310. [Google Scholar] [CrossRef]
- Anand Paramadhas, B.D.; Tiroyakgosi, C.; Mpinda-Joseph, P.; Morokotso, M.; Matome, M.; Sinkala, F.; Gaolebe, M.; Malone, B.; Molosiwa, E.; Shanmugam, M.G.; et al. Point prevalence study of antimicrobial use among hospitals across Botswana; findings and implications. Expert Rev. Anti-Infect. Ther. 2019, 17, 535–546. [Google Scholar] [CrossRef]
- Guma, S.P.; Godman, B.; Campbell, S.M.; Mahomed, O. Determinants of the Empiric Use of Antibiotics by General practitioners in South Africa: Observational, Analytic, Cross-Sectional Study. Antibiotics 2022, 11, 1423. [Google Scholar] [CrossRef]
- Ndaki, P.M.; Mushi, M.F.; Mwanga, J.R.; Konje, E.T.; Ntinginya, N.E.; Mmbaga, B.T.; Keenan, K.; Sabiiti, W.; Kesby, M.; Benitez-Paez, F.; et al. Dispensing Antibiotics without Prescription at Community Pharmacies and Accredited Drug Dispensing Outlets in Tanzania: A Cross-Sectional Study. Antibiotics 2021, 10, 1025. [Google Scholar] [CrossRef]
- Auta, A.; Hadi, M.A.; Oga, E.; Adewuyi, E.O.; Abdu-Aguye, S.N.; Adeloye, D.; Strickland-Hodge, B.; Morgan, D.J. Global access to antibiotics without prescription in community pharmacies: A systematic review and meta-analysis. J. Infect. 2019, 78, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Batista, A.D.; Rodrigues, D.A.; Figueiras, A.; Zapata-Cachafeiro, M.; Roque, F.; Herdeiro, M.T. Antibiotic Dispensation without a Prescription Worldwide: A Systematic Review. Antibiotics 2020, 9, 786. [Google Scholar] [CrossRef] [PubMed]
- Kalungia, A.C.; Burger, J.; Godman, B.; Costa, J.O.; Simuwelu, C. Non-prescription sale and dispensing of antibiotics in community pharmacies in Zambia. Expert Rev. Anti-Infect. Ther. 2016, 14, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Kibuule, D.; Kagoya, H.R.; Godman, B. Antibiotic use in acute respiratory infections in under-fives in Uganda: Findings and implications. Expert Rev. Anti-Infect. Ther. 2016, 14, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Sakeena, M.H.F.; Bennett, A.A.; McLachlan, A.J. Non-prescription sales of antimicrobial agents at community pharmacies in developing countries: A systematic review. Int. J. Antimicrob. Agents 2018, 52, 771–782. [Google Scholar] [CrossRef]
- Afari-Asiedu, S.; Oppong, F.B.; Tostmann, A.; Abdulai, M.A.; Boamah-Kaali, E.; Gyaase, S.; Agyei, O.; Kinsman, J.; Hulscher, M.; Wertheim, H.F.L.; et al. Determinants of Inappropriate Antibiotics Use in Rural Central Ghana Using a Mixed Methods Approach. Front Public Health 2020, 8, 90. [Google Scholar] [CrossRef]
- Klein, E.Y.; Tseng, K.K.; Pant, S.; Laxminarayan, R. Tracking global trends in the effectiveness of antibiotic therapy using the Drug Resistance Index. BMJ Glob. Health 2019, 4, e001315. [Google Scholar] [CrossRef]
- Mokwele, R.N.; Schellack, N.; Bronkhorst, E.; Brink, A.J.; Schweickerdt, L.; Godman, B. Using mystery shoppers to determine practices pertaining to antibiotic dispensing without a prescription among community pharmacies in South Africa—A pilot survey. JAC-Antimicrob. Resist. 2022, 4, dlab196. [Google Scholar] [CrossRef]
- Kaupitwa, C.J.; Nowaseb, S.; Godman, B.; Kibuule, D. Analysis of policies for use of medically important antibiotics in animals in Namibia: Implications for antimicrobial stewardship. Expert Rev. Anti-Infect. Ther. 2022, 20, 1365–1379. [Google Scholar] [CrossRef]
- Mudenda, S.; Mukosha, M.; Godman, B.; Fadare, J.; Malama, S.; Munyeme, M.; Hikaambo, C.N.; Kalungia, A.C.; Hamachila, A.; Kainga, H.; et al. Knowledge, Attitudes, and Practices of Community Pharmacy Professionals on Poultry Antibiotic Dispensing, Use, and Bacterial Antimicrobial Resistance in Zambia: Implications on Antibiotic Stewardship and WHO AWaRe Classification of Antibiotics. Antibiotics 2022, 11, 1210. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Pires, J.; Silvester, R.; Zhao, C.; Song, J.; Criscuolo, N.G.; Gilbert, M.; Bonhoeffer, S.; Laxminarayan, R. Global trends in antimicrobial resistance in animals in low- and middle-income countries. Science 2019, 365, eaaw1944. [Google Scholar] [CrossRef]
- Jibril, A.H.; Okeke, I.N.; Dalsgaard, A.; Olsen, J.E. Association between antimicrobial usage and resistance in Salmonella from poultry farms in Nigeria. BMC Vet. Res. 2021, 17, 234. [Google Scholar] [CrossRef]
- Njoga, E.O.; Ogugua, A.J.; Nwankwo, I.O.; Awoyomi, O.J.; Okoli, C.E.; Buba, D.M.; Oyeleye, F.A.; Ajibo, F.E.; Azor, N.; Ogunniran, T.M. Antimicrobial drug usage pattern in poultry farms in Nigeria: Implications for food safety, public health and poultry disease management. Vet. Ital. 2021, 57, 5–12. [Google Scholar]
- Hedman, H.D.; Vasco, K.A.; Zhang, L. A Review of Antimicrobial Resistance in Poultry Farming within Low-Resource Settings. Animals 2020, 10, 264. [Google Scholar] [CrossRef]
- Aslam, B.; Khurshid, M.; Arshad, M.I.; Muzammil, S.; Rasool, M.; Yasmeen, N.; Shah, T.; Chaudhry, T.H.; Rasool, M.H.; Shahid, A.; et al. Antibiotic Resistance: One Health One World Outlook. Front. Cell. Infect. Microbiol. 2021, 11, 771510. [Google Scholar] [CrossRef]
- Hernando-Amado, S.; Coque, T.M.; Baquero, F.; Martínez, J.L. Defining and combating antibiotic resistance from One Health and Global Health perspectives. Nat. Microbiol. 2019, 4, 1432–1442. [Google Scholar] [CrossRef]
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef]
- The World Bank. Final Report—DRUG-RESISTANT INFECTIONS. A Threat to Our Economic Future March 2017. Available online: http://documents1.worldbank.org/curated/en/323311493396993758/pdf/final-report.pdf (accessed on 15 November 2022).
- Gautam, A. Antimicrobial Resistance: The Next Probable Pandemic. JNMA J. Nepal Med. Assoc. 2022, 60, 225–228. [Google Scholar] [CrossRef]
- Lim, J.M.; Singh, S.R.; Duong, M.C.; Legido-Quigley, H.; Hsu, L.Y.; Tam, C.C. Impact of national interventions to promote responsible antibiotic use: A systematic review. J. Antimicrob. Chemother. 2020, 75, 14–29. [Google Scholar] [CrossRef]
- Kamere, N.; Garwe, S.T.; Akinwotu, O.O.; Tuck, C.; Krockow, E.M.; Yadav, S.; Olawale, A.G.; Diyaolu, A.H.; Munkombwe, D.; Muringu, E.; et al. Scoping Review of National Antimicrobial Stewardship Activities in Eight African Countries and Adaptable Recommendations. Antibiotics 2022, 11, 1149. [Google Scholar] [CrossRef]
- Interagency Coordination Group on Antimicrobial Resistance. No Time to Wait: Securing the Future from Drug-Resistant Infections—Report to the Secretary-General of the United Nations. 2019. Available online: https://www.who.int/antimicrobial-resistance/interagency-coordination-group/IACG_final_report_EN.pdf?ua=1 (accessed on 15 November 2022).
- OECD Health Policy Studies. Stemming the Superbug Tide. 2018. Available online: https://www.oecd-ilibrary.org/sites/9789264307599-en/index.html?itemId=/content/publication/9789264307599-en&mimeType=text/html (accessed on 15 November 2022).
- World Health Organization (WHO); Food and Agriculture Organization of the United Nations (FAO); World Organisation for Animal Health (OIE). Monitoring Global Progress on Antimicrobial Resistance: Tripartite Amr Country Self-Assessment Survey (Tracss) 2019–2020 Global Analysis Report. 2021. Available online: https://www.who.int/publications/i/item/monitoring-global-progress-on-antimicrobial-resistance-tripartite-amr-country-self-assessment-survey-(tracss)-2019-2020 (accessed on 14 November 2022).
- BSAC. Global Antimicrobial Stewardship Accreditation Scheme. 2021. Available online: https://bsac.org.uk/global-antimicrobial-stewardship-accreditation-scheme/ (accessed on 15 November 2022).
- World Bank Group. Pulling Together to Beat Superbugs Knowledge and Implementation Gaps in Addressing Antimicrobial Resistance. 2019. Available online: https://openknowledge.worldbank.org/bitstream/handle/10986/32552/Pulling-Together-to-Beat-Superbugs-Knowledge-and-Implementation-Gaps-in-Addressing-Antimicrobial-Resistance.pdf?sequence=1&isAllowed=y (accessed on 14 November 2022).
- WHO. Global Action Plan on Antimicrobial Resistance—Report by the Secretariat. 2016. Available online: https://apps.who.int/gb/ebwha/pdf_files/WHA69/A69_24-en.pdf (accessed on 15 November 2022).
- Matee, M. Antimicrobial resistance (AMR) at the Southern Africa Centre for Infectious Disease Surveillance. 2018. Available online: https://www.openaccessgovernment.org/southern-africa-centre-for-infectious-disease/52063/ (accessed on 14 November 2022).
- Craig, J.; Frost, I.; Sriram, A.; Nuttall, J.; Kapoor, G.; Alimi, Y.; Varma, J. Development of the first edition of African treatment guidelines for common bacterial infections and syndromes. J. Public Health Afr. 2021, 12, 2009. [Google Scholar] [CrossRef] [PubMed]
- Africa Centres for Disease Control and Prevention and Center for Disease Dynamics, Economics & Policy. African Antibiotic Treatment Guidelines for Common Bacterial Infections and Syndromes—Recommended Antibiotic Treatments in Neonatal and Pediatric Patients. 2021. Available online: https://africaguidelines.cddep.org/wp-content/uploads/2021/11/Quick-Reference-Guide_Peds_English.pdf (accessed on 15 November 2022).
- ASLM. The African Society for Laboratory Medicine. Available online: https://aslm.org/ (accessed on 14 November 2022).
- Tornimbene, B.; Eremin, S.; Abednego, R.; Abualas, E.O.; Boutiba, I.; Egwuenu, A.; Fuller, W.; Gahimbare, L.; Githii, S.; Kasambara, W.; et al. Global Antimicrobial Resistance and Use Surveillance System on the African continent: Early implementation 2017–2019. Afr. J. Lab. Med. 2022, 11, 1594. [Google Scholar] [CrossRef]
- Fraser, J.L.; Alimi, Y.H.; Varma, J.K.; Muraya, T.; Kujinga, T.; Carter, V.K.; Schultsz, C.; Vilas, V.J.D.R. Antimicrobial resistance control efforts in Africa: A survey of the role of Civil Society Organisations. Glob. Health Action. 2021, 14, 1868055. [Google Scholar] [CrossRef] [PubMed]
- Sharland, M.; Pulcini, C.; Harbarth, S.; Zeng, M.; Gandra, S.; Mathur, S.; Magrini, N. Classifying antibiotics in the WHO Essential Medicines List for optimal use-be AWaRe. Lancet Infect. Dis. 2018, 18, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Sharland, M.; Gandra, S.; Huttner, B.; Moja, L.; Pulcini, C.; Zeng, M.; Mendelson, M.; Cappello, B.; Cooke, G.; Magrini, N.; et al. Encouraging AWaRe-ness and discouraging inappropriate antibiotic use-the new 2019 Essential Medicines List becomes a global antibiotic stewardship tool. Lancet Infect. Dis. 2019, 19, 1278–1280. [Google Scholar] [CrossRef]
- Klein, E.Y.; Milkowska-Shibata, M.; Tseng, K.K.; Sharland, M.; Gandra, S.; Pulcini, C.; Laxminarayan, R. Assessment of WHO antibiotic consumption and access targets in 76 countries, 2000–2015: An analysis of pharmaceutical sales data. Lancet Infect. Dis. 2021, 21, 107–115. [Google Scholar] [CrossRef]
- Hsia, Y.; Lee, B.R.; Versporten, A.; Yang, Y.; Bielicki, J.; Jackson, C.; Newland, J.; Goossens, H.; Magrini, N.; Sharland, M.; et al. Use of the WHO Access, Watch, and Reserve classification to define patterns of hospital antibiotic use (AWaRe): An analysis of paediatric survey data from 56 countries. Lancet Glob. Health 2019, 7, e861–e871. [Google Scholar] [CrossRef]
- Adekoya, I.; Maraj, D.; Steiner, L.; Yaphe, H.; Moja, L.; Magrini, N.; Cooke, G.; Loeb, M.; Persaud, N. Comparison of antibiotics included in national essential medicines lists of 138 countries using the WHO Access, Watch, Reserve (AWaRe) classification: A cross-sectional study. Lancet Infect. Dis. 2021, 21, 1429–1440. [Google Scholar] [CrossRef]
- Pauwels, I.; Versporten, A.; Drapier, N.; Vlieghe, E.; Goossens, H. Hospital antibiotic prescribing patterns in adult patients according to the WHO Access, Watch and Reserve classification (AWaRe): Results from a worldwide point prevalence survey in 69 countries. J. Antimicrob. Chemother. 2021, 76, 1614–1624. [Google Scholar] [CrossRef]
- Skosana, P.; Schellack, N.; Godman, B.; Kurdi, A.; Bennie, M.; Kruger, D.; Meyer, J. A national, multicentre, web-based point prevalence survey of antimicrobial use and quality indices among hospitalised paediatric patients across South Africa. J. Glob. Antimicrob. Resist. 2022, 29, 542–550. [Google Scholar] [CrossRef]
- Skosana, P.P.; Schellack, N.; Godman, B.; Kurdi, A.; Bennie, M.; Kruger, D.; Meyer, J.C. A national, multicentre web-based point prevalence survey of antimicrobial use in community healthcare centres across South Africa and the implications. Hosp. Pract. 2022, 50, 306–317. [Google Scholar] [CrossRef]
- Amponsah, O.K.O.; Buabeng, K.O.; Owusu-Ofori, A.; Ayisi-Boateng, N.K.; Hämeen-Anttila, K.; Enlund, H. Point prevalence survey of antibiotic consumption across three hospitals in Ghana. JAC Antimicrob. Resist. 2021, 3, dlab008. [Google Scholar] [CrossRef]
- Seni, J.; Mapunjo, S.G.; Wittenauer, R.; Valimba, R.; Stergachis, A.; Werth, B.J.; Saitoti, S.; Mhadu, N.H.; Lusaya, E.; Konduri, N. Antimicrobial use across six referral hospitals in Tanzania: A point prevalence survey. BMJ Open 2020, 10, e042819. [Google Scholar] [CrossRef]
- Hsia, Y.; Sharland, M.; Jackson, C.; Wong, I.C.K.; Magrini, N.; Bielicki, J.A. Consumption of oral antibiotic formulations for young children according to the WHO Access, Watch, Reserve (AWaRe) antibiotic groups: An analysis of sales data from 70 middle-income and high-income countries. Lancet Infect. Dis. 2019, 19, 67–75. [Google Scholar] [CrossRef]
- Moolla, M.S.; Whitelaw, A.; Decloedt, E.H.; Koegelenberg, C.F.N.; Parker, A. Opportunities to enhance antibiotic stewardship: Colistin use and outcomes in a low-resource setting. JAC Antimicrob. Resist. 2021, 3, dlab169. [Google Scholar] [CrossRef]
- Prusakov, P.; Goff, D.A.; Wozniak, P.S.; Cassim, A.; Scipion, C.E.; Urzúa, S.; Ronchi, A.; Zeng, L.; Ladipo-Ajayi, O.; Aviles-Otero, N.; et al. A global point prevalence survey of antimicrobial use in neonatal intensive care units: The no-more-antibiotics and resistance (NO-MAS-R) study. eClinicalMedicine 2021, 32, 100727. [Google Scholar] [CrossRef]
- Munkholm, L.; Rubin, O. The global governance of antimicrobial resistance: A cross-country study of alignment between the global action plan and national action plans. Glob. Health 2020, 16, 109. [Google Scholar] [CrossRef]
- Iwu, C.D.; Patrick, S.M. An insight into the implementation of the global action plan on antimicrobial resistance in the WHO African region: A roadmap for action. Int. J. Antimicrob. Agents 2021, 58, 106411. [Google Scholar] [CrossRef]
- Godman, B.; Egwuenu, A.; Wesangula, E.; Schellack, N.; Kalungia, A.C.; Tiroyakgosi, C.; Kgatlwane, J.; Mwita, J.C.; Patrick, O.; Niba, L.L.; et al. Tackling antimicrobial resistance across sub-Saharan Africa: Current challenges and implications for the future. Expert Opin. Drug Saf. 2022, 21, 1089–1111. [Google Scholar] [CrossRef]
- Federal Ministries of Agriculture, Rural Development, Environment and Health, Abuja, Nigeria. National Action Plan for Antimicrobial Resistance, 2017–2022. 2017. Available online: https://ncdc.gov.ng/themes/common/docs/protocols/77_1511368219.pdf (accessed on 15 November 2022).
- Ghana Ministry of Health, Ministry of Food and Agriculture, Ministry of Environment, Science, Technology and Innovation, Ministry of Fisheries and Aquaculture Development. Ghana National Action Plan for Antimicrobial Use and Resistance. 2017–2021. Available online: http://www.moh.gov.gh/wp-content/uploads/2018/04/NAP_FINAL_PDF_A4_19.03.2018-SIGNED-1.pdf (accessed on 14 November 2022).
- Sangeda, R.Z.; Kibona, J.; Munishi, C.; Arabi, F.; Manyanga, V.P.; Mwambete, K.D.; Horumpende, P.G. Assessment of Implementation of Antimicrobial Resistance Surveillance and Antimicrobial Stewardship Programs in Tanzanian Health Facilities a Year After Launch of the National Action Plan. Front. Public Health 2020, 8, 454. [Google Scholar] [CrossRef]
- Koduah, A.; Gyansa-Lutterodt, M.; Hedidor, G.K.; Sekyi-Brown, R.; Asiedu-Danso, M.; Asare, B.A.; Ackon, A.A.; Annan, E.A. Antimicrobial resistance national level dialogue and action in Ghana: Setting and sustaining the agenda and outcomes. One Health Outlook 2021, 3, 18. [Google Scholar] [CrossRef] [PubMed]
- Labi, A.-K.; Obeng-Nkrumah, N.; Dayie, N.T.K.D.; Egyir, B.; Sampane-Donkor, E.; Newman, M.J.; Opintan, J.A. Antimicrobial use in hospitalized patients: A multicentre point prevalence survey across seven hospitals in Ghana. JAC Antimicrob. Resist. 2021, 3, dlab087. [Google Scholar] [CrossRef] [PubMed]
- Afriyie, D.K.; Sefah, I.A.; Sneddon, J.; Malcolm, W.; McKinney, R.; Cooper, L.; Kurdi, A.; Godman, B.; Seaton, R.A. Antimicrobial point prevalence surveys in two Ghanaian hospitals: Opportunities for antimicrobial stewardship. JAC Antimicrob. Resist. 2020, 2, dlaa001. [Google Scholar] [CrossRef] [PubMed]
- Dodoo, C.; Orman, E.; Alalbila, T.; Mensah, A.; Jato, J.; Mfoafo, K.; Folitse, I.; Hutton-Nyameaye, A.; Ben, I.O.; Mensah-Kane, P.; et al. Antimicrobial Prescription Pattern in Ho Teaching Hospital, Ghana: Seasonal Determination Using a Point Prevalence Survey. Antibiotics 2021, 10, 199. [Google Scholar] [CrossRef] [PubMed]
- Okoth, C.; Opanga, S.; Okalebo, F.; Oluka, M.; Baker Kurdi, A.; Godman, B. Point prevalence survey of antibiotic use and resistance at a referral hospital in Kenya: Findings and implications. Hosp. Pract. 2018, 46, 128–136. [Google Scholar] [CrossRef]
- Momanyi, L.; Opanga, S.; Nyamu, D.; Oluka, M.; Kurdi, A.; Godman, B. Antibiotic Prescribing Patterns at a Leading Referral Hospital in Kenya: A Point Prevalence Survey. J. Res. Pharm. Pract. 2019, 8, 149–154. [Google Scholar]
- Omulo, S.; Oluka, M.; Ombajo, L.; Osoro, E.; Kinuthia, R.; Guantai, A.; Ndegwa, L.; Verani, J.; Opanga, S.; Wesangula, E.; et al. Point-Prevalence Surveys of Antibiotic Use at Three Large Public Hospitals in Kenya. Infect. Control Hosp. Epidemiol. 2020, 41, s353–s354. [Google Scholar] [CrossRef]
- D’Arcy, N.; Ashiru-Oredope, D.; Olaoye, O.; Afriyie, D.; Akello, Z.; Ankrah, D.; Asima, D.M.; Banda, D.C.; Barrett, S.; Brandish, C.; et al. Antibiotic Prescribing Patterns in Ghana, Uganda, Zambia and Tanzania Hospitals: Results from the Global Point Prevalence Survey (G-PPS) on Antimicrobial Use and Stewardship Interventions Implemented. Antibiotics 2021, 10, 1122. [Google Scholar] [CrossRef]
- Abubakar, U. Antibiotic use among hospitalized patients in northern Nigeria: A multicenter point-prevalence survey. BMC Infect. Dis. 2020, 20, 86. [Google Scholar] [CrossRef]
- Mwita, J.C.; Ogunleye, O.O.; Olalekan, A.; Kalungia, A.C.; Kurdi, A.; Saleem, Z.; Sneddon, J.; Godman, B. Key Issues Surrounding Appropriate Antibiotic Use for Prevention of Surgical Site Infections in Low- and Middle-Income Countries: A Narrative Review and the Implications. Int. J. Gen. Med. 2021, 14, 515–530. [Google Scholar] [CrossRef]
- Bediako-Bowan, A.; Owusu, E.; Debrah, S.; Kjerulf, A.; Newman, M.; Kurtzhals, J.; Mølbak, K. Surveillance of surgical site infection in a teaching hospital in Ghana: A prospective cohort study. J. Hosp. Infect. 2020, 104, 321–327. [Google Scholar] [CrossRef]
- Alshaikh, F.S.; Godman, B.; Sindi, O.N.; Seaton, R.A.; Kurdi, A. Prevalence of bacterial coinfection and patterns of antibiotics prescribing in patients with COVID-19: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0272375. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.-P.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.-P.R.; Westwood, D.; Daneman, N.; MacFadden, D.R. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 520–531. [Google Scholar] [CrossRef]
- Jeon, K.; Jeong, S.; Lee, N.; Park, M.-J.; Song, W.; Kim, H.-S.; Kim, H.S.; Kim, J.-S. Impact of COVID-19 on Antimicrobial Consumption and Spread of Multidrug-Resistance in Bacterial Infections. Antibiotics 2022, 11, 535. [Google Scholar] [CrossRef]
- Fukushige, M.; Ngo, N.-H.; Lukmanto, D.; Fukuda, S.; Ohneda, O. Effect of the COVID-19 pandemic on antibiotic consumption: A systematic review comparing 2019 and 2020 data. Front. Public Health 2022, 10, 946077. [Google Scholar] [CrossRef]
- Adebisi, Y.A.; Jimoh, N.D.; Ogunkola, I.O.; Uwizeyimana, T.; Olayemi, A.H.; Ukor, N.A.; Lucero-Prisno, D.E. The use of antibiotics in COVID-19 management: A rapid review of national treatment guidelines in 10 African countries. Trop. Med. Health 2021, 49, 51. [Google Scholar] [CrossRef]
- Al-Hadidi, S.H.; Alhussain, H.; Hadi, H.A.; Johar, A.; Yassine, H.M.; Al Thani, A.A.; Eltai, N.O. The Spectrum of Antibiotic Prescribing During COVID-19 Pandemic: A Systematic Literature Review. Microb. Drug Resist. 2021, 27, 1705–1725. [Google Scholar] [CrossRef]
- Founou, R.C.; Blocker, A.J.; Noubom, M.; Tsayem, C.; Choukem, S.P.; Dongen, M.V.; Founou, L.L. The COVID-19 pandemic: A threat to antimicrobial resistance containment. Future Sci. OA 2021, 7, Fso736. [Google Scholar] [CrossRef]
- Ghosh, S.; Bornman, C.; Zafer, M.M. Antimicrobial Resistance Threats in the emerging COVID-19 pandemic: Where do we stand? J. Infect. Public Health 2021, 14, 555–560. [Google Scholar] [CrossRef]
- Quincho-Lopez, A.; Benites-Ibarra, C.A.; Hilario-Gomez, M.M.; Quijano-Escate, R.; Taype-Rondan, A. Self-medication practices to prevent or manage COVID-19: A systematic review. PLoS ONE 2021, 16, e0259317. [Google Scholar] [CrossRef] [PubMed]
- Sefah, I.A.; Ogunleye, O.O.; Essah, D.O.; Opanga, S.A.; Butt, N.; Wamaitha, A.; Guantai, A.N.; Chikowe, I.; Khuluza, F.; Kibuule, D.; et al. Rapid Assessment of the Potential Paucity and Price Increases for Suggested Medicines and Protection Equipment for COVID-19 Across Developing Countries With a Particular Focus on Africa and the Implications. Front. Pharmacol. 2020, 11, 588106. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J. How COVID-19 is accelerating the threat of antimicrobial resistance. Bmj 2020, 369, m1983. [Google Scholar] [CrossRef] [PubMed]
- Iwu, C.J.; Jordan, P.; Jaja, I.F.; Iwu, C.D.; Wiysonge, C.S. Treatment of COVID-19: Implications for antimicrobial resistance in Africa. Pan Afr. Med. J. 2020, 35 (Suppl. S2), 119. [Google Scholar] [CrossRef]
- Lai, C.C.; Chen, S.Y.; Ko, W.C.; Hsueh, P.R. Increased antimicrobial resistance during the COVID-19 pandemic. Int. J. Antimicrob. Agents 2021, 57, 106324. [Google Scholar] [CrossRef]
- Ramzan, K.; Shafiq, S.; Raees, I.; Mustafa, Z.U.; Salman, M.; Khan, A.H.; Meyer, J.C.; Godman, B. Co-Infections, Secondary Infections, and Antimicrobial Use in Patients Hospitalized with COVID-19 during the First Five Waves of the Pandemic in Pakistan; Findings and Implications. Antibiotics 2022, 11, 789. [Google Scholar] [CrossRef]
- Do, N.T.T.; Vu, H.T.L.; Nguyen, C.T.K.; Punpuing, S.; Khan, W.A.; Gyapong, M.; Asante, K.P.; Munguambe, K.; Gómez-Olivé, F.X.; John-Langba, J.; et al. Community-based antibiotic access and use in six low-income and middle-income countries: A mixed-method approach. Lancet Glob. Health 2021, 9, e610–e619. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Matsoso, P.; Pant, S.; Brower, C.; Røttingen, J.-A.; Klugman, K.; Davies, S. Access to effective antimicrobials: A worldwide challenge. Lancet 2016, 387, 168–175. [Google Scholar] [CrossRef]
- Kållberg, C.; Årdal, C.; Salvesen Blix, H.; Klein, E.; MMartinez, E.; Lindbæk, M.; Outterson, K.; Røttingen, J.A.; Laxminarayan, R. Introduction and geographic availability of new antibiotics approved between 1999 and 2014. PLoS ONE 2018, 13, e0205166. [Google Scholar] [CrossRef]
- Wang, K.; Wang, L.; Li, M.; Xie, B.; He, L.; Wang, M.; Zhang, R.; Hou, N.; Zhang, Y.; Jia, F. Real-Word Effectiveness of Global COVID-19 Vaccines Against SARS-CoV-2 Variants: A Systematic Review and Meta-Analysis. Front. Med. 2022, 9, 820544. [Google Scholar] [CrossRef]
- Mohammed, I.; Nauman, A.; Paul, P.; Ganesan, S.; Chen, K.-H.; Jalil, S.M.S.; Jaouni, S.H.; Kawas, H.; Khan, W.A.; Vattoth, A.L.; et al. The efficacy and effectiveness of the COVID-19 vaccines in reducing infection, severity, hospitalization, and mortality: A systematic review. Hum. Vaccines Immunother. 2022, 18, 2027160. [Google Scholar] [CrossRef]
- Martínez-Baz, I.; Miqueleiz, A.; Casado, I.; Navascués, A.; Trobajo-Sanmartín, C.; Burgui, C.; Guevara, M.; Ezpeleta, C.; Castilla, J. Effectiveness of COVID-19 vaccines in preventing SARS-CoV-2 infection and hospitalisation, Navarre, Spain, January to April 2021. Eurosurveillance 2021, 26, 2100438. [Google Scholar] [CrossRef]
- Korang, S.K.; von Rohden, E.; Veroniki, A.A.; Ong, G.; Ngalamika, O.; Siddiqui, F.; Juul, S.; Nielsen, E.E.; Feinberg, J.B.; Petersen, J.J.; et al. Vaccines to prevent COVID-19: A living systematic review with Trial Sequential Analysis and network meta-analysis of randomized clinical trials. PLoS ONE 2022, 17, e0260733. [Google Scholar] [CrossRef]
- Ogunleye, O.O.; Godman, B.; Fadare, J.O.; Mudenda, S.; Adeoti, A.O.; Yinka-Ogunleye, A.F.; Ogundele, S.O.; Oyawole, M.R.; Schönfeldt, M.; Rashed, W.M.; et al. Coronavirus Disease 2019 (COVID-19) Pandemic across Africa: Current Status of Vaccinations and Implications for the Future. Vaccines 2022, 10, 1553. [Google Scholar] [CrossRef]
- Njoga, E.O.; Mshelbwala, P.P.; Abah, K.O.; Awoyomi, O.J.; Wangdi, K.; Pewan, S.B.; Oyeleye, F.A.; Galadima, H.B.; Alhassan, S.A.; Okoli, C.E.; et al. COVID-19 Vaccine Hesitancy and Determinants of Acceptance among Healthcare Workers, Academics and Tertiary Students in Nigeria. Vaccines 2022, 10, 626. [Google Scholar] [CrossRef]
- Njoga, E.O.; Awoyomi, O.J.; Onwumere-Idolor, O.S.; Awoyomi, P.O.; Ugochukwu, I.C.I.; Ozioko, S.N. Persisting Vaccine Hesitancy in Africa: The Whys, Global Public Health Consequences and Ways-Out-COVID-19 Vaccination Acceptance Rates as Case-in-Point. Vaccines 2022, 10, 1934. [Google Scholar] [CrossRef]
- Nathwani, D.; Varghese, D.; Stephens, J.; Ansari, W.; Martin, S.; Charbonneau, C. Value of hospital antimicrobial stewardship programs [ASPs]: A systematic review. Antimicrob. Resist. Infect. Control 2019, 8, 35. [Google Scholar] [CrossRef]
- de Carvalho, F.R.T.; Telles, J.P.; Tuon, F.F.B.; Rabello Filho, R.; Caruso, P.; Correa, T.D. Antimicrobial Stewardship Programs: A Review of Strategies to Avoid Polymyxins and Carbapenems Misuse in Low Middle-Income Countries. Antibiotics 2022, 11, 378. [Google Scholar] [CrossRef]
- Versporten, A.; Zarb, P.; Caniaux, I.; Gros, M.-F.; Drapier, N.; Miller, M.; Jarlier, V.; Nathwani, D.; Goossens, H.; Koraqi, A.; et al. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: Results of an internet-based global point prevalence survey. Lancet Glob. Health 2018, 6, e619–e629. [Google Scholar] [CrossRef]
- Le Maréchal, M.; Tebano, G.; Monnier, A.A.; Adriaenssens, N.; Gyssens, I.C.; Huttner, B.; Milanic, R.; Schouten, J.; Benic, M.S.; Versporten, A.; et al. Quality indicators assessing antibiotic use in the outpatient setting: A systematic review followed by an international multidisciplinary consensus procedure. J. Antimicrob. Chemother. 2018, 73 (Suppl. S6), vi40–vi49. [Google Scholar] [CrossRef]
- Nnadozie, U.U.; Umeokonkwo, C.D.; Maduba, C.C.; Igwe-Okomiso, D.; Onah, C.K.; Madubueze, U.C.; Anikwe, C.C.; Versporten, A.; Pauwels, I.; Goossens, H.; et al. Antibiotic use among surgical inpatients at a tertiary health facility: A case for a standardized protocol for presumptive antimicrobial therapy in the developing world. Infect. Prev. Pract. 2020, 2, 100078. [Google Scholar] [CrossRef] [PubMed]
- Niaz, Q.; Godman, B.; Massele, A.; Campbell, S.; Kurdi, A.; Kagoya, H.R.; Kibuule, D. Validity of World Health Organisation prescribing indicators in Namibia’s primary healthcare: Findings and implications. Int. J. Qual. Health Care 2018, 31, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.M.; Meyer, J.C.; Godman, B. Why Compliance to National Prescribing Guidelines is Important Especially across Sub-Saharan Africa and Suggestions for the Future. Biomed. Pharm. Sci. 2021, 4, 1–7. [Google Scholar]
- Sulis, G.; Sayood, S.; Gandra, S. Antimicrobial resistance in low- and middle-income countries: Current status and future directions. Expert Rev. Anti-Infect. Ther. 2022, 20, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Haldeman, M.S.; Kishimbo, P.; Seddon, M.; Sangare, A.; Mwasomola, D.; Hall, J.; Shaffer, M.; Leclair, R.; Caulder, C.; Bookstaver, P.B.; et al. Evaluation of Antimicrobial Utilization and Concordance with National Guidelines at a Tertiary Hospital in the Southern Highlands Zone of Tanzania. Am. J. Trop. Med. Hyg. 2020, 102, 370–376. [Google Scholar] [CrossRef]
- Alsaeed, O.M.; Bukhari, A.A.; Alshehri, A.A.; Alsumairi, F.A.; Alnami, A.M.; Elsheikh, H.A. The Use of Antibiotics for the Prevention of Surgical Site Infections in Two Government Hospitals in Taif, Saudi Arabia: A Retrospective Study. Cureus 2022, 14, e26731. [Google Scholar] [CrossRef]
- Alagha, H.Z.; Al Telbani, M.J. Investigating antibiotic use in Gaza Strip hospitals: A retrospective cross-sectional analysis. J. Infect. Dev. Ctries. 2022, 16, 1739–1747. [Google Scholar] [CrossRef]
- Eticha, E.M.; Gemechu, W.D. Adherence to Guidelines for Assessment and Empiric Antibiotics Recommendations for Community-Acquired Pneumonia at Ambo University Referral Hospital: Prospective Observational Study. Patient Prefer. Adherence 2021, 15, 467–473. [Google Scholar] [CrossRef]
- Mugada, V.; Mahato, V.; Andhavaram, D.; Vajhala, S.M. Evaluation of Prescribing Patterns of Antibiotics Using Selected Indicators for Antimicrobial Use in Hospitals and the Access, Watch, Reserve (AWaRe) Classification by the World Health Organization. Turk. J. Pharm. Sci. 2021, 18, 282–288. [Google Scholar] [CrossRef]
- Dechasa, M.; Chelkeba, L.; Jorise, A.; Sefera, B.; Melaku, T. Antibiotics use evaluation among hospitalized adult patients at Jimma Medical Center, southwestern Ethiopia: The way to pave for antimicrobial stewardship. J. Pharm. Policy Pract. 2022, 15, 84. [Google Scholar] [CrossRef]
- WHO; World Bank; OECD. Delivering Quality Health Services: A Global Imperative for Universal Health Coverage. 2018. Available online: https://documents1.worldbank.org/curated/en/482771530290792652/pdf/127816-REVISED-quality-joint-publication-July2018-Complete-vignettes-ebook-L.pdf (accessed on 14 November 2022).
- Campbell, S.M.; Braspenning, J.; Hutchinson, A.; Marshall, M. Research methods used in developing and applying quality indicators in primary care. Qual. Saf. Health Care 2002, 11, 358–364. [Google Scholar] [CrossRef]
- Campbell, S.M.; Kontopantelis, E.; Hannon, K.; Burke, M.; Barber, A.; Lester, H.E. Framework and indicator testing protocol for developing and piloting quality indicators for the UK quality and outcomes framework. BMC Fam. Pract. 2011, 12, 85. [Google Scholar] [CrossRef]
- Campbell, S.M.; Godman, B.; Diogene, E.; Fürst, J.; Gustafsson, L.L.; MacBride-Stewart, S.; Malmström, R.E.; Pedersen, H.; Selke, G.; Vlahović-Palčevski, V.; et al. Quality indicators as a tool in improving the introduction of new medicines. Basic Clin. Pharmacol. Toxicol. 2015, 116, 146–157. [Google Scholar] [CrossRef]
- Cox, J.A.; Vlieghe, E.; Mendelson, M.; Wertheim, H.; Ndegwa, L.; Villegas, M.V.; Gould, I.; Hara, G.L. Antibiotic stewardship in low- and middle-income countries: The same but different? Clin. Microbiol. Infect. 2017, 23, 812–818. [Google Scholar] [CrossRef]
- Fadare, J.O.; Ogunleye, O.; Iliyasu, G.; Adeoti, A.; Schellack, N.; Engler, D.; Massele, A.; Godman, B. Status of antimicrobial stewardship programmes in Nigerian tertiary healthcare facilities: Findings and implications. J. Glob. Antimicrob. Resist. 2019, 17, 132–136. [Google Scholar] [CrossRef]
- Kalungia, A.C.; Mwambula, H.; Munkombwe, D.; Marshall, S.; Schellack, N.; May, C.; Jones, A.S.C.; Godman, B. Antimicrobial stewardship knowledge and perception among physicians and pharmacists at leading tertiary teaching hospitals in Zambia: Implications for future policy and practice. J. Chemother. 2019, 31, 378–387. [Google Scholar] [CrossRef]
- Hall, J.W.; Bouchard, J.; Bookstaver, P.B.; Haldeman, M.S.; Kishimbo, P.; Mbwanji, G.; Mwakyula, I.; Mwasomola, D.; Seddon, M.; Shaffer, M.; et al. The Mbeya Antimicrobial Stewardship Team: Implementing Antimicrobial Stewardship at a Zonal-Level Hospital in Southern Tanzania. Pharmacy 2020, 8, 107. [Google Scholar] [CrossRef]
- Akpan, M.R.; Isemin, N.U.; Udoh, A.E.; Ashiru-Oredope, D. Implementation of antimicrobial stewardship programmes in African countries: A systematic literature review. J. Glob. Antimicrob. Resist. 2020, 22, 317–324. [Google Scholar] [CrossRef]
- Ackers, L.; Ackers-Johnson, G.; Seekles, M.; Odur, J.; Opio, S. Opportunities and Challenges for Improving Anti-Microbial Stewardship in Low- and Middle-Income Countries; Lessons Learnt from the Maternal Sepsis Intervention in Western Uganda. Antibiotics 2020, 9, 315. [Google Scholar] [CrossRef]
- Alabi, A.S.; Picka, S.W.; Sirleaf, R.; Ntirenganya, P.R.; Ayebare, A.; Correa, N.; Anyango, S.; Ekwen, G.; Agu, E.; Cook, R.; et al. Implementation of an antimicrobial stewardship programme in three regional hospitals in the south-east of Liberia: Lessons learned. JAC Antimicrob. Resist. 2022, 4, dlac069. [Google Scholar] [CrossRef]
- Ashiru-Oredope, D.; Garraghan, F.; Olaoye, O.; Krockow, E.M.; Matuluko, A.; Nambatya, W.; Babigumira, P.A.; Tuck, C.; Amofah, G.; Ankrah, D.; et al. Development and Implementation of an Antimicrobial Stewardship Checklist in Sub-Saharan Africa: A Co-Creation Consensus Approach. Healthcare 2022, 10, 170. [Google Scholar] [CrossRef] [PubMed]
- Gulumbe, B.H.; Haruna, U.A.; Almazan, J.; Ibrahim, I.H.; Faggo, A.A.; Bazata, A.Y. Combating the menace of antimicrobial resistance in Africa: A review on stewardship, surveillance and diagnostic strategies. Biol. Proced. Online 2022, 24, 19. [Google Scholar] [CrossRef] [PubMed]
- Siachalinga, L.; Mufwambi, W.; Lee, I.H. Impact of antimicrobial stewardship interventions to improve antibiotic prescribing for hospital inpatients in Africa: A systematic review and meta-analysis. J. Hosp. Infect. 2022, 129, 124–143. [Google Scholar] [CrossRef] [PubMed]
- Otieno, P.A.; Campbell, S.; Maley, S.; Obinju Arunga, T.; Otieno Okumu, M. A Systematic Review of Pharmacist-Led Antimicrobial Stewardship Programs in Sub-Saharan Africa. Int. J. Clin. Pract. 2022, 2022, 3639943. [Google Scholar] [CrossRef] [PubMed]
- WHO. Antimicrobial stewardship programmes in health-care facilities in low- and middle-income countries: A WHO practical toolkit. JAC Antimicrob. Resist. 2019, 1, dlz072. [Google Scholar] [CrossRef]
- WHO. Antimicrobial Stewardship Programmes in Health-Care Facilities in Low- and Middle-Income Countries. A WHO Practical Toolkit. 2019. Available online: https://apps.who.int/iris/bitstream/handle/10665/329404/9789241515481-eng.pdf (accessed on 15 November 2022).
- Sefah, I.A.; Denoo, E.Y.; Bangalee, V.; Kurdi, A.; Sneddon, J.; Godman, B. Appropriateness of surgical antimicrobial prophylaxis in a teaching hospital in Ghana: Findings and implications. JAC Antimicrob. Resist. 2022, 4, dlac102. [Google Scholar] [CrossRef]
- Abubakar, U. Point-prevalence survey of hospital acquired infections in three acute care hospitals in Northern Nigeria. Antimicrob. Resist. Infect. Control 2020, 9, 63. [Google Scholar] [CrossRef]
- Martinez-Sobalvarro, J.V.; Júnior, A.A.P.; Pereira, L.B.; Baldoni, A.O.; Ceron, C.S.; Dos Reis, T.M. Antimicrobial stewardship for surgical antibiotic prophylaxis and surgical site infections: A systematic review. Int. J. Clin. Pharm. 2022, 44, 301–319. [Google Scholar] [CrossRef]
- Forrester, J.D.; Maggio, P.M.; Tennakoon, L. Cost of Health Care-Associated Infections in the United States. J. Patient Saf. 2022, 18, e477–e479. [Google Scholar] [CrossRef]
- Liu, X.; Cui, D.; Li, H.; Wang, Q.; Mao, Z.; Fang, L.; Ren, N.; Sun, J. Direct medical burden of antimicrobial-resistant healthcare-associated infections: Empirical evidence from China. J. Hosp. Infect. 2020, 105, 295–305. [Google Scholar] [CrossRef]
- Branch-Elliman, W.; O’Brien, W.; Strymish, J.; Itani, K.; Wyatt, C.; Gupta, K. Association of Duration and Type of Surgical Prophylaxis With Antimicrobial-Associated Adverse Events. JAMA Surg. 2019, 154, 590–598. [Google Scholar] [CrossRef]
- Kalungia, A.C.; Mukosha, M.; Mwila, C.; Banda, D.; Mwale, M.; Kagulura, S.; Ogunleye, O.O.; Meyer, J.C.; Godman, B. Antibiotic Use and Stewardship Indicators in the First- and Second-Level Hospitals in Zambia: Findings and Implications for the Future. Antibiotics 2022, 11, 1626. [Google Scholar] [CrossRef]
- Kiguba, R.; Karamagi, C.; Bird, S.M. Extensive antibiotic prescription rate among hospitalized patients in Uganda: But with frequent missed-dose days. J. Antimicrob. Chemother. 2016, 71, 1697–1706. [Google Scholar] [CrossRef]
- Butt, S.Z.; Ahmad, M.; Saeed, H.; Saleem, Z.; Javaid, Z. Post-surgical antibiotic prophylaxis: Impact of pharmacist’s educational intervention on appropriate use of antibiotics. J. Infect. Public Health 2019, 12, 854–860. [Google Scholar] [CrossRef]
- Cooper, L.; Sneddon, J.; Afriyie, D.K.; Sefah, I.A.; Kurdi, A.; Godman, B.; Seaton, R.A. Supporting global antimicrobial stewardship: Antibiotic prophylaxis for the prevention of surgical site infection in low- and middle-income countries (LMICs): A scoping review and meta-analysis. JAC-Antimicrob. Resist. 2020, 2, dlaa070. [Google Scholar] [CrossRef]
- Abubakar, U.; Syed Sulaiman, S.A.; Adesiyun, A.G. Utilization of surgical antibiotic prophylaxis for obstetrics and gynaecology surgeries in Northern Nigeria. Int. J. Clin. Pharm. 2018, 40, 1037–1043. [Google Scholar] [CrossRef]
- Aiken, A.M.; Wanyoro, A.K.; Mwangi, J.; Juma, F.; Mugoya, I.K.; Scott, J.A. Changing use of surgical antibiotic prophylaxis in Thika Hospital, Kenya: A quality improvement intervention with an interrupted time series design. PLoS ONE 2013, 8, e78942. [Google Scholar] [CrossRef]
- Bediako-Bowan, A.A.A.; Mølbak, K.; Kurtzhals, J.A.L.; Owusu, E.; Debrah, S.; Newman, M.J. Risk factors for surgical site infections in abdominal surgeries in Ghana: Emphasis on the impact of operating rooms door openings. Epidemiol. Infect. 2020, 148, e147. [Google Scholar] [CrossRef]
- Ouedraogo, A.S.; Versporten, A.; Nagalo, A.; Pauwels, I.; Goossens, H.; Ouedraogo, A.; Poda, A. The Global Point Prevalence Survey of Antimicrobial Consumptionand Resistance (Global-PPS)—Results of antimicrobial prescribing in Burkina Faso. 2019. Available online: https://www.global-pps.com/wp-content/uploads/2021/02/The-Global-PPS_results-of-antimicrobial-prescribing-in-Burkina-Faso.pdf (accessed on 15 November 2022).
- Halawi, E.; Assefa, T.; Hussen, S. Pattern of antibiotics use, incidence and predictors of surgical site infections in a Tertiary Care Teaching Hospital. BMC Res. Notes 2018, 11, 538. [Google Scholar] [CrossRef]
- Alemkere, G. Antibiotic usage in surgical prophylaxis: A prospective observational study in the surgical ward of Nekemte referral hospital. PLoS ONE 2018, 13, e0203523. [Google Scholar] [CrossRef]
- Fentie, A.M.; Degefaw, Y.; Asfaw, G.; Shewarega, W.; Woldearegay, M.; Abebe, E.; Gebretekle, G.B. Multicentre point-prevalence survey of antibiotic use and healthcare-associated infections in Ethiopian hospitals. BMJ Open 2022, 12, e054541. [Google Scholar] [CrossRef] [PubMed]
- Nkurunziza, T.; Kateera, F.; Sonderman, K.; Gruendl, M.; Nihiwacu, E.; Ramadhan, B.; Cherian, T.; Nahimana, E.; Ntakiyiruta, G.; Habiyakare, C.; et al. Prevalence and predictors of surgical-site infection after caesarean section at a rural district hospital in Rwanda. Br. J. Surg. 2019, 106, e121–e128. [Google Scholar] [CrossRef] [PubMed]
- Horumpende, P.G.; Mshana, S.E.; Mouw, E.F.; Mmbaga, B.T.; Chilongola, J.O.; de Mast, Q. Point prevalence survey of antimicrobial use in three hospitals in North-Eastern Tanzania. Antimicrob. Resist. Infect. Control 2020, 9, 149. [Google Scholar] [CrossRef] [PubMed]
- Gentilotti, E.; De Nardo, P.; Nguhuni, B.; Piscini, A.; Damian, C.; Vairo, F.; Chaula, Z.; Mencarini, P.; Torokaa, P.; Zumla, A.; et al. Implementing a combined infection prevention and control with antimicrobial stewardship joint program to prevent caesarean section surgical site infections and antimicrobial resistance: A Tanzanian tertiary hospital experience. Antimicrob. Resist. Infect. Control 2020, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Inoue, K.; Ditai, J.; Weeks, A.D. Pattern of Peri-Operative Antibiotic Use among Surgical Patients in a Regional Referral and Teaching Hospital in Uganda. Surg. Infect. 2020, 21, 540–546. [Google Scholar] [CrossRef]
- Bunduki, G.K.; Mukululi, M.P.; Masumbuko, C.K.; Uwonda, S.A. Compliance of antibiotics used for surgical site infection prophylaxis among patients undergoing surgery in a Congolese teaching hospital. Infect. Prev. Pract. 2020, 2, 100075. [Google Scholar] [CrossRef]
- Bediako-Bowan, A.A.A.; Owusu, E.; Labi, A.-K.; Obeng-Nkrumah, N.; Sunkwa-Mills, G.; Bjerrum, S.; Opintan, J.A.; Bannerman, C.; Mølbak, K.; Kurtzhals, J.A.L.; et al. Antibiotic use in surgical units of selected hospitals in Ghana: A multi-centre point prevalence survey. BMC Public Health 2019, 19, 797. [Google Scholar] [CrossRef]
- Ankrah, D.; Owusu, H.; Aggor, A.; Osei, A.; Ampomah, A.; Harrison, M.; Nelson, F.; Aboagye, G.O.; Ekpale, P.; Laryea, J.; et al. Point Prevalence Survey of Antimicrobial Utilization in Ghana’s Premier Hospital: Implications for Antimicrobial Stewardship. Antibiotics 2021, 10, 1528. [Google Scholar] [CrossRef]
- Opanga, S.A.; Mwangombe, N.J.; Okalebo, F.A.; Godman, B.; Oluka, M.; Kuria, K.A.M. Determinants of the Effectiveness of Antimicrobial Prophylaxis among Neurotrauma Patients at a Referral Hospital in Kenya: Findings and Implications. Infect. Dis. Prev. Med. 2017, 5, 169. [Google Scholar]
- Talaam, R.C.; Abungana, M.M.; Ooko, P.B. An antibiotic audit of the surgical department at a rural hospital in Western Kenya. Pan Afr. Med. J. 2018, 29, 219. [Google Scholar] [CrossRef]
- Nsofor, C.; Es, A.; Obijuru, C.; Ohalete, C.; Ukwandu, N. Prevalence of Antimicrobial Use in Major Hospitals in Owerri, Nigeria. EC Microbiol. 2016, 3, 522–527. [Google Scholar]
- Oduyebo, O.; Olayinka, A.; Iregbu, K.; Versporten, A.; Goossens, H.; Nwajiobi-Princewill, P.; Jimoh, O.; Ige, T.; Aigbe, A.; Ola-Bello, O.; et al. A point prevalence survey of antimicrobial prescribing in four Nigerian Tertiary Hospitals. Ann. Trop. Pathol. 2017, 8, 42–46. [Google Scholar] [CrossRef]
- Fowotade, A.; Fasuyi, T.; Aigbovo, O.; Versporten, A.; Adekanmbi, O.; Akinyemi, O.; Goossens, H.; Kehinde, A.; Oduyebo, O. Point Prevalence Survey of Antimicrobial Prescribing in a Nigerian Hospital: Findings and Implications on Antimicrobial Resistance. West Afr. J. Med. 2020, 37, 216–220. [Google Scholar]
- Mwita, J.C.; Souda, S.; Magafu, M.; Massele, A.; Godman, B.; Mwandri, M. Prophylactic antibiotics to prevent surgical site infections in Botswana: Findings and implications. Hosp. Pract. 2018, 46, 97–102. [Google Scholar] [CrossRef]
- Skosana, P.; Schellack, N.; Godman, B.; Kurdi, A.; Bennie, M.; Kruger, D.; Meyer, J. A point prevalence survey of antimicrobial utilisation patterns and quality indices amongst hospitals in South Africa; findings and implications. Expert Rev. Anti-Infect. Ther. 2021, 19, 1353–1366. [Google Scholar] [CrossRef]
- Kruger, D.; Dlamini, N.; Meyer, J.; Godman, B.; Kurdi, A.; Lennon, M.; Bennie, M.; Schellack, N. Development of a web-based application to improve data collection of antimicrobial utilization in the public health care system in South Africa. Hosp. Pract. 2021, 49, 184–193. [Google Scholar] [CrossRef]
- Bashar, M.A.; Miot, J.; Shoul, E.; van Zyl, R.L. Impact of an antibiotic stewardship programme in a surgical setting. S. Afr. J. Infect. Dis. 2021, 36, 307. [Google Scholar] [CrossRef]
- Brink, A.J.; Messina, A.P.; Feldman, C.; Richards, G.A.; Becker, P.J.; Goff, D.A.; Bauer, K.A.; Nathwani, D.; van den Bergh, D.; on behalf of the Netcare Antimicrobial Stewardship Study Alliance. Antimicrobial stewardship across 47 South African hospitals: An implementation study. Lancet Infect. Dis. 2016, 16, 1017–1025. [Google Scholar] [CrossRef]
- Boyles, T.H.; Naicker, V.; Rawoot, N.; Raubenheimer, P.J.; Eick, B.; Mendelson, M. Sustained reduction in antibiotic consumption in a South African public sector hospital; Four year outcomes from the Groote Schuur Hospital antibiotic stewardship program. S. Afr. Med. J. 2017, 107, 115–118. [Google Scholar] [CrossRef]
- Gebretekle, G.B.; Mariam, D.H.; Taye, W.A.; Fentie, A.M.; Degu, W.A.; Alemayehu, T.; Beyene, T.; Libman, M.; Fenta, T.G.; Yansouni, C.P.; et al. Half of Prescribed Antibiotics Are Not Needed: A Pharmacist-Led Antimicrobial Stewardship Intervention and Clinical Outcomes in a Referral Hospital in Ethiopia. Front. Public Health 2020, 8, 109. [Google Scholar] [CrossRef]
- Messina, A.P.; van den Bergh, D.; Goff, D.A. Antimicrobial Stewardship with Pharmacist Intervention Improves Timeliness of Antimicrobials Across Thirty-three Hospitals in South Africa. Infect. Dis. Ther. 2015, 4 (Suppl. S1), 5–14. [Google Scholar] [CrossRef] [PubMed]
- Labi, A.-K.; Obeng-Nkrumah, N.; Nartey, E.T.; Bjerrum, S.; Adu-Aryee, N.A.; Ofori-Adjei, Y.A.; Yawson, A.E.; Newman, M.J. Antibiotic use in a tertiary healthcare facility in Ghana: A point prevalence survey. Antimicrob. Resist. Infect. Control 2018, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Nakwatumbah, S.; Kibuule, D.; Godman, B.; Haakuria, V.; Kalemeera, F.; Baker, A.; Mubita, M. Compliance to guidelines for the prescribing of antibiotics in acute infections at Namibia’s national referral hospital: A pilot study and the implications. Expert Rev. Anti-Infect. Ther. 2017, 15, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Dlamini, N.N.; Meyer, J.C.; Kruger, D.; Kurdi, A.; Godman, B.; Schellack, N. Feasibility of using point prevalence surveys to assess antimicrobial utilisation in public hospitals in South Africa: A pilot study and implications. Hosp. Pract. 2019, 47, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Amdany, H.K.; Mcmillan, M. Metronidazole intravenous formulation use in in-patients in Kapkatet District Hospital, Kenya: A best practice implementation project. JBI Evid. Synth. 2014, 12, 419–432. [Google Scholar] [CrossRef]
- Umar, L.W.; Isah, A.; Musa, S.; Umar, B. Prescribing pattern and antibiotic use for hospitalized children in a Northern Nigerian Teaching Hospital. Ann. Afr. Med. 2018, 17, 26–32. [Google Scholar] [CrossRef]
- Amaha, N.D.; Berhe, Y.H.; Kaushik, A. Assessment of inpatient antibiotic use in Halibet National Referral Hospital using WHO indicators: A retrospective study. BMC Res. Notes 2018, 11, 904. [Google Scholar] [CrossRef]
- van den Bergh, D.; Messina, A.P.; Goff, D.A.; van Jaarsveld, A.; Coetzee, R.; de Wet, Y.; Bronkhorst, E.; Brink, A.; Mendelson, M.; Richards, G.A.; et al. A pharmacist-led prospective antibiotic stewardship intervention improves compliance to community-acquired pneumonia guidelines in 39 public and private hospitals across South Africa. Int. J. Antimicrob. Agents 2020, 56, 106189. [Google Scholar] [CrossRef]
- Niaz, Q.; Godman, B.; Campbell, S.; Kibuule, D. Compliance to prescribing guidelines among public health care facilities in Namibia; findings and implications. Int. J. Clin. Pharm. 2020, 42, 1227–1236. [Google Scholar] [CrossRef]
- Maina, M.; Mwaniki, P.; Odira, E.; Kiko, N.; McKnight, J.; Schultsz, C.; English, M.; Tosas-Auguet, O. Antibiotic use in Kenyan public hospitals: Prevalence, appropriateness and link to guideline availability. Int. J. Infect. Dis. 2020, 99, 10–18. [Google Scholar] [CrossRef]
- Ayieko, P.; Irimu, G.; Ogero, M.; Mwaniki, P.; Malla, L.; Julius, T.; Chepkirui, M.; Mbevi, G.; Oliwa, J.; Agweyu, A.; et al. Effect of enhancing audit and feedback on uptake of childhood pneumonia treatment policy in hospitals that are part of a clinical network: A cluster randomized trial. Implement. Sci. 2019, 14, 20. [Google Scholar] [CrossRef]
- Suliman, S.M.; Yousef, B.A.; Hamadelnil, A.A. Impact of guidelines implementation for the rational use of prophylactic antibiotics in elective cesarean sections at Elqutainah Teaching Hospital. J. Fam. Med. Prim. Care 2020, 9, 162–167. [Google Scholar]
- Brink, A.J.; Messina, A.P.; Feldman, C.; Richards, G.A.; van den Bergh, D. From guidelines to practice: A pharmacist-driven prospective audit and feedback improvement model for peri-operative antibiotic prophylaxis in 34 South African hospitals. J. Antimicrob. Chemother. 2017, 72, 1227–1234. [Google Scholar] [CrossRef]
- Sefah, I.A.; Essah, D.O.; Kurdi, A.; Sneddon, J.; Alalbila, T.M.; Kordorwu, H.; Godman, B. Assessment of adherence to pneumonia guidelines and its determinants in an ambulatory care clinic in Ghana: Findings and implications for the future. JAC Antimicrob. Resist. 2021, 3, dlab080. [Google Scholar] [CrossRef]
- Nambasa, V.; Ndagije, H.B.; Serwanga, A.; Manirakiza, L.; Atuhaire, J.; Nakitto, D.; Kiguba, R.; Figueras, A. Prescription of Levofloxacin and Moxifloxacin in Select Hospitals in Uganda: A Pilot Study to Assess Guideline Concordance. Antibiotics 2020, 9, 439. [Google Scholar] [CrossRef]
- Junaid, E.; Jenkins, L.; Swanepoel, H.; North, Z.; Gould, T. Antimicrobial stewardship in a rural regional hospital—Growing a positive culture. S. Afr. Med. J. 2018, 108, 546–550. [Google Scholar] [CrossRef]
- Lester, R.; Haigh, K.; Wood, A.; MacPherson, E.E.; Maheswaran, H.; Bogue, P.; Hanger, S.; Kalizang’oma, A.; Srirathan, V.; Kulapani, D.; et al. Sustained Reduction in Third-generation Cephalosporin Usage in Adult Inpatients Following Introduction of an Antimicrobial Stewardship Program in a Large, Urban Hospital in Malawi. Clin. Infect. Dis. 2020, 71, e478–e486. [Google Scholar] [CrossRef]
- Ntumba, P.; Mwangi, C.; Barasa, J.; Aiken, A.; Kubilay, Z.; Allegranzi, B. Multimodal approach for surgical site infection prevention—Results from a pilot site in Kenya. Antimicrob. Resist. Infect. Control 2015, 4, P87. [Google Scholar] [CrossRef]
- Allegranzi, B.; Aiken, A.M.; Kubilay, N.Z.; Nthumba, P.; Barasa, J.; Okumu, G.; Mugarura, R.; Elobu, A.E.; Jombwe, J.; Maimbo, M.; et al. A multimodal infection control and patient safety intervention to reduce surgical site infections in Africa: A multicentre, before-after, cohort study. Lancet Infect. Dis. 2018, 18, 507–515. [Google Scholar] [CrossRef]
- Chigome, A.K.; Matlala, M.; Godman, B.; Meyer, J.C. Availability and Use of Therapeutic Interchange Policies in Managing Antimicrobial Shortages among South African Public Sector Hospitals; Findings and Implications. Antibiotics 2020, 9, 4. [Google Scholar] [CrossRef]
- Ngonzi, J.; Bebell, L.M.; Boatin, A.A.; Owaraganise, A.; Tiibajuka, L.; Fajardo, Y.; Lugobe, H.M.; Wylie, B.J.; Jacquemyn, Y.; Obua, C.; et al. Impact of an educational intervention on WHO surgical safety checklist and pre-operative antibiotic use at a referral hospital in southwestern Uganda. Int. J. Qual. Health Care 2021, 33, mzab089. [Google Scholar] [CrossRef] [PubMed]
- BSAC. Antimicrobial Stewardship from Principles to Practice. 2018. Available online: https://www.bsac.org.uk/antimicrobialstewardshipebook/BSAC-AntimicrobialStewardship-FromPrinciplestoPractice-eBook.pdf (accessed on 14 November 2022).
- Olaoye, O.; Tuck, C.; Khor, W.P.; McMenamin, R.; Hudson, L.; Northall, M.; Panford-Quainoo, E.; Asima, D.M.; Ashiru-Oredope, D. Improving Access to Antimicrobial Prescribing Guidelines in 4 African Countries: Development and Pilot Implementation of an App and Cross-Sectional Assessment of Attitudes and Behaviour Survey of Healthcare Workers and Patients. Antibiotics 2020, 9, 555. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, U.; Syed Sulaiman, S.A.; Adesiyun, A.G. Impact of pharmacist-led antibiotic stewardship interventions on compliance with surgical antibiotic prophylaxis in obstetric and gynecologic surgeries in Nigeria. PLoS ONE 2019, 14, e0213395. [Google Scholar] [CrossRef] [PubMed]
- Curtis, C.E.; Al Bahar, F.; Marriott, J.F. The effectiveness of computerised decision support on antibiotic use in hospitals: A systematic review. PLoS ONE 2017, 12, e0183062. [Google Scholar] [CrossRef] [PubMed]
- Zahlanie, Y.; Mang, N.S.; Lin, K.; Hynan, L.S.; Prokesch, B.C. Improved Antibiotic Prescribing Practices for Respiratory Infections Through Use of Computerized Order Sets and Educational Sessions in Pediatric Clinics. Open Forum Infect. Dis. 2021, 8, ofaa601. [Google Scholar] [CrossRef]
- Holstiege, J.; Mathes, T.; Pieper, D. Effects of computer-aided clinical decision support systems in improving antibiotic prescribing by primary care providers: A systematic review. J. Am. Med. Inform. Assoc. 2015, 22, 236–242. [Google Scholar] [CrossRef]
- Sulis, G.; Sayood, S.; Katukoori, S.; Bollam, N.; George, I.; Yaeger, L.H.; Chavez, M.A.; Tetteh, E.; Yarrabelli, S.; Pulcini, C.; et al. Exposure to World Health Organization’s AWaRe antibiotics and isolation of multidrug resistant bacteria: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2022, 28, 1193–1202. [Google Scholar] [CrossRef]
- Charani, E.; Smith, I.; Skodvin, B.; Perozziello, A.; Lucet, J.C.; Lescure, F.X.; Birgand, G.; Poda, A.; Ahmad, R.; Singh, S.; et al. Investigating the cultural and contextual determinants of antimicrobial stewardship programmes across low-, middle- and high-income countries-A qualitative study. PLoS ONE 2019, 14, e0209847. [Google Scholar] [CrossRef]
- Kakkar, A.K.; Shafiq, N.; Singh, G.; Ray, P.; Gautam, V.; Agarwal, R.; Muralidharan, J.; Arora, P. Antimicrobial Stewardship Programs in Resource Constrained Environments: Understanding and Addressing the Need of the Systems. Front. Public Health 2020, 8, 140. [Google Scholar] [CrossRef]
- Pokharel, S.; Raut, S.; Adhikari, B. Tackling antimicrobial resistance in low-income and middle-income countries. BMJ Glob. Health 2019, 4, e002104. [Google Scholar] [CrossRef]
- Björkhem-Bergman, L.; Andersén-Karlsson, E.; Laing, R.; Diogene, E.; Melien, O.; Jirlow, M.; Malmström, R.E.; Vogler, S.; Godman, B.; Gustafsson, L.L. Interface management of pharmacotherapy. Joint hospital and primary care drug recommendations. Eur. J. Clin. Pharmacol. 2013, 69 (Suppl. S1), 73–78. [Google Scholar] [CrossRef]
- Yoon, C.H.; Ritchie, S.R.; Duffy, E.J.; Thomas, M.G.; McBride, S.; Read, K.; Chen, R.; Humphrey, G. Impact of a smartphone app on prescriber adherence to antibiotic guidelines in adult patients with community acquired pneumonia or urinary tract infections. PLoS ONE 2019, 14, e0211157. [Google Scholar] [CrossRef]
- Eriksen, J.; Gustafsson, L.L.; Ateva, K.; Bastholm-Rahmner, P.; Ovesjö, M.L.; Jirlow, M.; Juhasz-Haverinen, M.; Lärfars, G.; Malmström, R.E.; Wettermark, B.; et al. High adherence to the ‘Wise List’ treatment recommendations in Stockholm: A 15-year retrospective review of a multifaceted approach promoting rational use of medicines. BMJ Open 2017, 7, e014345. [Google Scholar] [CrossRef]
- Gustafsson, L.L.; Wettermark, B.; Godman, B.; Andersén-Karlsson, E.; Bergman, U.; Hasselström, J.; Hensjö, L.-O.; Hjemdahl, P.; Jägre, I.; Julander, M.; et al. The ‘wise list’- a comprehensive concept to select, communicate and achieve adherence to recommendations of essential drugs in ambulatory care in Stockholm. Basic Clin. Pharmacol. Toxicol. 2011, 108, 224–233. [Google Scholar] [CrossRef]
- Foxlee, N.D.; Townell, N.; Heney, C.; McIver, L.; Lau, C.L. Strategies Used for Implementing and Promoting Adherence to Antibiotic Guidelines in Low- and Lower-Middle-Income Countries: A Systematic Review. Trop. Med. Infect. Dis. 2021, 6, 166. [Google Scholar] [CrossRef]
- Commonwealth Partnerships for Antimicrobial Stewardship Programme (CwPAMS). CwPAMS Antimicrobial Prescribing App—Antimicrobial Stewardship Mobile Application. Available online: https://commonwealthpharmacy.org/cwpams-ams-app/ (accessed on 15 November 2022).
- Mashaba, T.P.; Matlala, M.; Godman, B.; Meyer, J.C. Implementation and monitoring of decisions by pharmacy and therapeutics committees in South African public sector hospitals. Expert Rev. Clin. Pharmacol. 2019, 12, 159–168. [Google Scholar] [CrossRef]
- Matlala, M.; Gous, A.G.S.; Meyer, J.C.; Godman, B. Formulary Management Activities and Practice Implications Among Public Sector Hospital Pharmaceutical and Therapeutics Committees in a South African Province. Front. Pharmacol. 2020, 11, 1267. [Google Scholar] [CrossRef]
- Fadare, J.O.; Ogunleye, O.; Obiako, R.; Orubu, S.; Enwere, O.; Ajemigbitse, A.A.; Meyer, J.C.; Enato, E.; Massele, A.; Godman, B.; et al. Drug and therapeutics committees in Nigeria: Evaluation of scope and functionality. Expert Rev. Clin. Pharmacol. 2018, 11, 1255–1262. [Google Scholar] [CrossRef]
- Zulu, A.; Matafwali, S.K.; Banda, M.; Mudenda, S. Assessment of knowledge, attitude and practices on antibiotic resistance among undergraduate medical students in the school of medicine at the University of Zambia. Int. J. Basic Clin. Pharmacol. 2020, 9, 263–270. [Google Scholar] [CrossRef]
- Nisabwe, L.; Brice, H.; Umuhire, M.C.; Gwira, O.; Harelimana, J.D.D.; Nzeyimana, Z.; Sebatunzi, O.R.; Rusingiza, E.K.; Hahirwa, I.; Muvunyi, C.M. Knowledge and attitudes towards antibiotic use and resistance among undergraduate healthcare students at University of Rwanda. J. Pharm. Policy Pract. 2020, 13, 7. [Google Scholar] [CrossRef]
- Wasserman, S.; Potgieter, S.; Shoul, E.; Constant, D.; Stewart, A.; Mendelson, M.; Boyles, T.H. South African medical students’ perceptions and knowledge about antibiotic resistance and appropriate prescribing: Are we providing adequate training to future prescribers? S. Afr. Med. J. 2017, 107, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Lubwama, M.; Onyuka, J.; Ayazika, K.T.; Ssetaba, L.J.; Siboko, J.; Daniel, O.; Mushi, M.F. Knowledge, attitudes, and perceptions about antibiotic use and antimicrobial resistance among final year undergraduate medical and pharmacy students at three universities in East Africa. PLoS ONE 2021, 16, e0251301. [Google Scholar] [CrossRef] [PubMed]
- Etando, A.; Amu, A.A.; Haque, M.; Schellack, N.; Kurdi, A.; Alrasheedy, A.A.; Timoney, A.; Mwita, J.C.; Rwegerera, G.M.; Patrick, O.; et al. Challenges and Innovations Brought about by the COVID-19 Pandemic Regarding Medical and Pharmacy Education Especially in Africa and Implications for the Future. Healthcare 2021, 9, 1722. [Google Scholar] [CrossRef] [PubMed]
- Sithole, T.; Mahlangu, G.; Walker, S.; Salek, S. Regulatory Authority Evaluation of the Effectiveness and Efficiency of the ZaZiBoNa Collaborative Medicines Registration Initiative: The Way Forward. Front. Med. 2022, 9, 898743. [Google Scholar] [CrossRef]
- WHO. Launch of the Lomé Initiative. 2020. Available online: https://www.who.int/dg/speeches/detail/launch-of-the-lom%C3%A9-initiative (accessed on 14 November 2022).
- Africa Centres for Disease Control and Prevention and Center for Disease Dynamics, Economics & Policy. African Antibiotic Treatment Guidelines for Common Bacterial Infections and Syndromes—Recommended Antibiotic Treatments in Adult Patients. 2021. Available online: https://africaguidelines.cddep.org/wp-content/uploads/2021/11/Quick-Reference-Guide_Adults_English.pdf (accessed on 15 November 2022).
- GASPH. Global Antimicrobial Stewardship Partnership Hub. 2022. Available online: https://global-asp-hub.com/ (accessed on 15 November 2022).
- Lorencatto, F.; Charani, E.; Sevdalis, N.; Tarrant, C.; Davey, P. Driving sustainable change in antimicrobial prescribing practice: How can social and behavioural sciences help? J. Antimicrob. Chemother. 2018, 73, 2613–2624. [Google Scholar] [CrossRef]
- Schellack, N.; Bronkhorst, E.; Coetzee, R.; Godman, B.; Gous, A.G.S.; Kolman, S.; Labuschagne, Q.; Malan, L.; Messina, A.P.; Naested, C.; et al. SASOCP position statement on the pharmacist’s role in antibiotic stewardship 2018. S. Afr. J. Infect. Dis. 2018, 33, 28–35. [Google Scholar] [CrossRef]
- Ogunleye, O.O.; Fadare, J.O.; Yinka-Ogunleye, A.F.; Anand Paramadhas, B.D.; Godman, B. Determinants of antibiotic prescribing among doctors in a Nigerian urban tertiary hospital. Hosp. Pract. 2019, 47, 53–58. [Google Scholar] [CrossRef]
- Fadare, J.O.; Bankole, I.; Babatola, A.; Simeon Olatunya, O.; Aina, F.; Godman, B. Adherence to WHO Criteria on Drug Promotion Literature: An Exploratory Study from a Tertiary Healthcare Facility in South-West Nigeria. Hosp. Pharm. 2022, 00185787221123217. [Google Scholar] [CrossRef]
- Modisakeng, C.; Matlala, M.; Godman, B.; Meyer, J.C. Medicine shortages and challenges with the procurement process among public sector hospitals in South Africa; findings and implications. BMC Health Serv. Res. 2020, 20, 234. [Google Scholar] [CrossRef]
- Fürst, J.; Čižman, M.; Mrak, J.; Kos, D.; Campbell, S.; Coenen, S.; Gustafsson, L.L.; Fürst, L.; Godman, B. The influence of a sustained multifaceted approach to improve antibiotic prescribing in Slovenia during the past decade: Findings and implications. Expert Rev. Anti-Infect. Ther. 2015, 13, 279–289. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. 2016. Available online: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (accessed on 14 November 2022).
- Sartelli, M.; Hardcastle, T.C.; Catena, F.; Chichom-Mefire, A.; Coccolini, F.; Dhingra, S.; Haque, M.; Hodonou, A.; Iskandar, K.; Labricciosa, F.; et al. Antibiotic Use in Low and Middle-Income Countries and the Challenges of Antimicrobial Resistance in Surgery. Antibiotics 2020, 9, 497. [Google Scholar] [CrossRef]
- Tadesse, B.T.; Ashley, E.A.; Ongarello, S.; Havumaki, J.; Wijegoonewardena, M.; González, I.J.; Dittrich, S. Antimicrobial resistance in Africa: A systematic review. BMC Infect. Dis. 2017, 17, 616. [Google Scholar] [CrossRef]
- Bell, B.G.; Schellevis, F.; Stobberingh, E.; Goossens, H.; Pringle, M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect. Dis. 2014, 14, 13. [Google Scholar] [CrossRef]
- Llor, C.; Bjerrum, L. Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014, 5, 229–241. [Google Scholar] [CrossRef]
- Collignon, P.; Beggs, J.J.; Walsh, T.R.; Gandra, S.; Laxminarayan, R. Anthropological and socioeconomic factors contributing to global antimicrobial resistance: A univariate and multivariable analysis. Lancet Planet. Health 2018, 2, e398–e405. [Google Scholar] [CrossRef]
- WHO. WHO Methodology for Point Prevalence Survey on Antibiotic Use in Hospitals, Version 1.1. 2018. Available online: https://www.who.int/publications/i/item/WHO-EMP-IAU-2018.01 (accessed on 14 November 2022).
- Engler, D.; Meyer, J.C.; Schellack, N.; Kurdi, A.; Godman, B. Compliance with South Africa’s Antimicrobial Resistance National Strategy Framework: Are we there yet? J. Chemother. 2021, 33, 21–31. [Google Scholar] [CrossRef]
- de Wit, T.F.R.; Janssens, W.; Antwi, M.; Milimo, E.; Mutegi, N.; Marwa, H.; Ndili, N.; Owino, W.; Waiyaiya, E.; Garcia Roja, D.C.; et al. Digital health systems strengthening in Africa for rapid response to COVID-19. Front. Health Serv. 2022, 2, 2035–2043. [Google Scholar] [CrossRef]
- Godman, B.; Grobler, C.; Van-De-Lisle, M.; Wale, J.; Barbosa, W.B.; Massele, A.; Opondo, P.; Petrova, G.; Tachkov, K.; Sefah, I.; et al. Pharmacotherapeutic interventions for bipolar disorder type II: Addressing multiple symptoms and approaches with a particular emphasis on strategies in lower and middle-income countries. Expert Opin. Pharmacother. 2019, 20, 2237–2255. [Google Scholar] [CrossRef]
- Ogunleye, O.O.; Basu, D.; Mueller, D.; Sneddon, J.; Seaton, R.A.; Yinka-Ogunleye, A.F.; Wamboga, J.; Miljković, N.; Mwita, J.C.; Rwegerera, G.M.; et al. Response to the Novel Corona Virus (COVID-19) Pandemic Across Africa: Successes, Challenges, and Implications for the Future. Front. Pharmacol. 2020, 11, 1205. [Google Scholar] [CrossRef]
- Godman, B.; Haque, M.; Abubakar, A.R.; Ogunleye, O.O.; Sani, I.H.; Sefah, I.; Kurdi, A.; Islam, S. Changes in Availability, Utilization, and Prices of Medicines and Protection Equipment for COVID-19 in an Urban Population of Northern Nigeria. J. Res. Pharm. Pract. 2021, 10, 17–22. [Google Scholar] [CrossRef]
- Godman, B.; Basu, D.; Pillay, Y.; Mwita, J.C.; Rwegerera, G.M.; Paramadhas, B.D.A.; Tiroyakgosi, C.; Okwen, P.M.; Niba, L.L.; Nonvignon, J.; et al. Review of Ongoing Activities and Challenges to Improve the Care of Patients With Type 2 Diabetes Across Africa and the Implications for the Future. Front. Pharmacol. 2020, 11, 108. [Google Scholar] [CrossRef] [PubMed]
- Godman, B.; Basu, D.; Pillay, Y.; Almeida, P.H.R.F.; Mwita, J.C.; Rwegerera, G.M.; Paramadhas, B.D.A.; Tiroyakgosi, C.; Patrick, O.; Niba, L.L.; et al. Ongoing and planned activities to improve the management of patients with Type 1 diabetes across Africa; implications for the future. Hosp. Pract. 2020, 48, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.; Salem, A.; Oortwijn, W.; Hill, R.; Godman, B. Mapping of Current Obstacles for Rationalizing Use of Medicines (CORUM) in Europe: Current Situation and Potential Solutions. Front. Pharmacol. 2020, 11, 144. [Google Scholar] [CrossRef] [PubMed]
- Saleem, Z.; Hassali, M.A.; Godman, B.; Versporten, A.; Hashmi, F.K.; Saeed, H.; Saleem, F.; Salman, M.; Rehman, I.U.; Khan, T.M. Point prevalence surveys of antimicrobial use: A systematic review and the implications. Expert Rev. Anti-Infect. Ther. 2020, 18, 897–910. [Google Scholar] [CrossRef] [PubMed]
- WHO. Anatomical Therapeutic Chemical (ATC) Classification. 2021. Available online: https://www.who.int/tools/atc-ddd-toolkit/atc-classification (accessed on 10 November 2022).
- Wettermark, B.; Godman, B.; Jacobsson, B.; Haaijer-Ruskamp, F.M. Soft regulations in pharmaceutical policy making: An overview of current approaches and their consequences. Appl. Health Econ. Health Policy 2009, 7, 137–147. [Google Scholar] [CrossRef]
- Matsitse, T.B.; Helberg, E.; Meyer, J.C.; Godman, B.; Massele, A.; Schellack, N. Compliance with the primary health care treatment guidelines and the essential medicines list in the management of sexually transmitted infections in correctional centres in South Africa: Findings and implications. Expert Rev. Anti-Infect. Ther. 2017, 15, 963–972. [Google Scholar] [CrossRef]
- Alrasheedy, A.A.; Alsalloum, M.A.; Almuqbil, F.A.; Almuzaini, M.A.; Aba Alkhayl, B.S.; Albishri, A.S.; Alharbi, F.F.; Alharbi, S.R.; Alodhayb, A.K.; Alfadl, A.A.; et al. The impact of law enforcement on dispensing antibiotics without prescription: A multi-methods study from Saudi Arabia. Expert Rev. Anti-Infect. Ther. 2020, 18, 87–97. [Google Scholar] [CrossRef]
- Mendelson, M.; Brink, A.; Gouws, J.; Mbelle, N.; Naidoo, V.; Pople, T.; Schellack, N.; van Vuuren, M.; Rees, H.; Banoo, S.; et al. The One Health stewardship of colistin as an antibiotic of last resort for human health in South Africa. Lancet Infect. Dis. 2018, 18, e288–e294. [Google Scholar] [CrossRef]
- Godman, B.; McCabe, H.; Leong, T.D.; Mueller, D.; Martin, A.P.; Hoxha, I.; Mwita, J.C.; Rwegerera, G.M.; Massele, A.; Costa, J.D.O.; et al. Fixed dose drug combinations—are they pharmacoeconomically sound? Findings and implications especially for lower- and middle-income countries. Expert Rev. Pharmacoecon. Outcomes Res. 2020, 20, 1–26. [Google Scholar] [CrossRef]
- Godman, B.; Wettermark, B.; van Woerkom, M.; Fraeyman, J.; Alvarez-Madrazo, S.; Berg, C.; Bishop, I.; Bucsics, A.; Campbell, S.; Finlayson, A.E.; et al. Multiple policies to enhance prescribing efficiency for established medicines in Europe with a particular focus on demand-side measures: Findings and future implications. Front. Pharmacol. 2014, 5, 106. [Google Scholar] [CrossRef]
- WHO. Monitoring the Building Blocks of Health Systems: A Handbook of Indicators and Their Measurement Strategies. 2010. Available online: https://apps.who.int/iris/bitstream/handle/10665/258734/9789241564052-eng.pdf (accessed on 10 November 2022).
- Campbell, S.M.; Roland, M.O.; Buetow, S.A. Defining quality of care. Soc. Sci. Med. 2000, 51, 1611–1625. [Google Scholar] [CrossRef]
- NHS England and NHS Improvement. A Model for Measuring Quality Care. 2022. Available online: https://www.england.nhs.uk/wp-content/uploads/2022/02/qsir-measuring-quality-care.pdf (accessed on 10 November 2022).
- van den Bosch, C.M.; Hulscher, M.E.; Natsch, S.; Wille, J.; Prins, J.M.; Geerlings, S.E. Applicability of generic quality indicators for appropriate antibiotic use in daily hospital practice: A cross-sectional point-prevalence multicenter study. Clin. Microbiol. Infect. 2016, 22, 888.e1–888.e9. [Google Scholar] [CrossRef]
- van den Bosch, C.M.; Geerlings, S.E.; Natsch, S.; Prins, J.M.; Hulscher, M.E. Quality indicators to measure appropriate antibiotic use in hospitalized adults. Clin. Infect. Dis. 2015, 60, 281–291. [Google Scholar] [CrossRef]
- Kallen, M.C.; Prins, J.M. A Systematic Review of Quality Indicators for Appropriate Antibiotic Use in Hospitalized Adult Patients. Infect. Dis. Rep. 2017, 9, 6821. [Google Scholar] [CrossRef]
- Wambale, J.M.I.J.; Mathe, D.M.; Kavuo, S.K.; Kikuni, T. Point prevalence study of antibiotic use in hospitals in Butembo. Int. J. Med. Med. Sci. 2016, 8, 133–139. [Google Scholar]
- Labi, A.K.; Obeng-Nkrumah, N.; Sunkwa-Mills, G.; Bediako-Bowan, A.; Akufo, C.; Bjerrum, S.; Owusu, E.; Enweronu-Laryea, C.; Opintan, J.A.; Kurtzhals, J.A.L.; et al. Antibiotic prescribing in paediatric inpatients in Ghana: A multi-centre point prevalence survey. BMC Pediatr. 2018, 18, 391. [Google Scholar] [CrossRef]
- Garcia-Vello, P.; Brobbey, F.; Gonzalez-Zorn, B.; Setsoafia Saba, C.K. A cross-sectional study on antibiotic prescription in a teaching hospital in Ghana. Pan Afr. Med. J. 2020, 35, 12. [Google Scholar] [CrossRef]
- Umeokonkwo, C.D.; Madubueze, U.C.; Onah, C.K.; Okedo-Alex, I.N.; Adeke, A.S.; Versporten, A.; Goossens, H.; Igwe-Okomiso, D.; Okeke, K.; Azuogu, B.N.; et al. Point prevalence survey of antimicrobial prescription in a tertiary hospital in South East Nigeria: A call for improved antibiotic stewardship. J. Glob. Antimicrob. Resist. 2019, 17, 291–295. [Google Scholar] [CrossRef]
- Chioma, O. Rational Use of Antibiotics—A Point Prevalence Study Carried out at a Tertiary Hospital in South-South Nigeria. Int. J. Trop. Dis. Health 2020, 41, 39–47. [Google Scholar] [CrossRef]
- Masich, A.M.; Vega, A.D.; Callahan, P.; Herbert, A.; Fwoloshi, S.; Zulu, P.M.; Chanda, D.; Chola, U.; Mulenga, L.; Hachaambwa, L.; et al. Antimicrobial usage at a large teaching hospital in Lusaka, Zambia. PLoS ONE 2020, 15, e0228555. [Google Scholar] [CrossRef]
| Timescale | Potential Strategies |
|---|---|
| Short to Medium Term (e.g., 1 to 5 years) | Health authorities/Governments (if not already instigated)
|
| Long Term (5 to 10 years) | Potential long-term strategies include:
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleem, Z.; Godman, B.; Cook, A.; Khan, M.A.; Campbell, S.M.; Seaton, R.A.; Siachalinga, L.; Haseeb, A.; Amir, A.; Kurdi, A.; et al. Ongoing Efforts to Improve Antimicrobial Utilization in Hospitals among African Countries and Implications for the Future. Antibiotics 2022, 11, 1824. https://doi.org/10.3390/antibiotics11121824
Saleem Z, Godman B, Cook A, Khan MA, Campbell SM, Seaton RA, Siachalinga L, Haseeb A, Amir A, Kurdi A, et al. Ongoing Efforts to Improve Antimicrobial Utilization in Hospitals among African Countries and Implications for the Future. Antibiotics. 2022; 11(12):1824. https://doi.org/10.3390/antibiotics11121824
Chicago/Turabian StyleSaleem, Zikria, Brian Godman, Aislinn Cook, Muhammad Arslan Khan, Stephen M. Campbell, Ronald Andrew Seaton, Linda Siachalinga, Abdul Haseeb, Afreenish Amir, Amanj Kurdi, and et al. 2022. "Ongoing Efforts to Improve Antimicrobial Utilization in Hospitals among African Countries and Implications for the Future" Antibiotics 11, no. 12: 1824. https://doi.org/10.3390/antibiotics11121824
APA StyleSaleem, Z., Godman, B., Cook, A., Khan, M. A., Campbell, S. M., Seaton, R. A., Siachalinga, L., Haseeb, A., Amir, A., Kurdi, A., Mwita, J. C., Sefah, I. A., Opanga, S. A., Fadare, J. O., Ogunleye, O. O., Meyer, J. C., Massele, A., Kibuule, D., Kalungia, A. C., ... Moore, C. E. (2022). Ongoing Efforts to Improve Antimicrobial Utilization in Hospitals among African Countries and Implications for the Future. Antibiotics, 11(12), 1824. https://doi.org/10.3390/antibiotics11121824












