Multidisciplinary Collaboration for the Optimization of Antibiotic Prescription: Analysis of Clinical Cases of Pneumonia between Emergency, Internal Medicine, and Pharmacy Services
Abstract
1. Introduction
2. Objective
3. Materials and Methods
3.1. Treatment Revision Procedure
3.2. The Items and the Evaluation Criteria Were:
3.2.1. Indication of Antimicrobial Treatment
3.2.2. Choice of Antimicrobial Agent
3.2.3. Timing of Administration of the First Dose
3.2.4. Dosage and Frequency of Administration
3.2.5. De-Escalation/Oral Sequencing
3.2.6. Treatment Duration
3.2.7. Monitoring of Efficacy and Adverse Effects
3.3. Statistical Analysis
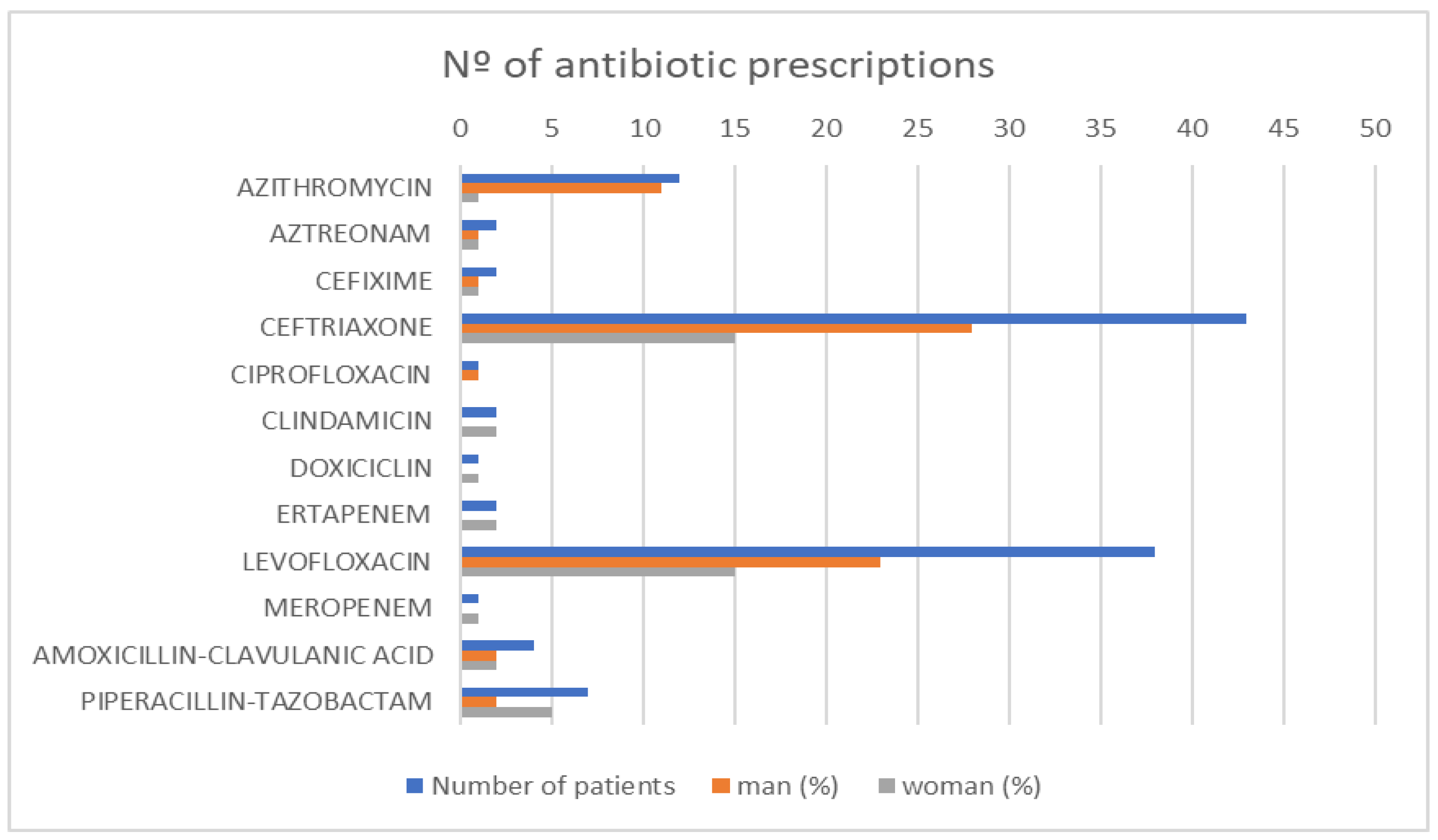
4. Results
5. Discussion/Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Metlay, J.P.; Waterer, G.W.; Long, A.C.; Anzueto, A.; Brozek, J.; Crothers, K.; Cooley, L.A.; Dean, N.C.; Fine, M.J.; Flanders, S.A.; et al. Diagnosis and Treatment of Adults with Community-Acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 2019, 200, e45–e67. [Google Scholar] [CrossRef] [PubMed]
- Jahanihashemi, H.; Babaie, M.; Bijani, S.; Bazzazan, M.; Bijani, B. Poverty as an Independent Risk Factor for In-Hospital Mortality in Community-Acquired Pneumonia: A Study in a Developing Country Population. Int. J. Clin. Pract. 2018, 72, e13085. [Google Scholar] [CrossRef] [PubMed]
- Millett, E.R.C.; Quint, J.K.; Smeeth, L.; Daniel, R.M.; Thomas, S.L. Incidence of Community-Acquired Lower Respiratory Tract Infections and Pneumonia among Older Adults in the United Kingdom: A Population-Based Study. PLoS ONE 2013, 8, e75131. [Google Scholar] [CrossRef] [PubMed]
- Partouche, H.; Lepoutre, A.; du Vaure, C.B.; Poisson, T.; Toubiana, L.; Gilberg, S. Incidence of All-Cause Adult Community-Acquired Pneumonia in Primary Care Settings in France. Med. Mal. Infect. 2018, 48, 389–395. [Google Scholar] [CrossRef]
- Bjarnason, A.; Westin, J.; Lindh, M.; Andersson, L.-M.; Kristinsson, K.G.; Löve, A.; Baldursson, O.; Gottfredsson, M. Incidence, Etiology, and Outcomes of Community-Acquired Pneumonia: A Population-Based Study. Open Forum Infect. Dis. 2018, 5, ofy010. [Google Scholar] [CrossRef]
- Rivero-Calle, I.; Pardo-Seco, J.; Aldaz, P.; Vargas, D.A.; Mascarós, E.; Redondo, E.; Díaz-Maroto, J.L.; Linares-Rufo, M.; Fierro-Alacio, M.J.; Gil, A.; et al. Incidence and Risk Factor Prevalence of Community-Acquired Pneumonia in Adults in Primary Care in Spain (NEUMO-ES-RISK Project). BMC Infect. Dis. 2016, 16, 645. [Google Scholar] [CrossRef]
- Molina, J.; González-Gamarra, A.; Ginel, L.; Peláez, M.E.; Juez, J.L.; Artuñedo, A.; Aldana, G.; Quesada, E.; Cabré, J.J.; Gómez, A.; et al. CAPPRIC Study-Characterization of Community-Acquired Pneumonia in Spanish Adults Managed in Primary Care Settings. Microorganisms 2021, 9, 508. [Google Scholar] [CrossRef]
- Chalmers, J.; Campling, J.; Ellsbury, G.; Hawkey, P.M.; Madhava, H.; Slack, M. Community-Acquired Pneumonia in the United Kingdom: A Call to Action. Pneumonia 2017, 9, 15. [Google Scholar] [CrossRef]
- Cillóniz, C.; Menéndez, R.; García-Vidal, C.; Péricas, J.M.; Torres, A. Defining Community-Acquired Pneumonia as a Public Health Threat: Arguments in Favor from Spanish Investigators. Med. Sci. 2020, 8, 6. [Google Scholar] [CrossRef]
- Kang, S.H.; Jo, Y.H.; Lee, J.H.; Jang, D.-H.; Kim, Y.J.; Park, I. Antibiotic Prescription Consistent with Guidelines in Emergency Department is Associated with 30-Day Survival in Severe Community-Acquired Pneumonia. BMC Emerg. Med. 2021, 21, 108. [Google Scholar] [CrossRef]
- Yu, J.; Wang, G.; Davidson, A.; Chow, I.; Chiu, A. Antibiotics Utilization for Community Acquired Pneumonia in a Community Hospital Emergency Department. J. Pharm. Pract. 2022, 35, 62–69. [Google Scholar] [CrossRef]
- Høgli, J.U.; Garcia, B.H.; Skjold, F.; Skogen, V.; Småbrekke, L. An Audit and Feedback Intervention Study Increased Adherence to Antibiotic Prescribing Guidelines at a Norwegian Hospital. BMC Infect. Dis. 2016, 16, 96. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.; Brancaccio, A.; Popova, K.; Nagel, J. Stewardship-Hospitalist Collaboration. Infect. Dis. Clin. N. Am. 2020, 34, 83–96. [Google Scholar] [CrossRef]
- Acquisto, N.M.; May, L. Collaborative Antimicrobial Stewardship in the Emergency Department. Infect. Dis. Clin. N. Am. 2020, 34, 109–127. [Google Scholar] [CrossRef]
- Prina, E.; Ranzani, O.T.; Torres, A. Community-Acquired Pneumonia. Lancet 2015, 386, 1097–1108. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- De Groot, V.; Beckerman, H.; Lankhorst, G.J.; Bouter, L.M. How to Measure Comorbidity. a Critical Review of Available Methods. J. Clin. Epidemiol. 2003, 56, 221–229. [Google Scholar] [CrossRef]
- Guilding, C.; Hardisty, J.; Randles, E.; Statham, L.; Green, A.; Bhudia, R.; Thandi, C.S.; Teodorczuk, A.; Scott, L.; Matthan, J. Designing and Evaluating an Interprofessional Education Conference Approach to Antimicrobial Education. BMC Med. Educ. 2020, 20, 360. [Google Scholar] [CrossRef]
- Gutiérrez-Urbón, J.M.; Arenere-Mendoza, M.; Fernández-de-Gamarra-Martínez, E.; Fernández-Polo, A.; González-Suárez, S.; Nicolás-Picó, J.; Rodríguez-Mateos, M.E.; Sánchez-Yáñez, E. PAUSATE Study: Prevalence and appropriateness of the use of antimicrobials in Spanish hospitals. Farm Hosp. 2022, 46, 271–281. [Google Scholar]
- McIntosh, K.A.; Maxwell, D.J.; Pulver, L.K.; Horn, F.; Robertson, M.B.; Kaye, K.I.; Peterson, G.M.; Dollman, W.B.; Wai, A.; Tett, S.E. A Quality Improvement Initiative to Improve Adherence to National Guidelines for Empiric Management of Community-Acquired Pneumonia in Emergency Departments. Int. J. Qual. Health Care 2011, 23, 142–150. [Google Scholar] [CrossRef]
- Postma, D.F.; van Werkhoven, C.H.; van Elden, L.J.R.; Thijsen, S.F.T.; Hoepelman, A.I.M.; Kluytmans, J.A.J.W.; Boersma, W.G.; Compaijen, C.J.; van der Wall, E.; Prins, J.M.; et al. Antibiotic Treatment Strategies for Community-Acquired Pneumonia in Adults. N. Engl. J. Med. 2015, 372, 1312–1323. [Google Scholar] [CrossRef] [PubMed]
- Carratalà, J.; Garcia-Vidal, C.; Ortega, L.; Fernández-Sabé, N.; Clemente, M.; Albero, G.; López, M.; Castellsagué, X.; Dorca, J.; Verdaguer, R.; et al. Effect of a 3-Step Critical Pathway to Reduce Duration of Intravenous Antibiotic Therapy and Length of Stay in Community-Acquired Pneumonia: A Randomized Controlled Trial. Arch. Intern. Med. 2012, 172, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Uranga, A.; España, P.P.; Bilbao, A.; Quintana, J.M.; Arriaga, I.; Intxausti, M.; Lobo, J.L.; Tomás, L.; Camino, J.; Nuñez, J.; et al. Duration of Antibiotic Treatment in Community-Acquired Pneumonia: A Multicenter Randomized Clinical Trial. JAMA Intern. Med. 2016, 176, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Dinh, A.; Ropers, J.; Duran, C.; Davido, B.; Deconinck, L.; Matt, M.; Senard, O.; Lagrange, A.; Makhloufi, S.; Mellon, G.; et al. Discontinuing β-Lactam Treatment after 3 Days for Patients with Community-Acquired Pneumonia in Non-Critical Care Wards (PTC): A Double-Blind, Randomised, Placebo-Controlled, Non-Inferiority Trial. Lancet 2021, 397, 1195–1203. [Google Scholar] [CrossRef]
- Rohde, J.M.; Jacobsen, D.; Rosenberg, D.J. Role of the Hospitalist in Antimicrobial Stewardship: A Review of Work Completed and Description of a Multisite Collaborative. Clin. Ther. 2013, 35, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Kuper, K.M.; Hamilton, K.W. Collaborative Antimicrobial Stewardship: Working with Information Technology. Infect. Dis. Clin. N. Am. 2020, 34, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Viasus, D.; Vecino-Moreno, M.; De La Hoz, J.M.; Carratalà, J. Antibiotic Stewardship in Community-Acquired Pneumonia. Expert Rev. Anti Infect. Ther. 2017, 15, 351–359. [Google Scholar] [CrossRef]
- Cercenado, E.; Rodríguez-Baño, J.; Alfonso, J.L.; Calbo, E.; Escosa, L.; Fernández-Polo, A.; García-Rodríguez, J.; Garnacho, J.; Gil-Navarro, M.V.; Grau, S.; et al. Antimicrobial stewardship in hospitals: Expert recommendation guidance document for activities in specific populations, syndromes and other aspects (PROA-2) from SEIMC, SEFH, SEMPSPGS, SEMICYUC and SEIP. Enferm. Infecc. Microbiol. Clínica 2022, in press. [Google Scholar] [CrossRef]
- Collado, R.; Losa, J.E.; Álvaro, E.A.; Toro, P.; Moreno, L.; Pérez, M. Evaluación del consumo de antimicrobianos mediante DDD/100 estancias versus DDD/100 altas en la implantación de un programa de optimización de la utilización de antibióticos. Rev. Esp. Quim. 2015, 28, 317–321. [Google Scholar]
- Momattin, H.; Al-Ali, A.Y.; Mohammed, K.; Al-Tawfiq, J.A. Benchmarking of Antibiotic Usage: An Adjustment to Reflect Antibiotic Stewardship Program Outcome in a Hospital in Saudi Arabia. J. Infect. Public Health 2018, 11, 310–313. [Google Scholar] [CrossRef]
- Dellit, T.H.; Owens, R.C.; McGowan, J.E.; Gerding, D.N.; Weinstein, R.A.; Burke, J.P.; Huskins, W.C.; Paterson, D.L.; Fishman, N.O.; Carpenter, C.F.; et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America Guidelines for Developing an Institutional Program to Enhance Antimicrobial Stewardship. Clin. Infect. Dis. 2007, 44, 159–177. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, V.M.; Flanders, S.A.; Snyder, A.; Conlon, A.; Rogers, M.A.M.; Malani, A.N.; McLaughlin, E.; Bloemers, S.; Srinivasan, A.; Nagel, J.; et al. Excess Antibiotic Treatment Duration and Adverse Events in Patients Hospitalized with Pneumonia: A Multihospital Cohort Study. Ann. Intern. Med. 2019, 171, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Robert, L.; Mark, V.; Moayed, A.; Nivashen, A.; Vinod, R.; Sophie, P.; Mohamed, E.W.; Rusheng, C. Antimicrobial Prescribing and Outcomes of Community-Acquired Pneumonia in Australian Hospitalized Patients: A Cross-Sectional Study. J. Int. Med. Res. 2021, 49, 3000605211058366. [Google Scholar] [CrossRef] [PubMed]
- Aspa, J.; Rajas, O.; de Castro, F.R.; Huertas, M.C.; Borderias, L.; Cabello, F.J.; Tabara, J.; Hernandez-Flix, S.; Martinez-Sanchis, A.; Torres, A.; et al. Impact of initial antibiotic choice on mortality from pneumococcal pneumonia. Eur. Respir. J. 2006, 27, 1010–1019. [Google Scholar] [CrossRef] [PubMed]
- Viasus, D.; Simonetti, A.F.; Garcia-Vidal, C.; Niubó, J.; Dorca, J.; Carratalà, J. Impact of Antibiotic De-Escalation on Clinical Outcomes in Community-Acquired Pneumococcal Pneumonia. J. Antimicrob. Chemother. 2017, 72, 547–553. [Google Scholar] [CrossRef]
- Yamana, H.; Matsui, H.; Tagami, T.; Hirashima, J.; Fushimi, K.; Yasunaga, H. De-Escalation versus Continuation of Empirical Antimicrobial Therapy in Community-Acquired Pneumonia. J. Infect. 2016, 73, 314–325. [Google Scholar] [CrossRef]
- Carugati, M.; Franzetti, F.; Wiemken, T.; Kelley, R.R.; Kelly, R.; Peyrani, P.; Blasi, F.; Ramirez, J.; Aliberti, S. De-Escalation Therapy among Bacteraemic Patients with Community-Acquired Pneumonia. Clin. Microbiol. Infect. 2015, 21, 936.e11–936.e18. [Google Scholar] [CrossRef]
- Uda, A.; Tokimatsu, I.; Koike, C.; Osawa, K.; Shigemura, K.; Kimura, T.; Miyara, T.; Yano, I. Antibiotic De-Escalation Therapy in Patients with Community-Acquired Nonbacteremic Pneumococcal Pneumonia. Int. J. Clin. Pharm. 2019, 41, 1611–1617. [Google Scholar] [CrossRef]
- Wald-Dickler, N.; Spellberg, B. Short-Course Antibiotic Therapy-Replacing Constantine Units with “Shorter is Better”. Clin. Infect. Dis. 2019, 69, 1476–1479. [Google Scholar] [CrossRef]
- Pasquau, J.; de Jesus, E.S.; Sadyrbaeva, S.; Aznarte, P.; Hidalgo-Tenorio, C. The Reduction in Duration of Antibiotic Therapy as a Key Element of Antibiotic Stewardship Programs. J. Antimicrob. 2015, 1, 103. [Google Scholar] [CrossRef]
- Tansarli, G.S.; Mylonakis, E. Systematic Review and Meta-Analysis of the Efficacy of Short-Course Antibiotic Treatments for Community-Acquired Pneumonia in Adults. Antimicrob. Agents Chemother. 2018, 62, e00635-18. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B.; Rice, L.B. Duration of Antibiotic Therapy: Shorter is Better. Ann. Intern. Med. 2019, 171, 210–211. [Google Scholar] [CrossRef] [PubMed]
- Pettigrew, M.M.; Kwon, J.; Gent, J.F.; Kong, Y.; Wade, M.; Williams, D.J.; Creech, C.B.; Evans, S.; Pan, Q.; Walter, E.B.; et al. Comparison of the Respiratory Resistomes and Microbiota in Children Receiving Short versus Standard Course Treatment for Community-Acquired Pneumonia. mBio 2022, 13, e0019522. [Google Scholar] [CrossRef] [PubMed]
| Comunity-Acquired Pneumonia (CAP) | Drug | Duration |
|---|---|---|
| Non-severe (PSI I–II) | Amoxicilina 1 g/8 h PO Amoxicilina-clavulanic acid 875/125 mg/8 h PO Azythromicin * 500 mg PO (atypical) | 5–7 days |
| Severe (PSI III) | ceftriaxone 2 g (pneumococcal antigenuria positive) | 5–7 days |
| ceftriaxone 2 g + (azythromicine * 500 mg or levofloxacin 500 mg) | 7–10 days | |
| Severe (PSI IV–V) | ceftriaxone 2 g (or 1 g/12 h) + (azythromicine * 500 mg or levofloxacin 500 mg) | 7–10 days |
| Variable | Values | Total Number (%) | Male (%) | Female (%) |
|---|---|---|---|---|
| Charlson index | 0 | 20 (36.36) | 13 (39.39) | 7 (31.82) |
| [1,2,3] | 20 (36.36) | 11 (33.33) | 9 (40.91) | |
| >3 | 14 (25.45) | 8 (24.24) | 6 (27.27) | |
| Not registered | 1 (1.82) | 1 (3.03) | 0 (0.00) | |
| PSI scale | I | 9 (16.36) | 5 (15.15) | 4 (18.18) |
| II | 10 (18.18) | 9 (27.27) | 1 (4.55) | |
| III | 9 (16.36) | 6 (18.18) | 3 (13.64) | |
| IV | 16 (29.09) | 8 (24.24) | 8 (36.36) | |
| V | 2 (3.64) | 2 (6.06) | 0 (0.00) | |
| Not registered | 9 (16.36) | 3 (9.09) | 6 (27.27) | |
| Mean age (years ± SD) | 65.07 ± 22.12 | 66.06 ± 21.20 | 69.58 ± 23.18 | |
| Variable | Values | Total (%) | Male (%) | Female (%) |
|---|---|---|---|---|
| Indication of the antibiotic | Adequate | 53 (96.36) | 31 (93.94) | 22 (100.00) |
| Doubtful | 1 (1.82) | 1 (3.03) | 0 (0.00) | |
| Inadequate | 1 (1.82) | 1 (3.03) | 0 (0.00) | |
| Choice of antibiotic | Optimal | 35 (63.64) | 20 (60.61) | 15 (68.18) |
| might be better | 14 (25.45) | 9 (27.27) | 5 (22.73) | |
| Inadequate | 1 (1.82) | 1 (3.03) | 0 (0.00) | |
| Doubtful | 5 (9.09) | 3 (9.09) | 2 (9.09) | |
| Time of administration of the first dose | Adequate | 50 (90.91) | 29 (87.88) | 21 (95.45) |
| Doubtful | 5 (9.09) | 4 (12.12) | 1 (4.55) | |
| De-escalation/ oral sequencing | Optimal | 23 (41.82) | 13 (39.39) | 10 (45.45) |
| might be better | 28 (50.91) | 19 (57.58) | 9 (40.91) | |
| Doubtful | 4 (7.27) | 1 (3.03) | 3 (13.64) | |
| Treatment duration | Optimal | 25 (45.45) | 15 (45.45) | 10 (45.45) |
| Excessive | 25 (45.45) | 14 (42.42) | 11 (50.00) | |
| Doubtful | 5 (9.09) | 4 (12.12) | 1 (4.55) | |
| Monitoring of efficacy and adverse effects | Adequate | 53 (96.36) | 31 (93.94) | 22 (100.00) |
| Doubtful | 2 (3.64) | 2 (6.06) | 0 (0.00) | |
| Registration in the medical record | Complete | 19 (34.55) | 10 (30.30) | 9 (40.91) |
| Partial | 32 (58.18) | 20 (60.61) | 12 (54.55) | |
| Lacking | 4 (7.27) | 3 (9.09) | 1 (4.55) | |
| Total | 55 (100.0) | 33 (60.0) | 22 (40.0) |
| PSI | Number of Patients | Mean | Standard Deviation | Confidence Interval |
|---|---|---|---|---|
| PSI I–II | 19 | 13.21 | 6.45 | (10.10–16.32) |
| PSI III | 9 | 10.78 | 3.38 | (8.18–13.38) |
| PSI IV | 16 | 12.56 | 3.41 | (10.75–14.38) |
| PSI V | 2 | 8.50 | 2.12 | (−10.56–27.56) |
| Missing PSI values | 9 | NA | NA | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arteche-Eguizabal, L.; Corcuera-Martínez de Tobillas, I.; Melgosa-Latorre, F.; Domingo-Echaburu, S.; Urrutia-Losada, A.; Eguiluz-Pinedo, A.; Rodriguez-Piacenza, N.V.; Ibarrondo-Olaguenaga, O. Multidisciplinary Collaboration for the Optimization of Antibiotic Prescription: Analysis of Clinical Cases of Pneumonia between Emergency, Internal Medicine, and Pharmacy Services. Antibiotics 2022, 11, 1336. https://doi.org/10.3390/antibiotics11101336
Arteche-Eguizabal L, Corcuera-Martínez de Tobillas I, Melgosa-Latorre F, Domingo-Echaburu S, Urrutia-Losada A, Eguiluz-Pinedo A, Rodriguez-Piacenza NV, Ibarrondo-Olaguenaga O. Multidisciplinary Collaboration for the Optimization of Antibiotic Prescription: Analysis of Clinical Cases of Pneumonia between Emergency, Internal Medicine, and Pharmacy Services. Antibiotics. 2022; 11(10):1336. https://doi.org/10.3390/antibiotics11101336
Chicago/Turabian StyleArteche-Eguizabal, Lorea, Iñigo Corcuera-Martínez de Tobillas, Federico Melgosa-Latorre, Saioa Domingo-Echaburu, Ainhoa Urrutia-Losada, Amaia Eguiluz-Pinedo, Natalia Vanina Rodriguez-Piacenza, and Oliver Ibarrondo-Olaguenaga. 2022. "Multidisciplinary Collaboration for the Optimization of Antibiotic Prescription: Analysis of Clinical Cases of Pneumonia between Emergency, Internal Medicine, and Pharmacy Services" Antibiotics 11, no. 10: 1336. https://doi.org/10.3390/antibiotics11101336
APA StyleArteche-Eguizabal, L., Corcuera-Martínez de Tobillas, I., Melgosa-Latorre, F., Domingo-Echaburu, S., Urrutia-Losada, A., Eguiluz-Pinedo, A., Rodriguez-Piacenza, N. V., & Ibarrondo-Olaguenaga, O. (2022). Multidisciplinary Collaboration for the Optimization of Antibiotic Prescription: Analysis of Clinical Cases of Pneumonia between Emergency, Internal Medicine, and Pharmacy Services. Antibiotics, 11(10), 1336. https://doi.org/10.3390/antibiotics11101336






