The Antimicrobial Resistance (AMR) Rates of Uropathogens in a Rural Western African Area—A Retrospective Single-Center Study from Kpando, Ghana
Abstract
1. Introduction
2. Methods
2.1. Local Procedure for Urine Microscopy and Establishment of Urine Cultures
2.2. Bacterial Identification and Count
- slight yellowish or greenish: Enterococcus faecalis
- yellow, opaque centre slightly deeper yellow: E. coli
- yellow to whitish blue: K. pneumonia
- Blue: Proteus vulgaris
- Bluish: Salmonella typhi
- Deep yellow: Staphylococcus aureus.
- Candida spp.
- Citrobacter koseri and Citrobacter NFC: referred to as Citrobacter spp.
- Corynebacterium
- Escherichia (E.) coli
- Klebsiella (K.) pneumonia, Klebsiella oxytoca and Klebsiella NFC: referred to as Klebsiella spp.
- Proteus vulgaris and Proteus mirabilis: referred to as Proteus spp.
- Pseudomonas aeruginosa and Pseudomonas NFC: referred to as Pseudomonas spp.
- Salmonella parathyphii and Salmonella NFC: referred to as Salmonella spp.
- Staphylococcus aureus and Staphylococcus epidermidis: referred to as Staphylococcus spp.
2.3. Antibacteril Susceptibility
2.4. Statistical Analysis
3. Results
3.1. Descriptive Statistics
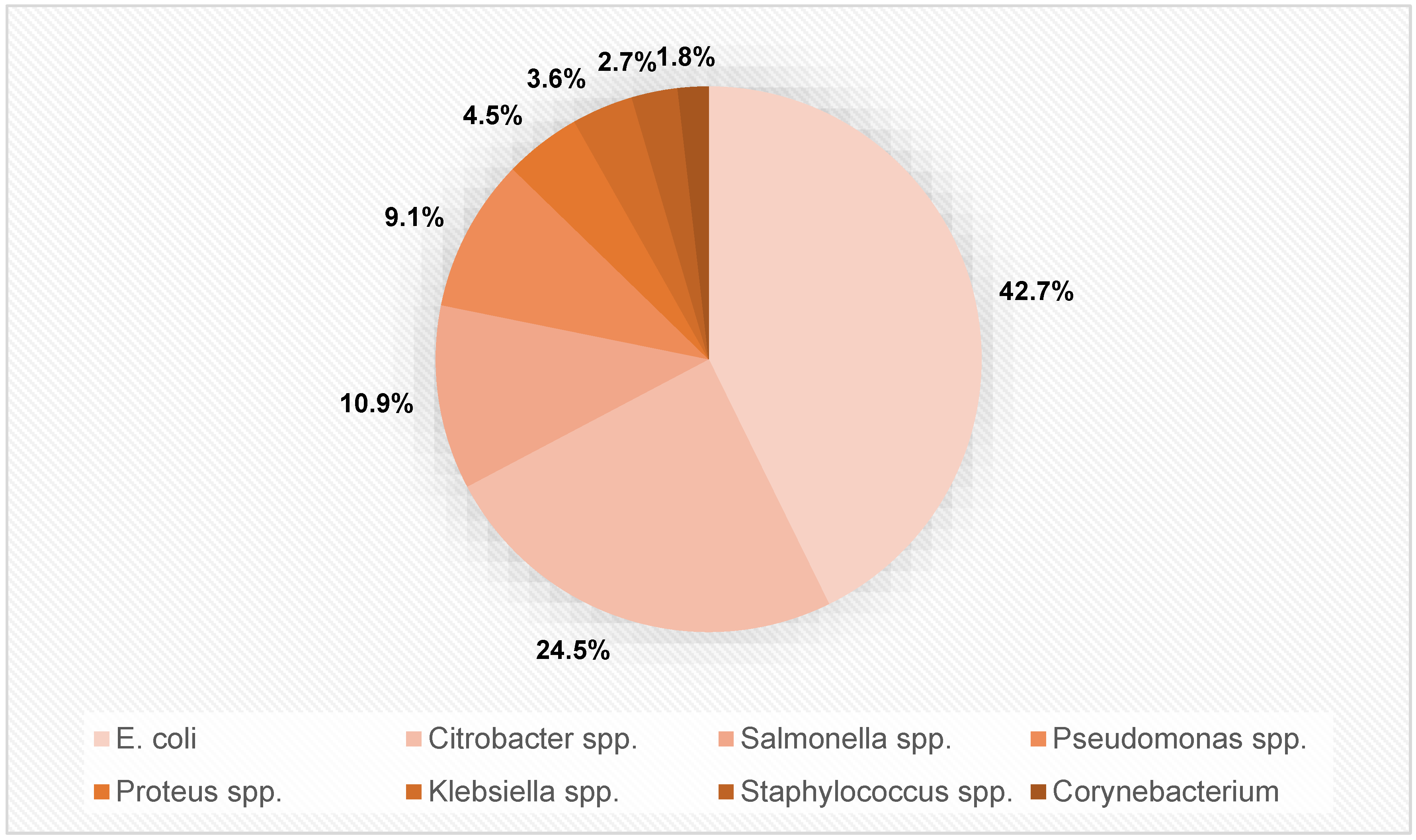
3.2. Urine Culture Results
3.3. AMR of Bacterial spp.
3.4. Clinical Predictors of Bacterial Growth
4. Discussion
Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AB(s) | Antibiotic(s) |
| AMK | Amikacin |
| AMP | Ampicillin |
| AMC | Amoxicillin + clavulanic acid |
| CXM | Cefuroxime |
| CFX | Cefotaxime |
| CAZ | Ceftazidim |
| CIP | Ciprofloxacin |
| CRO | Ceftriaxone |
| GEN | Gentamicin |
| LEV | Levofloxacin |
| NAL | Nalidixic acid |
| NFN | Nitrofurantoin |
| NOR | Norfloxacin |
| PIP | Piperacillin |
| SXT | Trimethoprim + Sulfamethoxazol |
| TET | Tetracyclin |
| AMC | antibiotic consumption |
| AMR | Antimicrobial Resistance |
| C.L.E.D. Agar | Cystine Lactose Electrolyte Deficient Agar |
| CLSI | Clinical and Laboratory Standards Institute |
| EAU | European Association of Urology |
| ECDC | European Centre for Disease Prevention |
| ESBL | extended-spectrum β-lactamase-producing |
| GLASS | Global Antimicrobial Resistance and Use Surveillance System |
| HIC(s) | High-income country/-ies |
| LIC(s) | Low-income country/-ies |
| Max. | Maximum |
| Min. | Minimum |
| MMCH | Margret Marquart Catholic Hospital |
| MP/CK | Mobile phones/computer keyboards |
| NA | Not available |
| OR | Odds ratio |
| SD | Standard deviation |
| spp. | Species |
| USA | United states of America |
| UTI | urinary tract infection |
| WHO | World Health Organization |
References
- Antibiotic Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance (accessed on 3 January 2022).
- Stekel, D. First report of antimicrobial resistance pre-dates penicillin. Nature 2018, 562, 192. [Google Scholar] [CrossRef]
- Miller, T.H. The Problem of Arsphenamine-Resistant Syphilis–Report Of A Case. JAMA 1931, 97, 11–14. [Google Scholar] [CrossRef]
- Abraham, E.P.; Chain, E. An enzyme from bacteria able to destroy penicillin. 1940. Rev. Infect. Dis. 1988, 10, 677–678. [Google Scholar] [PubMed]
- Hooper, D.C. Mechanisms of action and resistance of older and newer fluoroquinolones. Clin. Infect. Dis. 2000, 31, S24–S28. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, H. Multidrug resistance in bacteria. Annu. Rev. Biochem. 2009, 78, 119–146. [Google Scholar] [CrossRef]
- Castanheira, M.; Simner, P.J.; Bradford, P. Extended-spectrum beta-lactamases: An update on their characteristics, epidemiology and detection. JAC Antimicrob. Resist. 2021, 3, dlab092. [Google Scholar] [CrossRef]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; Salamat, M.K.F.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef]
- WHO. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2021; World Health Organization (WHO): Geneva, Switzerland, 2021. [Google Scholar]
- Tadesse, B.T.; Ashley, E.A.; Ongarello, S.; Havumaki, J.; Wijegoonewardena, M.; González, I.J.; Dittrich, S. Antimicrobial resistance in Africa: A systematic review. BMC Infect. Dis. 2017, 17, 616. [Google Scholar] [CrossRef]
- Morgenstern, S.; Salem, J.; Paffenholz, P.; Borgmann, H.; Horsch, R. Urological help under exceptional conditions. Doctors for Africa. Urologe A 2015, 54, 1446–1449. [Google Scholar] [CrossRef]
- Karah, N.; Rafei, R.; Elamin, W.; Ghazy, A.; Abbara, A.; Hamze, M.; Uhlin, B.E. Guideline for Urine Culture and Biochemical Identification of Bacterial Urinary Pathogens in Low-Resource Settings. Diagnostics 2020, 10, 832. [Google Scholar] [CrossRef]
- Sousa, A.M.; Machado, I.; Nicolau, A.; Pereira, M.O. Improvements on colony morphology identification towards bacterial profiling. J. Microbiol. Methods 2013, 95, 327–335. [Google Scholar] [CrossRef]
- C.L.E.D. Agar w/Bromo Thymol Blue (Product Code: CM0505). Available online: https://www.technopharmchem.com/ProductList/productTech/1642 (accessed on 29 November 2022).
- Tripathi, N.; Sapra, A. Gram Staining; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Lominski, I.; Grossfeld, E. Coagulase Test for Rapid Detection of Staph. aureus. Br. Med. J. 1944, 2, 854. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mueller Hinton Agar. Available online: https://www.himedialabs.com/eu/m173-mueller-hinton-agar.html (accessed on 29 November 2022).
- Clinical and Laboratory Standards Institute. M100-S25: Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fifth Informational Supplement; Clinical and laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- Forson, A.O.; Menkah, D.A.; Quarchie, M.N.; Dhikrullahi, S.B.; Olu-Taiwo, M.; Codjoe, F.S. Bacterial drug-resistance patterns and genetic diversity of bacteria-associated bacteriuria in diabetic patients in Ghana. IJID Reg. 2021, 1, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Forson, A.O.; Tsidi, W.B.; Nana-Adjei, D.; Quarchie, M.N.; Obeng-Nkrumah, N. Escherichia coli bacteriuria in pregnant women in Ghana: Antibiotic resistance patterns and virulence factors. BMC Res. Notes 2018, 11, 901. [Google Scholar] [CrossRef] [PubMed]
- Donkor, E.S.; Horlortu, P.Z.; Dayie, N.T.; Obeng-Nkrumah, N.; Labi, A.-K. Community acquired urinary tract infections among adults in Accra, Ghana. Infect. Drug Resist. 2019, 12, 2059–2067. [Google Scholar] [CrossRef] [PubMed]
- Afriyie, D.K.; Gyansa-Lutterodt, M.; Amponsah, S.; Asare, G.; Wiredu, V.; Wormenor, E.; Bugyei, K.A. Susceptibility pattern of uropathogens to ciprofloxacin at the Ghana police hospital. Pan. Afr. Med. J. 2015, 22, 87. [Google Scholar] [CrossRef]
- Critchley, I.A.; Cotroneo, N.; Pucci, M.J.; Jain, A.; Mendes, R.E. Resistance among urinary tract pathogens collected in Europe during 2018. J. Glob. Antimicrob. Resist. 2020, 23, 439–444. [Google Scholar] [CrossRef]
- Ministry of Social Affairs, Health, Care and Consumer Protection. Resistenzbericht Österreich AURES 2020—Antibiotikaresistenz und Verbrauch antimikrobieller Substanzen in Österreich; Bundesministerium für Soziales, Gesundheit, Pflege und Konsumentenschutz: Viena, Austria, 2020. [Google Scholar]
- Kaye, K.S.; Gupta, V.; Mulgirigama, A.; Joshi, A.V.; Scangarella-Oman, N.; Yu, K.; Ye, G.; Mitrani-Gold, F.S. Antimicrobial Resistance Trends in Urine Escherichia coli Isolates From Adult and Adolescent Females in the United States From 2011 to 2019: Rising ESBL Strains and Impact on Patient Management. Clin. Infect. Dis. 2021, 73, 1992–1999. [Google Scholar] [CrossRef]
- Bouza, E.; Juan, R.S.; Muñoz, P.; Voss, A.; Kluytmans, J. A European perspective on nosocomial urinary tract infections II. Report on incidence, clinical characteristics and outcome (ESGNI-004 study). European Study Group on Nosocomial Infection. Clin. Microbiol. Infect. 2001, 7, 532–542. [Google Scholar] [CrossRef]
- Paul, M.; Carrara, E.; Retamar, P.; Tängdén, T.; Bitterman, R.; Bonomo, R.A.; de Waele, J.; Daikos, G.L.; Akova, M.; Harbarth, S.; et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine). Clin. Microbiol. Infect. 2022, 28, 521–547. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, J.; Ju, Z.; Chang, W.; Sun, S. Molecular Characterization of Antimicrobial Resistance in Escherichia coli from Rabbit Farms in Tai’an, China. BioMed Res. Int. 2018, 2018, 8607647. [Google Scholar] [CrossRef] [PubMed]
- Olu-Taiwo, M.; Laryea, C.A.; Mykels, D.K.; Forson, A.O. Multidrug-Resistant Bacteria on the Mobile Phones and Computer Keyboards of Healthcare University Students in Ghana. Can. J. Infect. Dis. Med. Microbiol. 2021, 2021, 6647959. [Google Scholar] [CrossRef] [PubMed]
- Acolatse, J.E.E.; Portal, E.A.R.; Boostrom, I.; Akafity, G.; Dakroah, M.P.; Chalker, V.J.; Sands, K.; Spiller, O.B. Environmental surveillance of ESBL and carbapenemase-producing gram-negative bacteria in a Ghanaian Tertiary Hospital. Antimicrob. Resist. Infect. Control 2022, 11, 49. [Google Scholar] [CrossRef] [PubMed]
- EAU Guidelines. Edn. Presented at the EAU Annual Congress Amsterdam 2022; EAU Guidelines Office: Arnhem/Amsterdam, The Netherlands, 2022. [Google Scholar]
- Taxt, A.; Avershina, E.; Frye, S.A.; Naseer, U.; Ahmad, R. Rapid identification of pathogens, antibiotic resistance genes and plasmids in blood cultures by nanopore sequencing. Sci. Rep. 2020, 10, 7622. [Google Scholar] [CrossRef]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
| Aminoglycosides | Gentamicin (GEN) |
| Amikacin (AMK) | |
| Aminopenicillins +/− ß-lactam inhibitor | Piperacillin (PIP) |
| Amoxicillin + clavulanic acid (AMC) | |
| Cephalosporins | Due to the different composition of the antibiotic rings, various cephalosporins are grouped together under this heading (including cefuroxime, cefazolin, and ceftriaxone). A separate evaluation was not possible because of the inconsistent documentation. |
| Fluorchinolones and Diazanaphthaline | Ciprofloxacin (CIP) |
| Levofloxacin (LEV) | |
| Norfloxacin (NOR) | |
| Nalidixic acid (NAL) | |
| Nitrofurantoin (NFN) | |
| Tetracyclines | Tetracycline (TET) |
| Mean | SD | Min. | Max. | ||
|---|---|---|---|---|---|
| Patients’ characteristics | Patients’ age in years | 46.1 | 23.2 | 0 | 97 |
| Urine microscopy: cells/high power field | Pus cells | 13.0 | 27.0 | 0 | 250 |
| Epithelial cells | 4.4 | 4.8 | 0 | 31 | |
| Red blood cells | 6.8 | 19.2 | 0 | 150 | |
| Urine microbiology | Total number of AMR per patient | 8.6 | 2.1 | 2 | 11 |
| Bacterial spp. (Number of Isolates) | Antibiotics | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aminoglykosides | Aminopenicillins +/−ß-Lactam AB | Cephalosporins | Fluorchinolones and Diazanaphthaline | NFN | TET | |||||||
| AMK | GEN | AMC | PIP | CIP | LEV | NOR | NAL | |||||
| 1 | Citrobacter spp. (n = 27) | 0.0 | 66.7 | / | 100.0 | 88.9 | 74.1 | 55.6 | 81.5 | 77.8 | 85.2 | 77.8 |
| 2 | Corynebacterium (n = 2) | 50.0 | 100.0 | 100.0 | 100.0 | 100.0 | 50.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| 3 | Escherichia coli (n = 47) | 10.6 | 61.7 | 100.0 | 100.0 | 85.1 | 89.4 | 51.1 | 91.5 | 97.9 | 55.3 | 87.2 |
| 4 | Klebsiella spp. ° (n = 4) | 25.0 | 50.0 | 75.0 | 100.0 | 75.0 | 50.0 | 0.0 | 50.0 | 75.0 | 75.0 | 100.0 |
| 5 | Proteus spp. * (n = 5) | 80.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 80.0 | 100.0 | 100.0 | 80.0 | / |
| 6 | Pseudomonas (n = 10) | 0.0 | 80.0 | / | 100.0 | / | 100.0 | 60.0 | 100.0 | 100.0 | 70.0 | 80.0 |
| 7 | Salmonella spp. (n = 12) | 0.0 | 66.7 | 100.0 | 91.7 | 83.3 | 66.7 | 41.7 | 83.3 | 75.0 | 66.7 | 83.3 |
| 8 | Staphylococcus spp. (n = 3) | 0.0 | 66.7 | 100.0 | 100.0 | 100.0 | 66.7 | 33.3 | 66.7 | 100.0 | 66.7 | 66.7 |
 ° comment on Klebsiella spp. susceptibility: susceptibility of AMC but PIP resistance is questionable. * comment on Proteus spp. susceptibility: swarming phenomenon on disc diffusion AMR testing might be interpreted as resistant but might have shown an “outer zone diameter”. This might explain the high R rate in Proteus spp.
° comment on Klebsiella spp. susceptibility: susceptibility of AMC but PIP resistance is questionable. * comment on Proteus spp. susceptibility: swarming phenomenon on disc diffusion AMR testing might be interpreted as resistant but might have shown an “outer zone diameter”. This might explain the high R rate in Proteus spp.| Estimation | (1) | (2) | (3) | (4) | ||||
|---|---|---|---|---|---|---|---|---|
| Endog. Variable | Bacteria Growth | |||||||
| Predictors | OR | SE | OR | SE | OR | SE | OR | SE |
| Intercept | 0.11 *** | 0.48 | 0.07 *** | 0.53 | 0.09 *** | 0.50 | 0.11 *** | 0.49 |
| Age in yrs | 1.03 *** | 0.01 | 1.03 *** | 0.01 | 1.03 *** | 0.01 | 1.03 *** | 0.01 |
| Sex (0: male, 1: female) | 2.88 * | 0.61 | 3.84 ** | 0.66 | 2.31 | 0.63 | 2.52 | 0.63 |
| Age * Sex | 0.99 | 0.01 | 0.98 | 0.01 | 0.99 | 0.01 | 0.99 | 0.01 |
| Pus cells/HPF | 1.05 *** | 0.01 | ||||||
| Epithelial cells/HPF | 1.07 ** | 0.03 | ||||||
| Red blood cells(HPF) | 1.01 | 0.01 | ||||||
| Observations | 333 | 320 | 322 | 322 | ||||
| R2 Tjur | 0.070 | 0.164 | 0.090 | 0.078 | ||||
| Publication | Year | Origin of Specimen | Number of E. coli Isolates | Special Features of the Cohort | Antibiotics | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aminoglykosides | Aminopenicillins +/−ß-Lactam AB | Cephalosporins | Fluorochinolones and Diazanaphthaline | NFN | SXT | TET | |||||||||||
| AMK | GEN | AMC | AMP | PIP | CIP | LEV | NOR | NAL | |||||||||
| Ghana | |||||||||||||||||
| Deininger et al. | 2022 | Urine | 47 | 10.6 | 61.7 | 100.0 | NA | 100.0 | 85.1 | 89.4 | 51.1 | 91.5 | 97.9 | 55.3 | NA | 87.2 | |
| Forson et al. [19] | 2021 | 28 | Diabetics | NA | 14.3 | 21.4 | 85.7 | NA | 28.6 (CFX) 35.7 (CRO) | 21.4 | NA | NA | 50 | NA | 42.8 | NA | |
| Forson et al. [20] | 2018 | 82 | Pregnant women | NA | 41.5 | NA | 79.3 | NA | 32.9 (CMX) | NA | NA | NA | 48.8 | 35.4 | 59.8 | 70.7 | |
| Donkor et al. [21] | 2019 | 15 | 6.7 | 26.7 | 93.4 | NA | 93.4 | 26.7 (CAZ) 6.7 (CXM) | 20.0 | 20.0 | 40.0 | 73.4 | 26.7 | NA | 53.4 | ||
| Afriyie et al. [22] | 2015 | 52 | NA | NA | NA | NA | NA | NA | 38.5 | NA | NA | NA | NA | NA | NA | ||
| Europe | |||||||||||||||||
| Critchley et al. [23] | 2018 | Urine | 766 | 0.9 | 12 | 20.1 | 50.1 | 4.1 | 20.0 (CXM) 13.2 (CEP) 11.1 (CAZ) 15.9 (CRO) | 22.7 | 21.8 | NA | NA | NA | 32.7 | NA | |
| Austria | |||||||||||||||||
| AURES [24] | 2016 | Blood | 5.7 ° | 50.5 * | NA | 9.2 ∞ | 19.8 | NA | NA | NA | |||||||
| AURES [24] | 2020 | Blood | 6.4 ° | 46.1 * | NA | 10.1 ∞ | 17.8 | NA | NA | NA | |||||||
| USA | |||||||||||||||||
| Kaye et al. [25] | 2019 | Urine | 1 513 882 | NA | NA | NA | NA | NA | 3.2 (CEP) 11.9 (CEF) | 21.1 | NA | 3.8 | 25.4 | NA | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deininger, S.; Gründler, T.; Deininger, S.H.M.; Lütcke, K.; Lütcke, H.; Agbesi, J.; Ladzaka, W.; Gyamfi, E.; Wichlas, F.; Hofmann, V.; et al. The Antimicrobial Resistance (AMR) Rates of Uropathogens in a Rural Western African Area—A Retrospective Single-Center Study from Kpando, Ghana. Antibiotics 2022, 11, 1808. https://doi.org/10.3390/antibiotics11121808
Deininger S, Gründler T, Deininger SHM, Lütcke K, Lütcke H, Agbesi J, Ladzaka W, Gyamfi E, Wichlas F, Hofmann V, et al. The Antimicrobial Resistance (AMR) Rates of Uropathogens in a Rural Western African Area—A Retrospective Single-Center Study from Kpando, Ghana. Antibiotics. 2022; 11(12):1808. https://doi.org/10.3390/antibiotics11121808
Chicago/Turabian StyleDeininger, Susanne, Therese Gründler, Sebastian Hubertus Markus Deininger, Karina Lütcke, Harry Lütcke, James Agbesi, Williams Ladzaka, Eric Gyamfi, Florian Wichlas, Valeska Hofmann, and et al. 2022. "The Antimicrobial Resistance (AMR) Rates of Uropathogens in a Rural Western African Area—A Retrospective Single-Center Study from Kpando, Ghana" Antibiotics 11, no. 12: 1808. https://doi.org/10.3390/antibiotics11121808
APA StyleDeininger, S., Gründler, T., Deininger, S. H. M., Lütcke, K., Lütcke, H., Agbesi, J., Ladzaka, W., Gyamfi, E., Wichlas, F., Hofmann, V., Erne, E., Törzsök, P., Lusuardi, L., Kern, J. M., & Deininger, C. (2022). The Antimicrobial Resistance (AMR) Rates of Uropathogens in a Rural Western African Area—A Retrospective Single-Center Study from Kpando, Ghana. Antibiotics, 11(12), 1808. https://doi.org/10.3390/antibiotics11121808







