Diagnosis and Antifungal Prophylaxis for COVID-19 Associated Pulmonary Aspergillosis
Abstract
1. Background
2. Case Definitions and Diagnosis
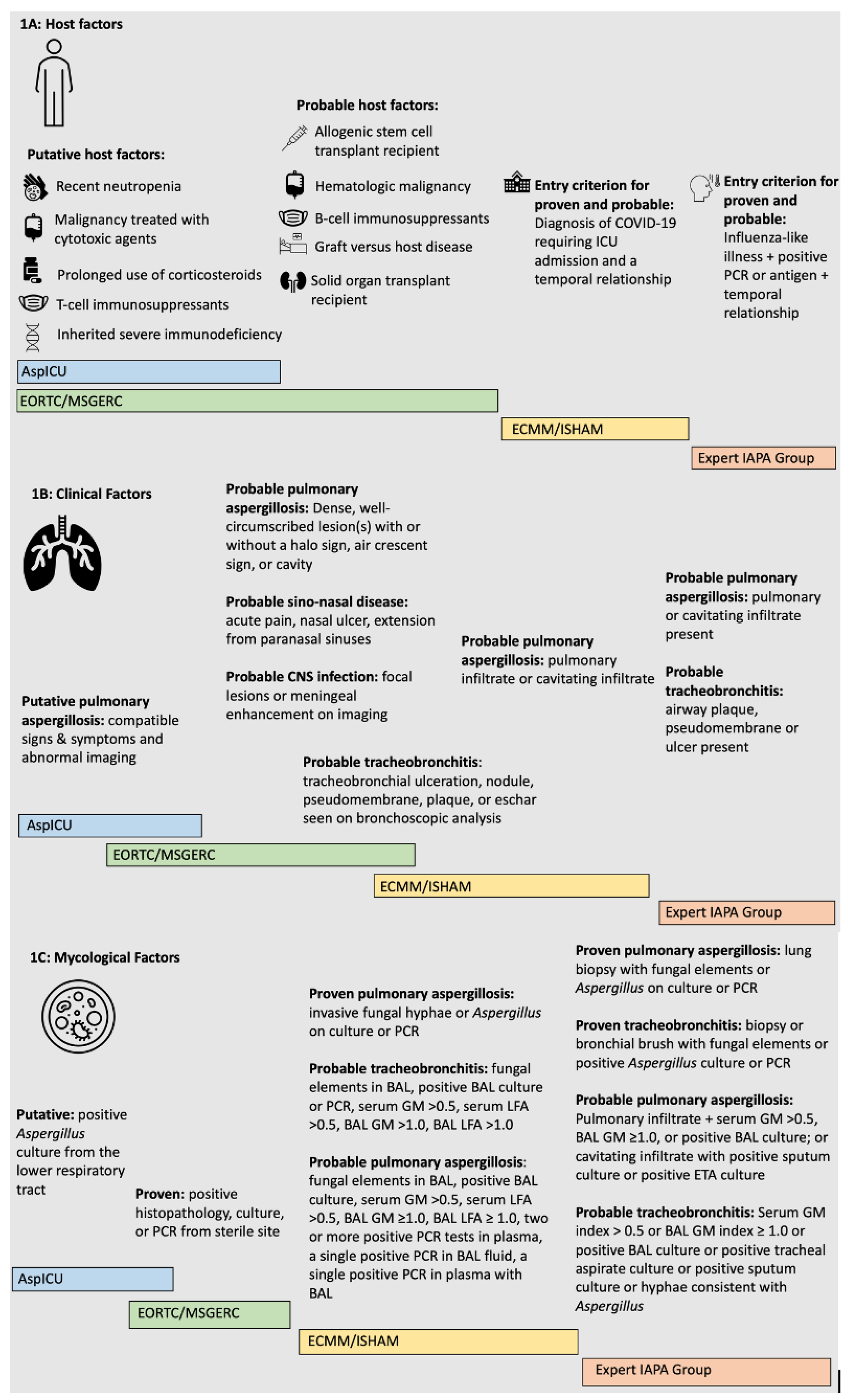
2.1. Case Definitions
2.1.1. EORTC/MSGERC
2.1.2. AspICU
2.1.3. Expert Case Definitions for IAPA
2.1.4. ECCM/ISHAM
2.2. Current CAPA Diagnostic Tests
2.2.1. Culture Data
2.2.2. PCR Diagnostics
2.2.3. Point of Care Tests
2.2.4. Fungal Biomarkers
3. Current Data on Antifungal Prophylaxis
4. Our Opinions on Antifungal Prophylaxis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hawes, A.M.; Desai, A.; Patel, P.K. Did Clostridioides difficile testing and infection rates change during the COVID-19 pandemic? Anaerobe 2021, 70, 102384. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, B.; Li, Q.; Wen, L.; Zhang, R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin. Infect. Dis. 2020, 71, 769–777. [Google Scholar] [CrossRef]
- Ripa, M.; Galli, L.; Poli, A.; Oltolini, C.; Spagnuolo, V.; Mastrangelo, A.; Muccini, C.; Monti, G.; De Luca, G.; Landoni, G. Secondary infections in patients hospitalized with COVID-19: Incidence and predictive factors. Clin. Microbiol. Infect. 2021, 27, 451–457. [Google Scholar] [CrossRef]
- Hatzl, S.; Reisinger, A.C.; Posch, F.; Prattes, J.; Stradner, M.; Pilz, S.; Eller, P.; Schoerghuber, M.; Toller, W.; Gorkiewicz, G. Antifungal prophylaxis for prevention of COVID-19-associated pulmonary aspergillosis in critically ill patients: An observational study. Crit. Care 2021, 25, 335. [Google Scholar] [CrossRef]
- Calderón-Parra, J.; Mills-Sanchez, P.; Moreno-Torres, V.; Tejado-Bravo, S.; Romero-Sánchez, I.; Balandin-Moreno, B.; Calvo-Salvador, M.; Portero-Azorín, F.; García-Masedo, S.; Muñez-Rubio, E. COVID-19-associated pulmonary aspergillosis (CAPA): Risk factors and development of a predictive score for critically ill COVID-19 patients. Mycoses 2022, 65, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Koehler, P.; Bassetti, M.; Chakrabarti, A.; Chen, S.C.; Colombo, A.L.; Hoenigl, M.; Klimko, N.; Lass-Flörl, C.; Oladele, R.O.; Vinh, D.C. Defining and managing COVID-19-associated pulmonary aspergillosis: The 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect. Dis. 2021, 21, e149–e162. [Google Scholar] [CrossRef]
- Permpalung, N.; Maertens, J.; Marr, K.A. Diagnostic dilemma in COVID-19-associated pulmonary aspergillosis. Lancet Infect. Dis. 2021, 21, 766–767. [Google Scholar] [CrossRef] [PubMed]
- Nitipong Permpalung, M.; Chiang, T.P.-Y.; Massie, A.B. COVID-19 Associated Pulmonary Aspergillosis in Mechanically Ventilated Patients. Clin. Infect. Dis. 2021, 74, 83–91. [Google Scholar]
- Baddley, J.W.; Thompson III, G.R.; Chen, S.C.-A.; White, P.L.; Johnson, M.D.; Nguyen, M.H.; Schwartz, I.S.; Spec, A.; Ostrosky-Zeichner, L.; Jackson, B.R. Coronavirus Disease 2019–Associated Invasive Fungal Infection. Open Forum Infect. Dis. 2021, 8, ofab510. [Google Scholar] [CrossRef] [PubMed]
- Bartoletti, M.; Pascale, R.; Cricca, M.; Rinaldi, M.; Maccaro, A.; Bussini, L.; Fornaro, G.; Tonetti, T.; Pizzilli, G.; Francalanci, E. Epidemiology of invasive pulmonary aspergillosis among intubated patients with COVID-19: A prospective study. Clin. Infect. Dis. 2021, 73, e3606–e3614. [Google Scholar] [CrossRef] [PubMed]
- Marr, K.A.; Platt, A.; Tornheim, J.A.; Zhang, S.X.; Datta, K.; Cardozo, C.; Garcia-Vidal, C. Aspergillosis complicating severe coronavirus disease. Emerg. Infect. Dis. 2021, 27, 18. [Google Scholar] [CrossRef] [PubMed]
- Lamoth, F.; Glampedakis, E.; Boillat-Blanco, N.; Oddo, M.; Pagani, J.-L. Incidence of invasive pulmonary aspergillosis among critically ill COVID-19 patients. Clin. Microbiol. Infect. 2020, 26, 1706–1708. [Google Scholar] [CrossRef] [PubMed]
- White, L.; Dhillon, R.; Cordey, A. A national strategy to diagnose COVID-19 associated invasive fungal disease in the ICU. Clin. Infect. Dis. 2021, 73, e1634–e1644. [Google Scholar] [CrossRef] [PubMed]
- Reizine, F.; Pinceaux, K.; Lederlin, M.; Autier, B.; Guegan, H.; Gacouin, A.; Luque-Paz, D.; Boglione-Kerrien, C.; Bacle, A.; Le Dare, B. Influenza-and COVID-19-associated pulmonary aspergillosis: Are the pictures different? J. Fungi 2021, 7, 388. [Google Scholar] [CrossRef]
- Schwartz, I.S.; Friedman, D.Z.; Zapernick, L.; Dingle, T.C.; Lee, N.; Sligl, W.; Zelyas, N.; Smith, S.W. High rates of influenza-associated invasive pulmonary aspergillosis may not be universal: A retrospective cohort study from Alberta, Canada. Clin. Infect. Dis. 2020, 71, 1760–1763. [Google Scholar] [CrossRef]
- Schauwvlieghe, A.F.; Rijnders, B.J.; Philips, N.; Verwijs, R.; Vanderbeke, L.; Van Tienen, C.; Lagrou, K.; Verweij, P.E.; Van de Veerdonk, F.L.; Gommers, D. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: A retrospective cohort study. Lancet Respir. Med. 2018, 6, 782–792. [Google Scholar] [CrossRef]
- Wauters, J.; Baar, I.; Meersseman, P.; Meersseman, W.; Dams, K.; De Paep, R.; Lagrou, K.; Wilmer, A.; Jorens, P.; Hermans, G. Invasive pulmonary aspergillosis is a frequent complication of critically ill H1N1 patients: A retrospective study. Intensive Care Med. 2012, 38, 1761–1768. [Google Scholar] [CrossRef]
- Levandowski, R.A.; Gerrity, T.R.; Garrard, C.S. Modifications of lung clearance mechanisms by acute influenza A infection. J. Lab. Clin. Med. 1985, 106, 424–427. [Google Scholar]
- Feys, S.; Gonçalves, S.M.; Khan, M.; Choi, S.; Boeckx, B.; Chatelain, D.; Cunha, C.; Debaveye, Y.; Hermans, G.; Hertoghs, M. Lung epithelial and myeloid innate immunity in influenza-associated or COVID-19-associated pulmonary aspergillosis: An observational study. Lancet Respir. Med. 2022, 22, 00259-4. [Google Scholar] [CrossRef]
- Latgé, J.-P.; Chamilos, G. Aspergillus fumigatus and Aspergillosis in 2019. Clin. Microbiol. Rev. 2019, 33, e00140-18. [Google Scholar] [CrossRef]
- Croft, C.A.; Culibrk, L.; Moore, M.M.; Tebbutt, S.J. Interactions of Aspergillus fumigatus conidia with airway epithelial cells: A critical review. Front. Microbiol. 2016, 7, 472. [Google Scholar] [CrossRef] [PubMed]
- Dewi, I.M.; Janssen, N.A.; Rosati, D.; Bruno, M.; Netea, M.G.; Brüggemann, R.J.; Verweij, P.E.; van de Veerdonk, F.L. Invasive pulmonary aspergillosis associated with viral pneumonitis. Curr. Opin. Microbiol. 2021, 62, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Somers, E.C.; Eschenauer, G.A.; Troost, J.P.; Golob, J.L.; Gandhi, T.N.; Wang, L.; Zhou, N.; Petty, L.A.; Baang, J.H.; Dillman, N.O. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin. Infect. Dis. 2021, 73, e445–e454. [Google Scholar] [CrossRef] [PubMed]
- Leistner, R.; Schroeter, L.; Adam, T.; Poddubnyy, D.; Stegemann, M.; Siegmund, B.; Maechler, F.; Geffers, C.; Schwab, F.; Gastmeier, P. Corticosteroids as risk factor for COVID-19-associated pulmonary aspergillosis in intensive care patients. Crit. Care 2022, 26, 30. [Google Scholar] [CrossRef]
- De Pauw, B.; Walsh, T.J.; Donnelly, J.P.; Stevens, D.A.; Edwards, J.E.; Calandra, T.; Pappas, P.G.; Maertens, J.; Lortholary, O.; Kauffman, C.A. Revised definitions of invasive fungal disease from the European organization for research and treatment of cancer/invasive fungal infections cooperative group and the national institute of allergy and infectious diseases mycoses study group (EORTC/MSG) consensus group. Clin. Infect. Dis. 2008, 46, 1813–1821. [Google Scholar]
- Verweij, P.E.; Brüggemann, R.J.; Azoulay, E.; Bassetti, M.; Blot, S.; Buil, J.B.; Calandra, T.; Chiller, T.; Clancy, C.J.; Cornely, O.A. Taskforce report on the diagnosis and clinical management of COVID-19 associated pulmonary aspergillosis. Intensive Care Med. 2021, 47, 819–834. [Google Scholar] [CrossRef]
- Lamoth, F.; Lewis, R.E.; Walsh, T.J.; Kontoyiannis, D.P. Navigating the Uncertainties of COVID-19–Associated Aspergillosis: A Comparison With Influenza-Associated Aspergillosis. J. Infect. Dis. 2021, 224, 1631–1640. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef]
- Blot, S.; Taccone, F.; Van den Abeele, A.; Bulpa, P.; Meersseman, W.; Brusselaers, N.; Dimopoulos, G.; Paiva, J.; Misset, B.; Rello, J.; et al. A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am. J. Respir. Crit. Care Med. 2012, 186, 56–64. [Google Scholar] [CrossRef]
- Verweij, P.E.; Rijnders, B.J.; Brüggemann, R.J.; Azoulay, E.; Bassetti, M.; Blot, S.; Calandra, T.; Clancy, C.J.; Cornely, O.A.; Chiller, T. Review of influenza-associated pulmonary aspergillosis in ICU patients and proposal for a case definition: An expert opinion. Intensive Care Med. 2020, 46, 1524–1535. [Google Scholar] [CrossRef]
- Bounhiol, A.; Pasquier, G.; Novara, A.; Bougnoux, M.-E.; Dannaoui, E. Aspergillus detection in airways of ICU COVID-19 patients: To treat or not to treat? J. Med. Mycol. 2022, 32, 101290. [Google Scholar] [CrossRef] [PubMed]
- Meersseman, W.; Lagrou, K.; Maertens, J.; Wijngaerden, E.V. Invasive aspergillosis in the intensive care unit. Clin. Infect. Dis. 2007, 45, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Hage, C.A.; Carmona, E.M.; Epelbaum, O.; Evans, S.E.; Gabe, L.M.; Haydour, Q.; Knox, K.S.; Kolls, J.K.; Murad, M.H.; Wengenack, N.L. Microbiological laboratory testing in the diagnosis of fungal infections in pulmonary and critical care practice. An official American Thoracic Society clinical practice guideline. Am. J. Respir. Crit. Care Med. 2019, 200, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Bussini, L.; Hoenigl, M.; Bartoletti, M. Prevalence of COVID-19-Associated Pulmonary Aspergillosis: Critical Review and Conclusions. J. Fungi 2022, 8, 390. [Google Scholar] [CrossRef] [PubMed]
- Cruciani, M.; Mengoli, C.; Barnes, R.; Donnelly, J.P.; Loeffler, J.; Jones, B.L.; Klingspor, L.; Maertens, J.; Morton, C.O.; White, L.P. Polymerase chain reaction blood tests for the diagnosis of invasive aspergillosis in immunocompromised people. Cochrane Database Syst. Rev. 2019, 9, CD009551. [Google Scholar] [CrossRef]
- Sharma, A.; Bisht, D.; Das, S.; Rai, G.; Dutt, S.; Arora, V. Molecular detection of Aspergillus in sputum of patients with lower respiratory tract infections. Int. J. Appl. Basic Med. Res. 2020, 10, 86. [Google Scholar]
- Imbert, S.; Meyer, I.; Palous, M.; Brossas, J.-Y.; Uzunov, M.; Touafek, F.; Gay, F.; Trosini-Desert, V.; Fekkar, A. Aspergillus PCR in bronchoalveolar lavage fluid for the diagnosis and prognosis of aspergillosis in patients with hematological and non-hematological conditions. Front. Microbiol. 2018, 9, 1877. [Google Scholar] [CrossRef]
- Janssen, N.; Nyga, R.; Vanderbeke, L.; Jacobs, C.; Ergün, M.; Buil, J.; van Dijk, K.; Altenburg, J.; Bouman, C.; van der Spoel, H.; et al. Multinational Observational Cohort Study of COVID-19–Associated Pulmonary Aspergillosis. Emerg. Infect. Dis. 2021, 27, 2892–2898. [Google Scholar] [CrossRef]
- Jenks, J.D.; Prattes, J.; Frank, J.; Spiess, B.; Mehta, S.R.; Boch, T.; Buchheidt, D.; Hoenigl, M. Performance of the bronchoalveolar lavage fluid aspergillus galactomannan lateral flow assay with cube reader for diagnosis of invasive pulmonary aspergillosis: A multicenter cohort study. Clin. Infect. Dis. 2021, 73, e1737–e1744. [Google Scholar] [CrossRef]
- Jenks, J.D.; Miceli, M.H.; Prattes, J.; Mercier, T.; Hoenigl, M. The aspergillus lateral flow assay for the diagnosis of invasive aspergillosis: An update. Curr. Fungal Infect. Rep. 2020, 14, 378–383. [Google Scholar] [CrossRef]
- Mercier, T.; Dunbar, A.; Veldhuizen, V.; Holtappels, M.; Schauwvlieghe, A.; Maertens, J.; Rijnders, B.; Wauters, J. Point of care aspergillus testing in intensive care patients. Crit. Care 2020, 24, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Autier, B.; Prattes, J.; White, P.L.; Valerio, M.; Machado, M.; Price, J.; Egger, M.; Gangneux, J.-P.; Hoenigl, M. Aspergillus lateral flow assay with digital reader for the diagnosis of COVID-19-associated pulmonary aspergillosis (CAPA): A multicenter study. J. Clin. Microbiol. 2022, 60, e01689-21. [Google Scholar] [CrossRef] [PubMed]
- D’Haese, J.; Theunissen, K.; Vermeulen, E.; Schoemans, H.; De Vlieger, G.; Lammertijn, L.; Meersseman, P.; Meersseman, W.; Lagrou, K.; Maertens, J. Detection of galactomannan in bronchoalveolar lavage fluid samples of patients at risk for invasive pulmonary aspergillosis: Analytical and clinical validity. J. Clin. Microbiol. 2012, 50, 1258–1263. [Google Scholar] [CrossRef] [PubMed]
- Talento, A.F.; Dunne, K.; Joyce, E.A.; Palmer, M.; Johnson, E.; White, P.L.; Springer, J.; Loeffler, J.; Ryan, T.; Collins, D. A prospective study of fungal biomarkers to improve management of invasive fungal diseases in a mixed specialty critical care unit. J. Crit. Care 2017, 40, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Prüller, F.; Krause, R.; Prattes, J.; Hoenigl, M. Utility of Serum 1, 3-β-d-Glucan Testing for Diagnosis and Prognostication in COVID-19-Associated Pulmonary Aspergillosis. Microbiol. Spectr. 2022, 10, e01373-22. [Google Scholar] [CrossRef]
- Maertens, J.; Verhaegen, J.; Demuynck, H.; Brock, P.; Verhoef, G.; Vandenberghe, P.; Van Eldere, J.; Verbist, L.; Boogaerts, M. Autopsy-controlled prospective evaluation of serial screening for circulating galactomannan by a sandwich enzyme-linked immunosorbent assay for hematological patients at risk for invasive aspergillosis. J. Clin. Microbiol. 1999, 37, 3223–3228. [Google Scholar] [CrossRef]
- Prattes, J.; Wauters, J.; Giacobbe, D.R.; Lagrou, K.; Hoenigl, M. Diagnosis and treatment of COVID-19 associated pulmonary apergillosis in critically ill patients: Results from a European confederation of medical mycology registry. Intensive Care Med. 2021, 47, 1158–1160. [Google Scholar] [CrossRef]
- Petraitiene, R.; Petraitis, V.; Bacher, J.D.; Finkelman, M.A.; Walsh, T.J. Effects of host response and antifungal therapy on serum and BAL levels of galactomannan and (1 → 3)-β-D-glucan in experimental invasive pulmonary aspergillosis. Med. Mycol. 2015, 53, 558–568. [Google Scholar] [CrossRef]
- Ghazanfari, M.; Yazdani Charati, J.; Davoodi, L.; Arastehfar, A.; Moazeni, M.; Abastabar, M.; Haghani, I.; Mayahi, S.; Hoenigl, M.; Pan, W. Comparative analysis of Galactomannan Lateral Flow Assay, Galactomannan Enzyme Immunoassay and BAL culture for Diagnosis of COVID-19 associated pulmonary aspergillosis. Mycoses 2022, 65, 960–968. [Google Scholar] [CrossRef]
- Bow, E.J.; Laverdière, M.; Lussier, N.; Rotstein, C.; Cheang, M.S.; Ioannou, S. Antifungal prophylaxis for severely neutropenic chemotherapy recipients: A meta-analysis of randomized-controlled clinical trials. Cancer 2002, 94, 3230–3246. [Google Scholar] [CrossRef]
- Teh, B.W.; Yeoh, D.K.; Haeusler, G.M.; Yannakou, C.K.; Fleming, S.; Lindsay, J.; Slavin, M.A.; Committee, A.A.G.S.; Thursky, K.A. Consensus guidelines for antifungal prophylaxis in haematological malignancy and haemopoietic stem cell transplantation, 2021. Intern. Med. J. 2021, 51, 67–88. [Google Scholar] [CrossRef] [PubMed]
- Cornely, O.A.; Maertens, J.; Winston, D.J.; Perfect, J.; Ullmann, A.J.; Walsh, T.J.; Helfgott, D.; Holowiecki, J.; Stockelberg, D.; Goh, Y.-T. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N. Engl. J. Med. 2007, 356, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, A.J.; Aguado, J.M.; Arikan-Akdagli, S.; Denning, D.W.; Groll, A.H.; Lagrou, K.; Lass-Flörl, C.; Lewis, R.E.; Munoz, P.; Verweij, P.E. Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect. 2018, 24, e1–e38. [Google Scholar] [CrossRef] [PubMed]
- Huggins, J.P.; Pease, R.; Stanly, K.; Workman, A.; Reynolds, J.; Alexander, B.D. Safety of Inhaled Amphotericin B Lipid Complex as Antifungal Prophylaxis in Lung Transplant Recipients. Antimicrob. Agents Chemother. 2022, 66, e00283-22. [Google Scholar] [CrossRef]
- Peghin, M.; Monforte, V.; Martin-Gomez, M.T.; Ruiz-Camps, I.; Berastegui, C.; Saez, B.; Riera, J.; Ussetti, P.; Solé, J.; Gavaldá, J. 10 years of prophylaxis with nebulized liposomal amphotericin B and the changing epidemiology of Aspergillus spp. infection in lung transplantation. Transpl. Int. 2016, 29, 51–62. [Google Scholar] [CrossRef]
- Monforte, V.; Ussetti, P.; Gavaldà, J.; Bravo, C.; Laporta, R.; Len, O.; García-Gallo, C.L.; Tenorio, L.; Solé, J.; Román, A. Feasibility, tolerability, and outcomes of nebulized liposomal amphotericin B for Aspergillus infection prevention in lung transplantation. J. Heart Lung Transplant. 2010, 29, 523–530. [Google Scholar] [CrossRef]
- Borro, J.; Sole, A.; De la Torre, M.; Pastor, A.; Fernandez, R.; Saura, A.; Delgado, M.; Monte, E.; Gonzalez, D. Efficiency and safety of inhaled amphotericin B lipid complex (Abelcet) in the prophylaxis of invasive fungal infections following lung transplantation. Transplant. Proc. 2008, 9, 3090–3093. [Google Scholar] [CrossRef]
- Lowry, C.; Marty, F.; Vargas, S.; Lee, J.; Fiumara, K.; Deykin, A.; Baden, L. Safety of aerosolized liposomal versus deoxycholate amphotericin B formulations for prevention of invasive fungal infections following lung transplantation: A retrospective study. Transpl. Infect. Dis. 2007, 9, 121–125. [Google Scholar] [CrossRef]
- Duckwall, M.J.; Gales, M.A.; Gales, B.J. Inhaled amphotericin B as aspergillosis prophylaxis in hematologic disease: An update. Microbiol. Insights 2019, 12, 1178636119869937. [Google Scholar] [CrossRef]
- Corcoran, T.; Venkataramanan, R.; Mihelc, K.; Marcinkowski, A.; Ou, J.; McCook, B.; Weber, L.; Carey, M.E.; Paterson, D.; Pilewski, J. Aerosol deposition of lipid complex amphotericin-B (Abelcet) in lung transplant recipients. Am. J. Transplant. 2006, 6, 2765–2773. [Google Scholar] [CrossRef]
- Vanderbeke, L.; Janssen, N.A.; Bergmans, D.C.; Bourgeois, M.; Buil, J.B.; Debaveye, Y.; Depuydt, P.; Feys, S.; Hermans, G.; Hoiting, O. Posaconazole for prevention of invasive pulmonary aspergillosis in critically ill influenza patients (POSA-FLU): A randomised, open-label, proof-of-concept trial. Intensive Care Med. 2021, 47, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Van Daele, R.; Wauters, J.; Dreesen, E.; Boelens, J.; Nulens, E.; Lormans, P.; Vanderbeke, L.; Jacobs, C.; Rijnders, B.; Verweij, P.E. Exposure to intravenous posaconazole in critically ill patients with influenza: A pharmacokinetic analysis of the POSA-FLU study. Mycoses 2022, 65, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Clancy, C.J.; Nguyen, M.-H. Rapid diagnosis of invasive candidiasis: Ready for prime-time? Curr. Opin. Infect. Dis. 2019, 32, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Playford, E.G.; Webster, A.C.; Sorrell, T.C.; Craig, J.C. Antifungal agents for preventing fungal infections in non-neutropenic critically ill and surgical patients: Systematic review and meta-analysis of randomized clinical trials. J. Antimicrob. Chemother. 2006, 57, 628–638. [Google Scholar] [CrossRef]
- Shorr, A.F.; Chung, K.; Jackson, W.L.; Waterman, P.E.; Kollef, M.H. Fluconazole prophylaxis in critically ill surgical patients: A meta-analysis. Crit. Care Med. 2005, 33, 1928–1935. [Google Scholar] [CrossRef]
- Eggimann, P.; Francioli, P.; Bille, J.; Schneider, R.; Wu, M.-M.; Chapuis, G.; Chiolero, R.; Pannatier, A.; Schilling, J.; Geroulanos, S. Fluconazole prophylaxis prevents intra-abdominal candidiasis in high-risk surgical patients. Crit. Care Med. 1999, 27, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Garbino, J.; Lew, D.P.; Romand, J.-A.; Hugonnet, S.; Auckenthaler, R.; Pittet, D. Prevention of severe Candida infections in nonneutropenic, high-risk, critically ill patients: A randomized, double-blind, placebo-controlled trial in patients treated by selective digestive decontamination. Intensive Care Med. 2002, 28, 1708–1717. [Google Scholar] [CrossRef]
- Pelz, R.K.; Hendrix, C.W.; Swoboda, S.M.; Diener-West, M.; Merz, W.G.; Hammond, J.; Lipsett, P.A. Double-blind placebo-controlled trial of fluconazole to prevent candidal infections in critically ill surgical patients. Ann. Surg. 2001, 233, 542. [Google Scholar] [CrossRef]
- Mian, P.; Trof, R.J.; Beishuizen, A.; Masselink, J.B.; Cornet, A.D.; Sportel, E.T. Suboptimal plasma concentrations with posaconazole suspension as prophylaxis in critically ill COVID-19 patients at risk of Covid-associated pulmonary aspergillosis. J. Clin. Pharm. Ther. 2022, 47, 383–385. [Google Scholar] [CrossRef]
- Rutsaert, L.; Steinfort, N.; Van Hunsel, T.; Bomans, P.; Naesens, R.; Mertes, H.; Dits, H.; Van Regenmortel, N. COVID-19-associated invasive pulmonary aspergillosis. Ann. Intensive Care 2020, 10, 71. [Google Scholar] [CrossRef]
- Soriano, M.C.; Narváez-Chávez, G.; López-Olivencia, M.; Fortún, J.; de Pablo, R. Inhaled amphotericin B lipid complex for prophylaxis against COVID-19-associated invasive pulmonary aspergillosis. Intensive Care Med. 2022, 48, 360–361. [Google Scholar] [CrossRef] [PubMed]
- Melchers, M.; van Zanten, A.R.; Heusinkveld, M.; Leeuwis, J.W.; Schellaars, R.; Lammers, H.J.; Kreemer, F.J.; Haas, P.-J.; Verweij, P.E.; van Bree, S.H. Nebulized Amphotericin B in Mechanically Ventilated COVID-19 Patients to Prevent Invasive Pulmonary Aspergillosis: A Retrospective Cohort Study. Crit. Care Explor. 2022, 4, e0696. [Google Scholar] [CrossRef] [PubMed]
- Van Ackerbroeck, S.; Rutsaert, L.; Roelant, E.; Dillen, K.; Wauters, J.; Van Regenmortel, N. Inhaled liposomal amphotericin-B as a prophylactic treatment for COVID-19-associated pulmonary aspergillosis/aspergillus tracheobronchitis. Crit. Care 2021, 25, 298. [Google Scholar] [CrossRef]
- van de Veerdonk, F.L.; Brüggemann, R.J.; Vos, S.; De Hertogh, G.; Wauters, J.; Reijers, M.H.; Netea, M.G.; Schouten, J.A.; Verweij, P.E. COVID-19-associated Aspergillus tracheobronchitis: The interplay between viral tropism, host defence, and fungal invasion. Lancet Respir. Med. 2021, 9, 795–802. [Google Scholar] [CrossRef]
- Nasir, N.; Farooqi, J.; Mahmood, S.F.; Jabeen, K. COVID-19-associated pulmonary aspergillosis (CAPA) in patients admitted with severe COVID-19 pneumonia: An observational study from Pakistan. Mycoses 2020, 63, 766–770. [Google Scholar] [CrossRef]
- Chong, W.H.; Neu, K.P. Incidence, diagnosis and outcomes of COVID-19-associated pulmonary aspergillosis (CAPA): A systematic review. J. Hosp. Infect. 2021, 113, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Mitaka, H.; Kuno, T.; Takagi, H.; Patrawalla, P. Incidence and mortality of COVID-19-associated pulmonary aspergillosis: A systematic review and meta-analysis. Mycoses 2021, 64, 993–1001. [Google Scholar] [CrossRef]
- Paramythiotou, E.; Dimopoulos, G.; Koliakos, N.; Siopi, M.; Vourli, S.; Pournaras, S.; Meletiadis, J. Epidemiology and incidence of COVID-19-associated pulmonary aspergillosis (CAPA) in a Greek tertiary care academic reference hospital. Infect. Dis. Ther. 2021, 10, 1779–1792. [Google Scholar] [CrossRef]
- Verweij, P.E.; Ananda-Rajah, M.; Andes, D.; Arendrup, M.C.; Brüggemann, R.J.; Chowdhary, A.; Cornely, O.A.; Denning, D.W.; Groll, A.H.; Izumikawa, K. International expert opinion on the management of infection caused by azole-resistant Aspergillus fumigatus. Drug Resist. Updat. 2015, 21, 30–40. [Google Scholar] [CrossRef]
- Ekinci, P.B.; Kara, E.; Er, A.G.; Inkaya, A.C.; Demirkan, K.; Uzun, O. Challenge in treating COVID-19 associate pulmonary aspergillosis: Supratherapeutic voriconazole levels. Br. J. Clin. Pharmacol. 2022, 88, 1387. [Google Scholar] [CrossRef]
- Permpalung, N.; Bazemore, K.; Chiang, T.P.-Y.; Mathew, J.; Barker, L.; Nematollahi, S.; Cochran, W.; Sait, A.S.; Avery, R.K.; Shah, P.D. Impact of COVID-19 on lung allograft and clinical outcomes in lung transplant recipients: A case-control study. Transplantation 2021, 105, 2072–2079. [Google Scholar] [CrossRef] [PubMed]
| Authors & Country | Study Design | CAPA Definition Used | N | Prophylaxis Used | CAPA Diagnosis (Prophylaxis vs. No Prophylaxis) | Mortality | Overall Summary & Author Recommendations |
|---|---|---|---|---|---|---|---|
| Mian et al. in the Netherlands | Prospective | Not specified | 7 | Oral Posaconazole 200 mg suspension three times daily ranging from 8–21 total doses | Not studied | Not studied | -Posaconazole suspension has poor bioavailability -Posaconazole suspension is not recommended to prevent CAPA |
| Hatzl et al. in Austria | Prospective | ECMM/ISHAM | 132 | Intravenous Posaconazole (dose, duration, and schedule not specified) | 1.4% vs. 17.5% | No difference | -CAPA is associated with poor outcomes -Antifungal prophylaxis can prevent CAPA |
| Rutsaert et al. in Belgium | Prospective | Not specified | N/A | Nebulized liposomal amphotericin B 12.5 mg (duration and schedule not specified) | Not specified | Not studied | -There should be a low threshold for screening, prophylaxis, and early antifungal treatment for CAPA |
| Soriano et al. in Spain | Prospective | ECMM/ISHAM | 45 | Nebulized amphotericin B lipid complex 50 mg every 48 h (total duration not specified) | Not studied | Not studied | -Prophylaxis should be considered to control an outbreak of CAPA |
| Melchers et al. in the Netherlands | Retrospective | ECMM/ISHAM | 39 | Nebulized conventional amphotericin B 10 mg twice daily for 22 days, followed by 5 mg four times daily (total duration not specified) | 27% vs. 67% | No difference | -Prophylaxis may need to start early during hospitalization -Antifungal prophylaxis can prevent CAPA |
| Van Ackerbroeck et al. in Belgium | Retrospective | Not specified | 78 | Nebulized liposomal amphotericin B 12.5 mg with 5 drops of salbutamol twice weekly | 9% vs. 61% | Not studied | -Antifungal prophylaxis can reduce the incidence of CAPA and reduce the incidence of aspergillus colonization |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hawes, A.M.; Permpalung, N. Diagnosis and Antifungal Prophylaxis for COVID-19 Associated Pulmonary Aspergillosis. Antibiotics 2022, 11, 1704. https://doi.org/10.3390/antibiotics11121704
Hawes AM, Permpalung N. Diagnosis and Antifungal Prophylaxis for COVID-19 Associated Pulmonary Aspergillosis. Antibiotics. 2022; 11(12):1704. https://doi.org/10.3390/antibiotics11121704
Chicago/Turabian StyleHawes, Armani M., and Nitipong Permpalung. 2022. "Diagnosis and Antifungal Prophylaxis for COVID-19 Associated Pulmonary Aspergillosis" Antibiotics 11, no. 12: 1704. https://doi.org/10.3390/antibiotics11121704
APA StyleHawes, A. M., & Permpalung, N. (2022). Diagnosis and Antifungal Prophylaxis for COVID-19 Associated Pulmonary Aspergillosis. Antibiotics, 11(12), 1704. https://doi.org/10.3390/antibiotics11121704







