Piperacillin–Tazobactam as an Adjuvant in the Mechanical Treatment of Patients with Periodontitis: A Randomized Clinical Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
- Patients older than 18 years;
- ASA I or II patients;
- Patients who had a bacterial load of at least one of the periodontal pathogens at the time of diagnosis.
- Patients younger than 18 years;
- ASA III and IV patients;
- Patients who had received periodontal treatment in the last 6 months;
- Smoking patients;
- Pregnant or lactating women;
- Immunocompromised patients or patients in treatment with bisphosphonates.
2.2. Timeline
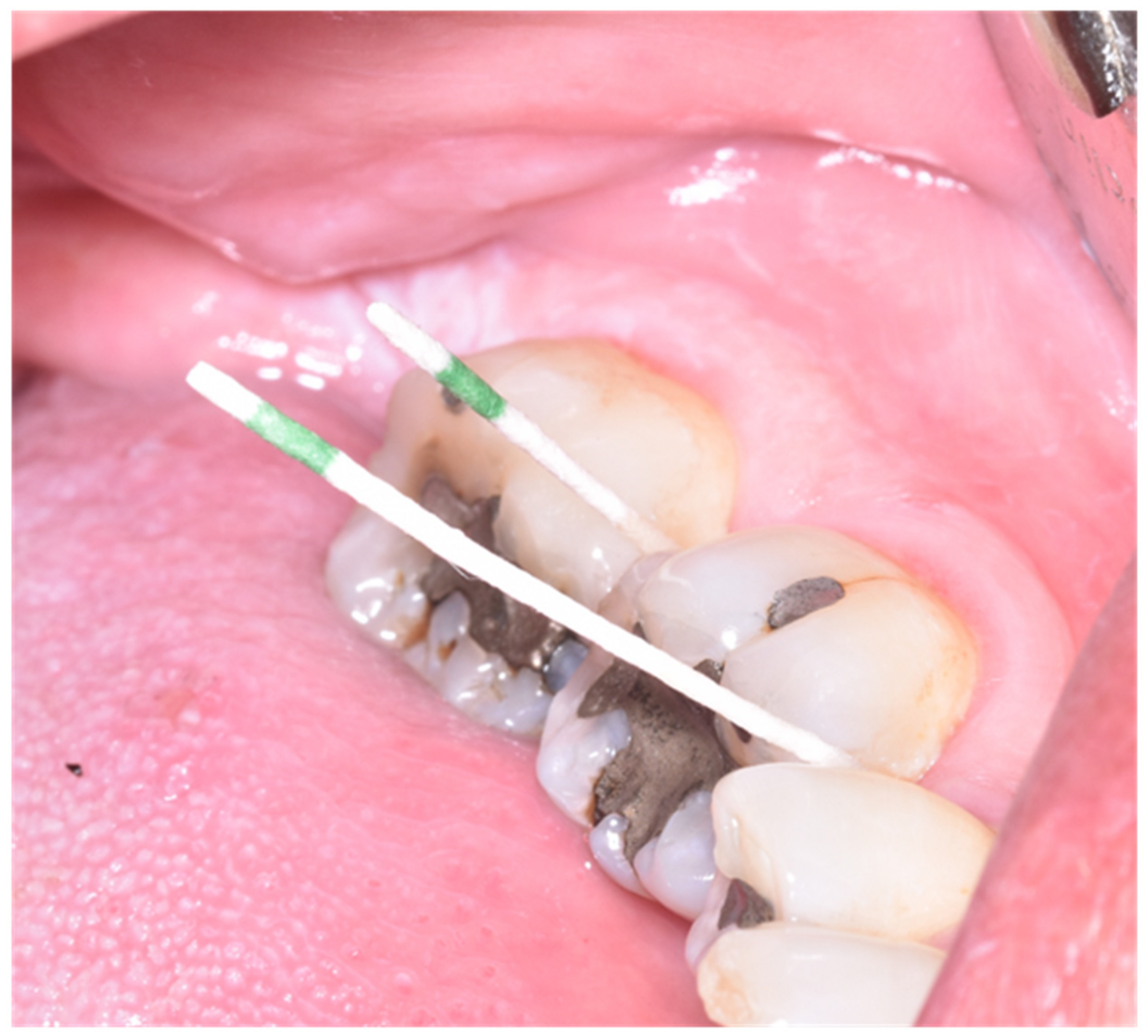
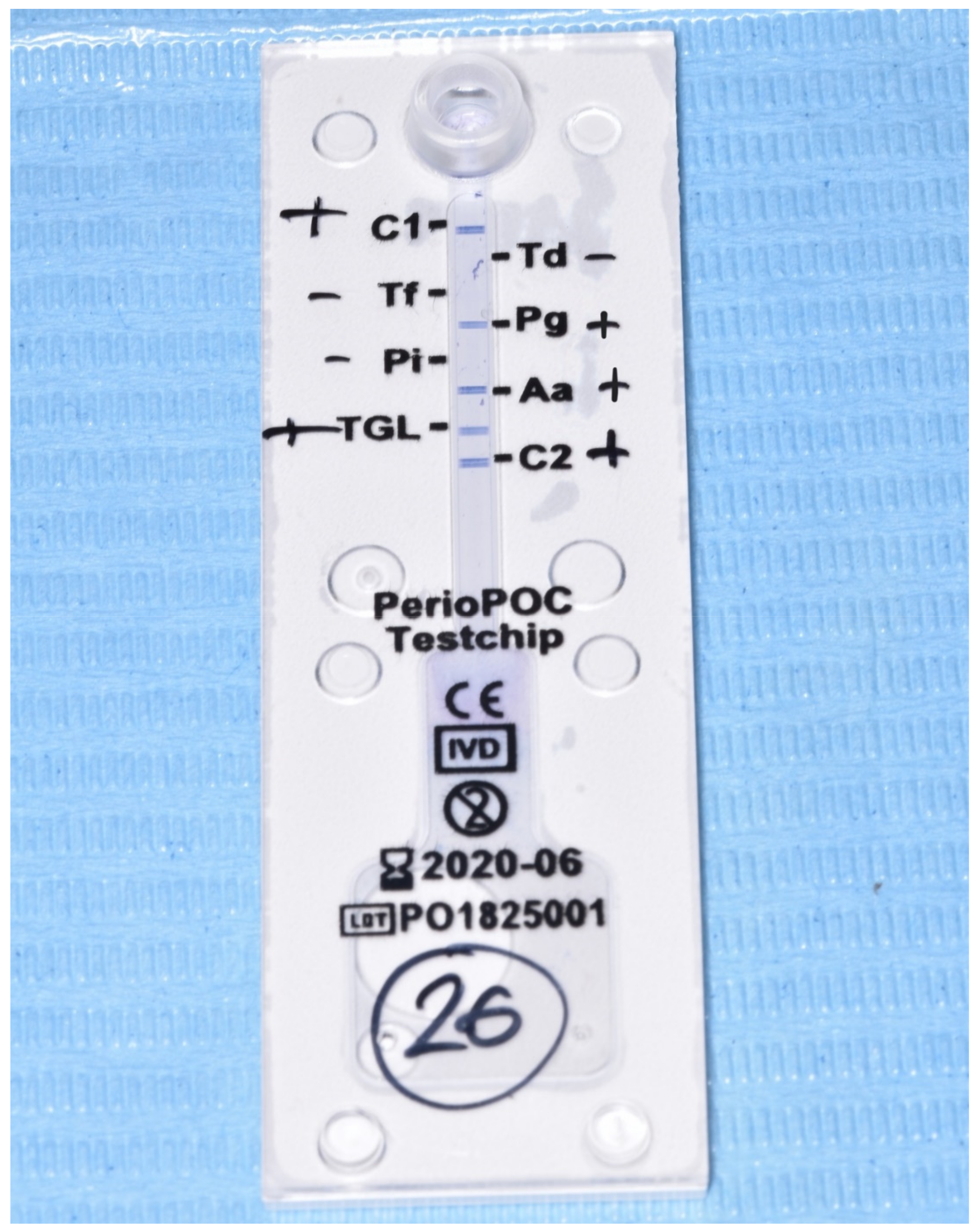
2.3. Evaluation of Microbiological Data
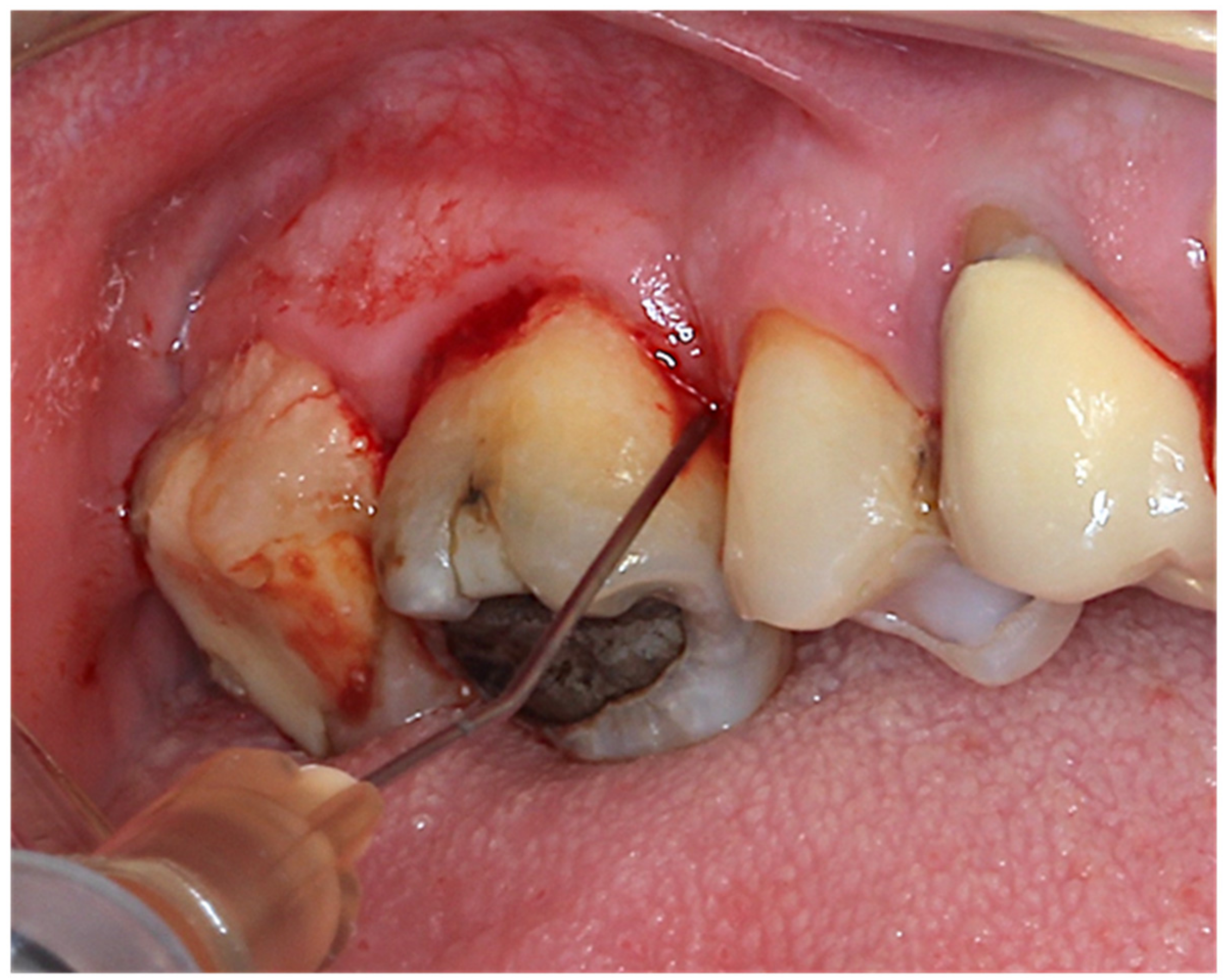
2.4. Intervention and Randomization
2.5. Evaluation of Clinical Data
- -
- Clinical attachment level (CAL): The CAL is the distance from the cementoenamel junction to the bottom of the subgingival sulcus. The measurements were obtained at 6 points on each tooth (mesial, middle, and distal on the buccal; mesial, middle, and distal on the palatal/lingual) in millimeters using a CP12 periodontal probe;
- -
- Probing pocket depth (PPD): The PPD is the distance from the gingival margin to the bottom of the subgingival sulcus. The measures were obtained at 6 points on each tooth (mesial, middle, and distal on the buccal; mesial, middle, and distal on the palatal/lingual) in millimeters using a CP12 periodontal probe;
- -
- Löe-Silness plaque index (IPL): All teeth were assessed in 4 gingival units (buccal, palatal/lingual, mesial, and distal), assigning a code to each of them. The index value was calculated by adding the numerical value of each gingival unit and dividing it by the number of units studied:
- 0 = No plaque in the gingival area;
- 1 = A thin film of plaque adhering to the free gingival margin and to the adjacent area of the tooth, which could only be recognized by passing a probe through the tooth surface or revealing it;
- 2 = Moderate accumulation of soft deposits within the gingival pocket, on the gingival margin, and/or adjacent to the tooth surface, which was recognizable at a glance;
- 3 = Abundance of soft 1–2 mm-thick material from the gingival pocket and/or on the gingival margin and adjacent tooth surface;
- -
- Löe -Silness gingival index (GI): Each tooth was divided into 4 gingival units (buccal, palatal/lingual, distal, and mesial). Each gingival unit was scored from 0 to 3. We assessed the average of all the values obtained:
- 0 = Normal gingiva, no swelling, no discoloration, no bleeding;
- 1 = Mild swelling, slight color change, slight edema, no bleeding on probing;
- 2 = Moderate swelling, redness, edema, bleeding on probing and pressure;
- 3 = Marked inflammation, marked redness, edema, ulceration, spontaneous bleeding, eventual ulceration.
2.6. Statistical Analysis
3. Results
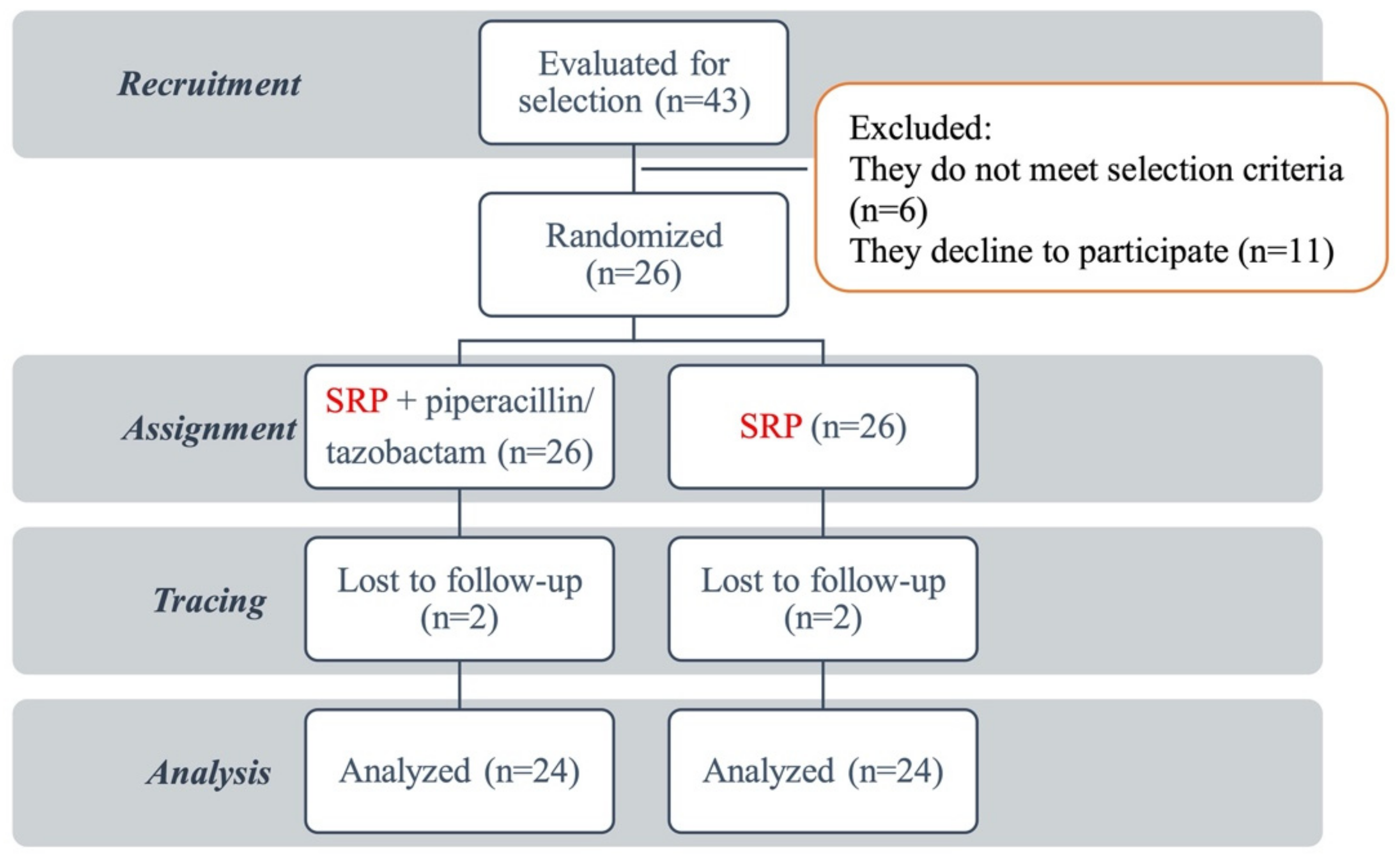
3.1. Study Sample
3.2. Clinical Results
3.3. Microbiological Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Magda Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. 1), S173–S182. [Google Scholar] [CrossRef] [PubMed]
- Kranendonk, A.; van der Reijden, W.; van Winkelhoff, A.; van der Weijden, G. The bactericidal effect of a Genius Nd:YAG laser. Int. J. Dent. Hyg. 2010, 8, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Keestra, J.A.J.; Grosjean, I.; Coucke, W.; Quirynen, M.; Teughels, W. Non-surgical periodontal therapy with systemic antibiotics in patients with untreated aggressive periodontitis: A systematic review and meta-analysis. J. Periodontal. Res. 2015, 50, 689–706. [Google Scholar] [CrossRef] [PubMed]
- Chambrone, L.; Vargas, M.; Arboleda, S.; Serna, M.; Guerrero, M.; de Sousa, J.; Lafaurie, G.I. Efficacy of local and systemic antimicrobials in the non-surgical treatment of smokers with chronic periodontitis: A systematic review. J. Periodontal. 2016, 87, 1320–1332. [Google Scholar] [CrossRef] [PubMed]
- Chisci, G.; Hatia, A. Antibiotics in orthognathic surgery and postoperative infections. Int. J. Oral Maxillofac. Surg. 2022. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosio, F.; Di Spirito, F.; De Caro, F.; Lanza, A.; Passarella, D.; Sbordone, L. Adherence to Antibiotic Prescription of Dental Patients: The Other Side of the Antimicrobial Resistance. Healthcare 2022, 10, 1636. [Google Scholar] [CrossRef]
- Bergamaschi, C.C.; Santamaria, M.P.; Berto, L.A.; Cogo-Müller, K.; Motta, R.H.L.; Salum, E.A.; Groppo, F.C. Full mouth periodontal debridement with or without adjunctive metronidazole gel in smoking patients with chronic periodontitis: A pilot study. J. Periodontal. Res. 2016, 51, 50–59. [Google Scholar] [CrossRef]
- Wang, C.Y.; Yang, Y.H.; Li, H.; Lin, P.Y.; Su, Y.T.; Kuo, M.Y.P.; Tu, Y.K. Adjunctive local treatments for patients with residual pockets during supportive periodontal care: A systematic review and network meta-analysis. J. Clin. Periodontol. 2020, 47, 1496–1510. [Google Scholar] [CrossRef]
- Trajano, V.C.D.C.; Brasileiro, C.B.; Henriques, J.A.S.; Cota, L.M.; Lanza, C.R.; Cortés, M.E. Doxycycline encapsulated in β-cyclodextrin for periodontitis: A clinical trial. Arm. Oral Res. 2020, 33, e112. [Google Scholar] [CrossRef]
- Killeen, A.C.; Harn, J.A.; Erickson, L.M.; Yu, F.; Reinhardt, R.A. Local minocycline effect on inflammation and clinical attachment during periodontal maintenance: Randomized clinical trial. J. Periodontal. 2016, 87, 1149–1157. [Google Scholar] [CrossRef]
- Agarwal, E.; Pradeep, A.R.; Bajaj, P.; Naik, S.B. Efficacy of local drug selivery of 0.5% clarithromycin gel as an adjunct to non-surgical periodontal therapy in the treatment of current smokers with chronic periodontitis: A randomized controlled clinical trial. J. Periodontal. 2012, 83, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Nadig, P.S.; Shah, M.A. Tetracycline as local drug delivery in treatment of chronic periodontitis: A systematic review and meta-analysis. J. Indian Soc. Periodontal. 2016, 20, 576. [Google Scholar] [CrossRef] [PubMed]
- Bashir, N.; Sharma, P. Clarithromycin as an adjunct to periodontal therapy: A systematic review and meta-analysis. Int. J. Dent. Hyg. 2022, 20, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Tan, O.L.; Safii, S.H.; Razali, M. Clinical Efficacy of Repeated Applications of Local Drug Delivery and Adjunctive Agents in Nonsurgical Periodontal Therapy: A Systematic Review. Antibiotics 2021, 10, 1178. [Google Scholar] [CrossRef]
- Busa, A.; Parrini, S.; Chisci, G.; Pozzi, T.; Burgassi, S.; Capuano, A. Local versus systemic antibiotics effectiveness: A comparative study of postoperative oral disability in lower third molar surgery. J. Craniofac. Surg. 2014, 25, 708–709. [Google Scholar] [CrossRef] [PubMed]
- Zirk, M.; Dreiiseidler, T.; Pohl, M.; Rothamel, D.; Buller, J.; Peters, F.; Zoller, J.E.; Kreppel, M. Odontogenic sinusitis maxillaris: A retrospective study of 121 cases with surgical intervention. J. Craniomaxillofac. Surg. 2017, 45, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Lauenstein, M.; Kaufmann, M.; Persson, G.R. Clinical and microbiological results following nonsurgical periodontal therapy with or without local administration of piperacillin/tazobactam. Clin. Oral Investig. 2013, 17, 1645–1660. [Google Scholar] [CrossRef][Green Version]
- Callow Pueyo, S.; Martínez-González, J.M. Evaluation of the Inhibitory Efficacy of Piperacillin in Periodontopathogens Related to Peri-Implant Diseases. 2018. Available online: https://eprints.ucm.es/50149 (accessed on 30 July 2022).
- Arweiler, N.B.; Marx, V.K.; Laugisch, O.; Sculean, A.; Auschill, T.M. Clinical evaluation of a newly developed chairside test to determine periodontal pathogens. J. Periodontal. 2020, 91, 387–395. [Google Scholar] [CrossRef]
- Suvan, J.; Leira, Y.; Moreno Sancho, F.M.; Graziani, F.; Derks, J.; Tomasi, C. Subgingival instrumentation for treatment of periodontitis: A systematic review. J. Clin. Periodontol. 2020, 47 (Suppl. 22), 155–175. [Google Scholar] [CrossRef]
- Park, S.H.; Song, Y.W.; Cha, J.K.; Lee, J.S.; Kim, Y.T.; Shin, H.S.; Kim, C.S. Adjunctive use of metronidazole-minocycline ointment in the nonsurgical treatment of peri-implantitis: A multicenter randomized controlled trial. Clin. Implant Dent. Relat. Res. 2021, 23, 543–554. [Google Scholar] [CrossRef]
- Yusri, S.; Elfana, A.; Elbattawy, W.; Fawzy El-Sayed, K.M. Effect of locally delivered adjunctive antibiotics during surgical periodontal therapy: A systematic review and meta-analysis. Clin. Oral Investig. 2021, 25, 5127–5138. [Google Scholar] [CrossRef] [PubMed]
- Toledano, M.; Osorio, M.T.; Vallecillo-Rivas, M.; Toledano-Osorio, M.; Rodríguez-Archilla, A.; Toledano, R.; Osorio, R. Efficacy of local antibiotic therapy in the treatment of peri-implantitis: A systematic review and meta-analysis. J. Dent. 2021, 113, 103790. [Google Scholar] [CrossRef] [PubMed]
- González Regueiro, I.; Martínez Rodríguez, N.; Barona Dorado, C.; Sanz-Sánchez, I.; Montero, E.; Ata-Ali, J.; Duarte, F.; Martínez-González, J.M. Surgical approach combining implantoplasty and reconstructive therapy with locally delivered antibiotic in the treatment of peri-implantitis: A prospective clinical case series. Clin. Implant Dent. Relat. Res. 2021, 23, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Laugisch, O.; Auschill, T.M.; Tumbrink, A.; Sculean, A.; Arweiler, N.B. Influence of Anti-Infective Periodontal Therapy on Subgingival Microbiota Evaluated by Chair-Side Test Compared to qPCR—A Clinical Follow-Up Study. Antibiotics 2022, 11, 577. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Taccardi, D.; Scribante, A. Home Oral Care of Periodontal Patients Using Antimicrobial Gel with Postbiotics, Lactoferrin, and Aloe Barbadensis Leaf Juice Powder vs. Conventional Chlorhexidine Gel: A Split-Mouth Randomized Clinical Trial. Antibiotics 2022, 11, 118. [Google Scholar] [CrossRef]
| Periodontitis Stage | Stage I | Stage II | Stage III | Stage IV | |
|---|---|---|---|---|---|
| Severity | Interdental CAL at site of greatest loss | 1 to 2 mm | 3 to 4 mm | ≥5 mm | ≥5 mm |
| Radiographic bone loss | Coronal third (<15%) | Coronal third (15 to 33%) | Extending to mid-third of root and beyond | Extending to mid-third of root and beyond | |
| Tooth loss | No tooth loss due to periodontitis | Tooth loss due to periodontitis of ≤4 teeth | Tooth loss due to periodontitis of ≥5 teeth | ||
| Complexity | Local | In addition to stage II complexity: | In addition to stage III complexity: | ||
|
|
|
| ||
| Extent and distribution | Add to stage as descriptor | For each stage, describe the extent as localized (<30% of teeth involved), generalized, or molar/incisor pattern | |||
| Clinical Parameter | Side Control (RAR) | Treatment Side (RAR + Piperacillin/Tazobactam) | |
|---|---|---|---|
| p-Value | |||
| Clinical attachment level (CAL) in mm | |||
| T0 (Basal) | 6.63 ± 1.44 | 6.38 ± 1.53 | 0.398 |
| T1 (15 days) | 5.33 ± 1.55 * | 4.96 ± 1.33 * | 0.131 |
| T2 (3 months) | 5.67 ± 1.40 * | 4.71 ± 1.27 * | 0.001a |
| T3 (6 months) | 5 ± 1.25 * | 4.25 ± 1.29 * | 0.002 a |
| ∆ T0–T3 | 1.63 ± 0.18 | 2.13 ± 0.17 | |
| Probing depth (PS) in mm | |||
| T0 (Basal) | 4.43 ± 0.84 | 4.34 ± 0.84 | 0.553 |
| T1 (15 days) | 3.50 ± 0.78 * | 3.29 ± 0.78 * | 0.139 |
| T2 (3 months) | 3.67 ± 0.84 * | 3.32 ± 0.8 * | 0.024 a |
| T3 (6 months) | 3.47 ± 0.73 * | 3.02 ± 0.83 * | 0.004 a |
| ∆ T0–T3 | 0.96 ± 0.14 | 1.32 ± 0.09 | |
| Plaque Index (IPL) | |||
| T0 (Basal) | 1.72 ± 0.64 | 1.67 ± 0.57 | 0.069 |
| T1 (15 days) | 1.42 ± 0.61 * | 1.3 ± 0.56 * | 0.000 a |
| T2 (3 months) | 1.46 ± 0.63 * | 1.3 ± 0.53 * | 0.002 a |
| T3 (6 months) | 1.41 ± 0.61 * | 1.21 ± 0.54 * | 0.000 a |
| ∆ T0–T3 | 0.31 ± 0.04 | 0.46 ± 0.04 | |
| Gingival index (GI) | |||
| T0 (Basal) | 1.62 ± 0.65 | 1.62 ± 0.64 | 0.992 |
| T1 (15 days) | 1.26 ± 0.58 * | 1.21 ± 0.56 * | 0.234 |
| T2 (3 months) | 1.37 ± 0.61 * | 1.28 ± 0.59 * | 0.105 |
| T3 (6 months) | 1.35 ± 0.59 * | 1.21 ± 0.6 * | 0.034 a |
| ∆ T0–T3 | 0.27 ± 0.03 | 0.40 ± 0.04 | |
| Clinical Parameter | Control Side (RAR) | Treatment Side (RAR + Piperacillin/Tazobactam) | |
|---|---|---|---|
| p-Value | |||
| Presence of Aa | |||
| T0 (Basal) | 5 (20.8%) | 5 (20.8%) | 1.000 |
| T1 (15 days) | 1 (4.2%) | 0 (0%) | 1.000 |
| T2 (3 months) | 4 (16.7%) | 1 (4.2%) | 0.250 |
| T3 (6 months) | 3 (12.5%) | 1 (4.2%) | 0.250 |
| ∆ T0–T3 | 2 (8.3%) | 4 (16.6%) | |
| Presence of Pg | |||
| T0 (Basal) | 14 (58.3%) | 14 (58.3%) | 1.000 |
| T1 (15 days) | 6 (25%) * | 4 (16.7%) * | 0.500 |
| T2 (3 months) | 13 (54.2%) | 10 (41.7%) | 0.250 |
| T3 (6 months) | 11 (45.8%) | 8 (33.3%) * | 0.250 |
| ∆ T0–T3 | 3 (12.5%) | 6 (25%) | |
| Presence of Pi | |||
| T0 (Basal) | 15 (62.5%) | 15 (62.5%) | 1.000 |
| T1 (15 days) | 9 (37.5%) * | 5 (20.8%) * | 0.125 |
| T2 (3 months) | 15 (62.5%) | 13 (54.2%) | 0.500 |
| T3 (6 months) | 14 (58.3%) | 11 (45.8%) | 0.250 |
| ∆ T0–T3 | 1 (4.2%) | 4 (16.7%) | |
| Presence of Td | |||
| T0 (Basal) | 14 (58.3%) | 14 (58.3) | 1.000 |
| T1 (15 days) | 6 (25%) * | 4 (16.7) * | 0.500 |
| T2 (3 months) | 14 (58.3%) | 8 (33.3) * | 0.031 a |
| T3 (6 months) | 11 (45.8%) | 7 (29.2) * | 0.125 |
| ∆ T0–T3 | 3 (12.5%) | 7 (29.1%) | |
| Presence of Tf | |||
| T0 (Basal) | 9 (37.5%) | 9 (37.5%) | 1.000 |
| T1 (15 days) | 1 (4.2%) * | 0 (0%) * | 1.000 |
| T2 (3 months) | 5 (20.8%) | 1 (4.2%) * | 0.219 |
| T3 (6 months) | 3 (12.5%) * | 1 (4.2%) * | 0.625 |
| ∆ T0–T3 | 6 (25%) | 8 (33.3%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hurtado-Celotti, D.; Martínez-Rodríguez, N.; Ruiz-Sáenz, P.L.; Barona-Dorado, C.; Santos-Marino, J.; Martínez-González, J.M. Piperacillin–Tazobactam as an Adjuvant in the Mechanical Treatment of Patients with Periodontitis: A Randomized Clinical Study. Antibiotics 2022, 11, 1689. https://doi.org/10.3390/antibiotics11121689
Hurtado-Celotti D, Martínez-Rodríguez N, Ruiz-Sáenz PL, Barona-Dorado C, Santos-Marino J, Martínez-González JM. Piperacillin–Tazobactam as an Adjuvant in the Mechanical Treatment of Patients with Periodontitis: A Randomized Clinical Study. Antibiotics. 2022; 11(12):1689. https://doi.org/10.3390/antibiotics11121689
Chicago/Turabian StyleHurtado-Celotti, Dolores, Natalia Martínez-Rodríguez, Pedro Luis Ruiz-Sáenz, Cristina Barona-Dorado, Juan Santos-Marino, and José María Martínez-González. 2022. "Piperacillin–Tazobactam as an Adjuvant in the Mechanical Treatment of Patients with Periodontitis: A Randomized Clinical Study" Antibiotics 11, no. 12: 1689. https://doi.org/10.3390/antibiotics11121689
APA StyleHurtado-Celotti, D., Martínez-Rodríguez, N., Ruiz-Sáenz, P. L., Barona-Dorado, C., Santos-Marino, J., & Martínez-González, J. M. (2022). Piperacillin–Tazobactam as an Adjuvant in the Mechanical Treatment of Patients with Periodontitis: A Randomized Clinical Study. Antibiotics, 11(12), 1689. https://doi.org/10.3390/antibiotics11121689






