Efficacy of Treatment with the Antibiotic Novobiocin against Infection with Bacillus anthracis or Burkholderia pseudomallei
Abstract
1. Introduction
2. Results
2.1. Antibiotic Efficacy against Parenteral Challenge with B. anthracis or B. pseudomallei
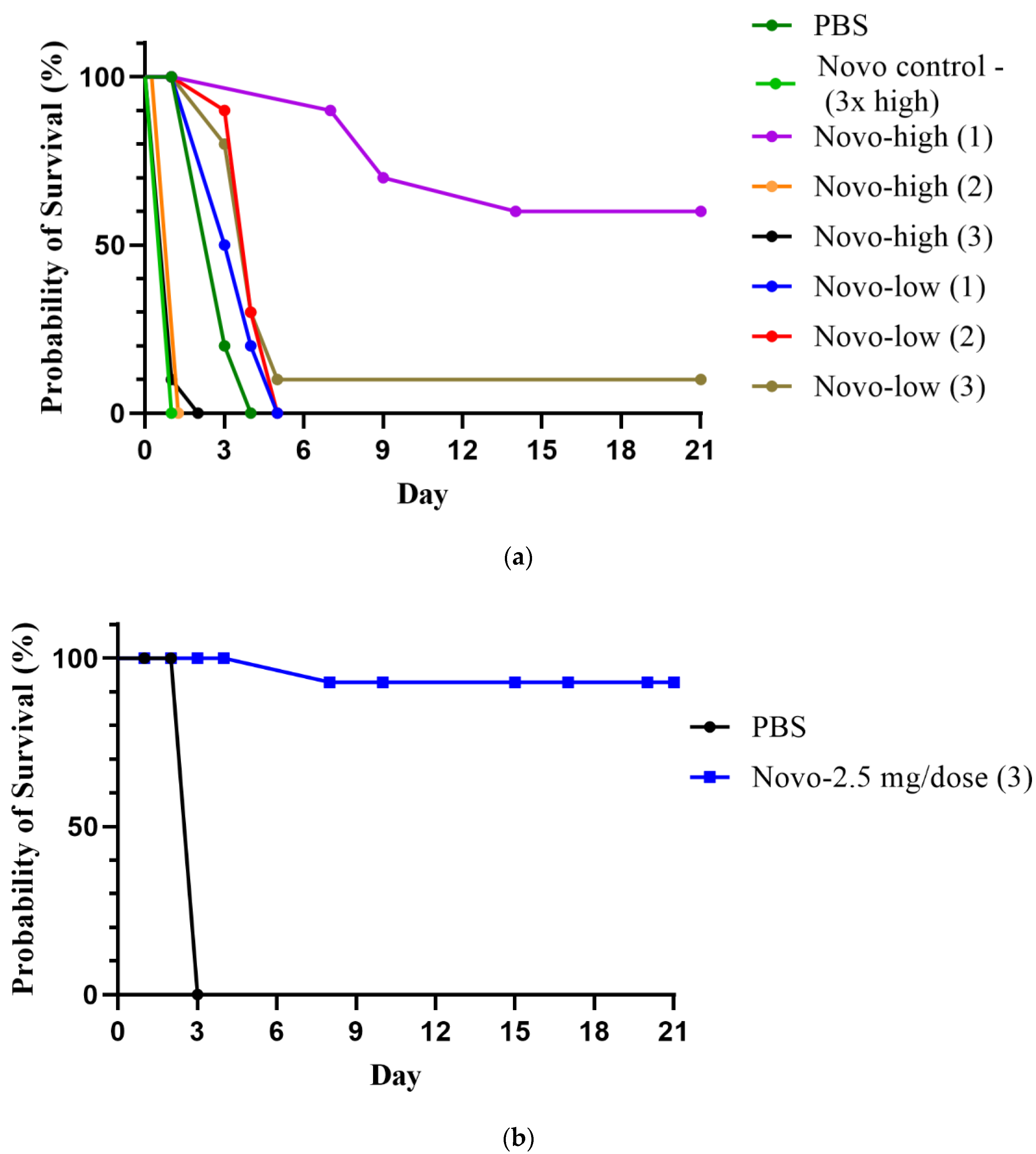
2.1.1. Anthrax Model
2.1.2. Melioidosis Model
2.2. Antibiotic Efficacy against Inhalational Exposure in Models of B. anthracis and B. pseudomallei
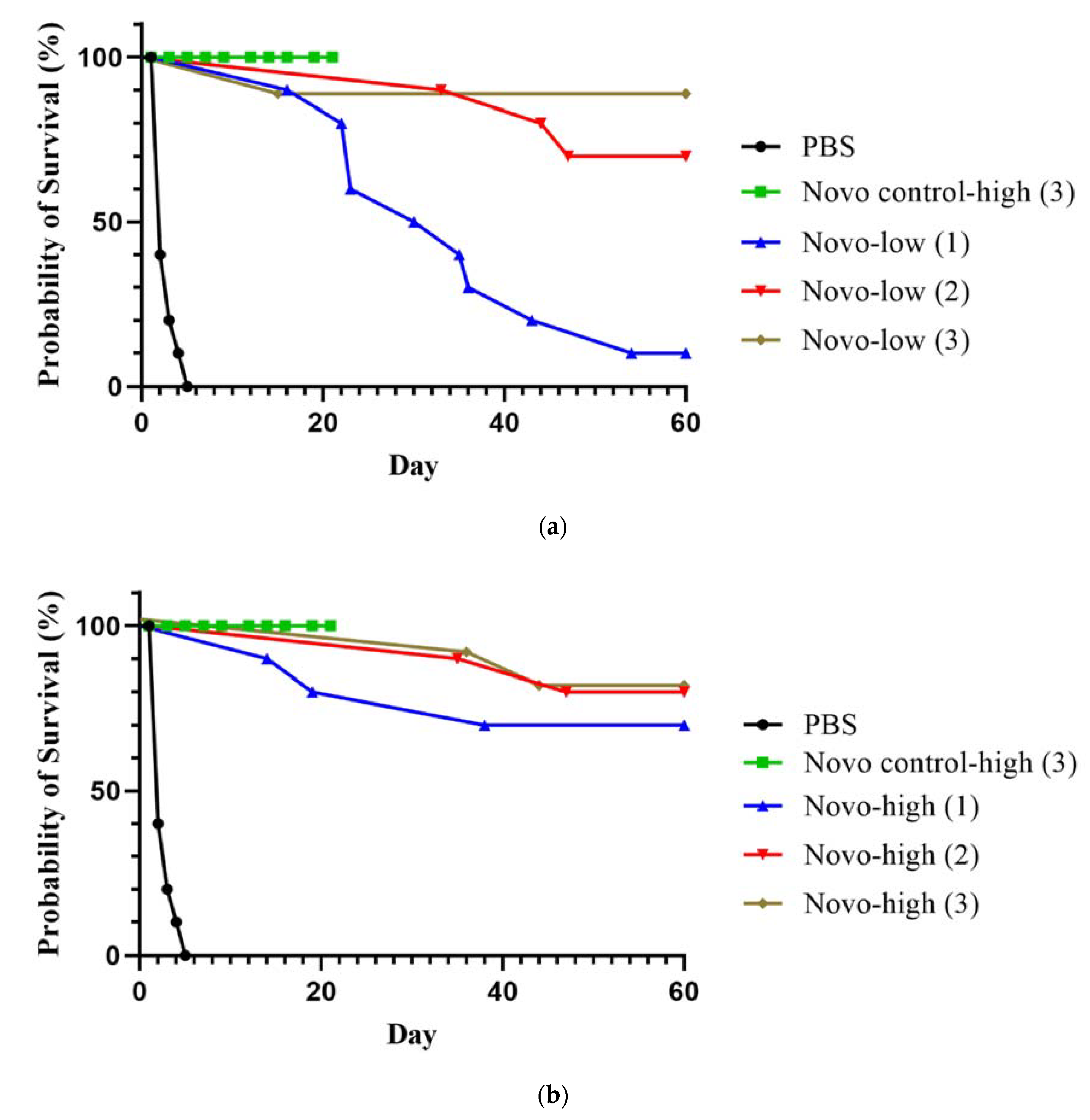
2.2.1. Anthrax Inhalational Model
2.2.2. Melioidosis Inhalational Model
2.3. Novobiocin in Combination with a Representative Partially Protective Vaccine
3. Discussion and Conclusions
4. Materials and Methods
4.1. Bacteria, Media, and Growth Conditions
4.2. Animal Challenges and Monitoring
4.3. Antibiotic Treatments and Vaccine
4.4. Bacteriology
4.5. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Disclaimer
References
- Dance, D.A.; Limmathurotsakul, D. Global Burden and Challenges of Melioidosis. Trop. Med. Infect Dis. 2018, 3, 13. [Google Scholar] [CrossRef]
- Wiersinga, W.J.; Currie, B.J.; Peacock, S.J. Melioidosis. N. Engl. J. Med. 2012, 367, 1035–1044. [Google Scholar] [CrossRef]
- Turnbull, P. Anthrax in Humans and Animals, 4th ed.; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Mock, M.; Fouet, A. Anthrax. Annu. Rev. Microbiol. 2001, 55, 647–671. [Google Scholar] [CrossRef]
- Welkos, S.; Bozue, J.; Twenhafel, N.; Cote, C. Animal Models for the Pathogenesis, Treatment, and Prevention of Infection by Bacillus anthracis. Microbiol. Spectr. 2015, 3, TBS-0001-2012. [Google Scholar] [CrossRef] [PubMed]
- 2020 Annual Report of the Federal Select Agent Program; United States Department of Agriculture: Washington, DC, USA, 2020; pp. 1–31. Available online: www.selectagents.gov (accessed on 9 November 2022).
- Bisacchi, G.S.; Manchester, J.I. A New-Class Antibacterial-Almost. Lessons in Drug Discovery and Development: A Critical Analysis of More than 50 Years of Effort toward ATPase Inhibitors of DNA Gyrase and Topoisomerase IV. ACS Infect Dis. 2015, 1, 4–41. [Google Scholar] [CrossRef]
- Cote, C.K.; Blanco, I.I.; Hunter, M.; Shoe, J.L.; Klimko, C.P.; Panchal, R.G.; Welkos, S.L. Combinations of early generation antibiotics and antimicrobial peptides are effective against a broad spectrum of bacterial biothreat agents. Microb. Pathog. 2020, 142, 104050. [Google Scholar] [CrossRef]
- Willcocks, S.J.; Cia, F.; Francisco, A.F.; Wren, B.W. Revisiting aminocoumarins for the treatment of melioidosis. Int. J. Antimicrob. Agents 2020, 56, 106002. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.L.; Rossi, L.; De Pascale, G.; Wright, G.D. A forward chemical screen identifies antibiotic adjuvants in Escherichia coli. ACS Chem. Biol. 2012, 7, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Thibault, F.M.; Hernandez, E.; Vidal, D.R.; Girardet, M.; Cavallo, J.D. Antibiotic susceptibility of 65 isolates of Burkholderia pseudomallei and Burkholderia mallei to 35 antimicrobial agents. J. Antimicrob. Chemother. 2004, 54, 1134–1138. [Google Scholar] [CrossRef]
- Heine, H.S.; Miller, L.; Halasohoris, S.; Purcell, B.K. In Vitro Antibiotic Susceptibilities of Francisella tularensis Determined by Broth Microdilution following CLSI Methods. Antimicrob. Agents Chemother. 2017, 61, e00612-17. [Google Scholar] [CrossRef]
- Bugrysheva, J.V.; Lascols, C.; Sue, D.; Weigel, L.M. Rapid Antimicrobial Susceptibility Testing of Bacillus anthracis, Yersinia pestis, and Burkholderia pseudomallei by Use of Laser Light Scattering Technology. J. Clin. Microbiol. 2016, 54, 1462–1471. [Google Scholar] [CrossRef][Green Version]
- Sarovich, D.S.; Webb, J.R.; Pitman, M.C.; Viberg, L.T.; Mayo, M.; Baird, R.W.; Robson, J.M.; Currie, B.J.; Price, E.P. Raising the Stakes: Loss of Efflux Pump Regulation Decreases Meropenem Susceptibility in Burkholderia pseudomallei. Clin. Infect Dis. 2018, 67, 243–250. [Google Scholar] [CrossRef]
- Somprasong, N.; Hall, C.M.; Webb, J.R.; Sahl, J.W.; Wagner, D.M.; Keim, P.; Currie, B.J.; Schweizer, H.P. Burkholderia ubonensis Meropenem Resistance: Insights into Distinct Properties of Class A beta-Lactamases in Burkholderia cepacia Complex and Burkholderia pseudomallei Complex Bacteria. mBio 2020, 11, e00592-20. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, A.M.; Welkos, S.L.; Pitt, M.L.; Ezzell, J.W.; Worsham, P.L.; Rose, K.J.; Ivins, B.E.; Lowe, J.R.; Howe, G.B.; Mikesell, P.; et al. Postexposure prophylaxis against experimental inhalation anthrax. J. Infect Dis. 1993, 167, 1239–1243. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.M.; Karchmer, A.W.; Fisher, M.C.; Muhammad, K.M.; Yu, P.A. Safety of Antimicrobials for Postexposure Prophylaxis and Treatment of Anthrax: A Review. Clin. Infect Dis. 2022, 75, S417–S431. [Google Scholar] [CrossRef] [PubMed]
- Vietri, N.J.; Purcell, B.K.; Lawler, J.V.; Leffel, E.K.; Rico, P.; Gamble, C.S.; Twenhafel, N.A.; Ivins, B.E.; Heine, H.S.; Sheeler, R.; et al. Short-course postexposure antibiotic prophylaxis combined with vaccination protects against experimental inhalational anthrax. Proc. Natl. Acad. Sci. USA 2006, 103, 7813–7816. [Google Scholar] [CrossRef] [PubMed]
- Vietri, N.J.; Purcell, B.K.; Tobery, S.A.; Rasmussen, S.L.; Leffel, E.K.; Twenhafel, N.A.; Ivins, B.E.; Kellogg, M.D.; Webster, W.M.; Wright, M.E.; et al. A short course of antibiotic treatment is effective in preventing death from experimental inhalational anthrax after discontinuing antibiotics. J. Infect Dis. 2009, 199, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Currie, B.J. Melioidosis: Evolving concepts in epidemiology, pathogenesis, and treatment. Semin. Respir. Crit. Care Med. 2015, 36, 111–125. [Google Scholar] [CrossRef]
- Dance, D. Treatment and prophylaxis of melioidosis. Int. J. Antimicrob. Agents 2014, 43, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.P.; Marshall, C.S.; Anstey, N.M.; Ward, L.; Currie, B.J. 2020 Review and revision of the 2015 Darwin melioidosis treatment guideline; paradigm drift not shift. PLoS Negl. Trop. Dis. 2020, 14, e0008659. [Google Scholar] [CrossRef]
- Sullivan, R.P.; Ward, L.; Currie, B.J. Oral eradication therapy for melioidosis: Important but not without risks. Int. J. Infect Dis. 2019, 80, 111–114. [Google Scholar] [CrossRef]
- Welkos, S.L.; Klimko, C.P.; Kern, S.J.; Bearss, J.J.; Bozue, J.A.; Bernhards, R.C.; Trevino, S.R.; Waag, D.M.; Amemiya, K.; Worsham, P.L.; et al. Characterization of Burkholderia pseudomallei Strains Using a Murine Intraperitoneal Infection Model and In Vitro Macrophage Assays. PLoS ONE 2015, 10, e0124667. [Google Scholar] [CrossRef]
- Bearss, J.J.; Hunter, M.; Dankmeyer, J.L.; Fritts, K.A.; Klimko, C.P.; Weaver, C.H.; Shoe, J.L.; Quirk, A.V.; Toothman, R.G.; Webster, W.M.; et al. Characterization of pathogenesis of and immune response to Burkholderia pseudomallei K96243 using both inhalational and intraperitoneal infection models in BALB/c and C57BL/6 mice. PLoS ONE 2017, 12, e0172627. [Google Scholar] [CrossRef]
- Amemiya, K.; Bozue, J.; Cote, C.; DeShazer, D.; Soffler, C.; Welkos, S.; Worsham, P.L. Animal models for melioidosis. In Melioidosis and Tropical Bacterology; Torres, A., Ed.; Current Tropical Medicine Reports; Springer: Berlin/Heidelberg, Germany, 2017; pp. 208–222. [Google Scholar] [CrossRef]
- Trevino, S.R.; Klimko, C.P.; Reed, M.C.; Aponte-Cuadrado, M.J.; Hunter, M.; Shoe, J.L.; Meyer, J.R.; Dankmeyer, J.L.; Biryukov, S.S.; Quirk, A.V.; et al. Disease progression in mice exposed to low-doses of aerosolized clinical isolates of Burkholderia pseudomallei. PLoS ONE 2018, 13, e0208277. [Google Scholar] [CrossRef]
- Limmathurotsakul, D.; Funnell, S.G.; Torres, A.G.; Morici, L.A.; Brett, P.J.; Dunachie, S.; Atkins, T.; Altmann, D.M.; Bancroft, G.; Peacock, S.J.; et al. Consensus on the development of vaccines against naturally acquired melioidosis. Emerg. Infect Dis. 2015, 21, e141480. [Google Scholar] [CrossRef] [PubMed]
- Conejero, L.; Patel, N.; de Reynal, M.; Oberdorf, S.; Prior, J.; Felgner, P.L.; Titball, R.W.; Salguero, F.J.; Bancroft, G.J. Low-dose exposure of C57BL/6 mice to burkholderia pseudomallei mimics chronic human melioidosis. Am. J. Pathol. 2011, 179, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Leakey, A.K.; Ulett, G.C.; Hirst, R.G. BALB/c and C57Bl/6 mice infected with virulent Burkholderia pseudomallei provide contrasting animal models for the acute and chronic forms of human melioidosis. Microb. Pathog. 1998, 24, 269–275. [Google Scholar] [CrossRef]
- Amemiya, K.; Dankmeyer, J.L.; Biryukov, S.S.; Trevino, S.R.; Klimko, C.P.; Mou, S.M.; Fetterer, D.P.; Garnes, P.G.; Cote, C.K.; Worsham, P.L.; et al. Deletion of Two Genes in Burkholderia pseudomallei MSHR668 That Target Essential Amino Acids Protect Acutely Infected BALB/c Mice and Promote Long Term Survival. Vaccines 2019, 7, 196. [Google Scholar] [CrossRef] [PubMed]
- Burtnick, M.N.; Brett, P.J.; Harding, S.V.; Ngugi, S.A.; Ribot, W.J.; Chantratita, N.; Scorpio, A.; Milne, T.S.; Dean, R.E.; Fritz, D.L.; et al. The cluster 1 type VI secretion system is a major virulence determinant in Burkholderia pseudomallei. Infect Immun. 2011, 79, 1512–1525. [Google Scholar] [CrossRef] [PubMed]
- Titball, R.W.; Burtnick, M.N.; Bancroft, G.J.; Brett, P. Burkholderia pseudomallei and Burkholderia mallei vaccines: Are we close to clinical trials? Vaccine 2017, 35, 5981–5989. [Google Scholar] [CrossRef]
- Patel, N.; Conejero, L.; De Reynal, M.; Easton, A.; Bancroft, G.J.; Titball, R.W. Development of vaccines against burkholderia pseudomallei. Front Microbiol. 2011, 2, 198. [Google Scholar] [CrossRef] [PubMed]
- Sarkar-Tyson, M.; Titball, R.W. Progress toward development of vaccines against melioidosis: A review. Clin. Ther. 2010, 32, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- Ndumnego, O.C.; Koehler, S.M.; Crafford, J.E.; Beyer, W.; van Heerden, H. Immunogenicity of anthrax recombinant peptides and killed spores in goats and protective efficacy of immune sera in A/J mouse model. Sci. Rep. 2018, 8, 16937. [Google Scholar] [CrossRef] [PubMed]
- Dhiensiri, T.; Puapairoj, S.; Susaengrat, W. Pulmonary melioidosis: Clinical-radiologic correlation in 183 cases in northeastern Thailand. Radiology 1988, 166, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Nor, M.B.M.; Richards, G.A.; McGloughlin, S.; Amin, P.R.; Council of the World Federation of Societies of, I.; Critical Care, M. Pneumonia in the tropics: Report from the Task Force on tropical diseases by the World Federation of Societies of Intensive and Critical Care Medicine. J. Crit. Care 2017, 42, 360–365. [Google Scholar] [CrossRef]
- Heine, H.S.; Hershfield, J.; Marchand, C.; Miller, L.; Halasohoris, S.; Purcell, B.K.; Worsham, P.L. In vitro antibiotic susceptibilities of Yersinia pestis determined by broth microdilution following CLSI methods. Antimicrob. Agents Chemother. 2015, 59, 1919–1921. [Google Scholar] [CrossRef][Green Version]
- Biryukov, S.S.; Cote, C.K.; Klimko, C.P.; Dankmeyer, J.L.; Rill, N.O.; Shoe, J.L.; Hunter, M.; Shamsuddin, Z.; Velez, I.; Hedrick, Z.; et al. Evaluation of two different vaccine platforms for immunization against melioidosis and glanders. Front. Microbiol. 2022, 28, 965518. [Google Scholar] [CrossRef] [PubMed]
- Klimko, C.P.; Shoe, J.L.; Rill, N.O.; Hunter, M.; Dankmeyer, J.L.; Talyanski, Y.; Schmidt, L.; Orne, C.; Fetterer, D.P.; Biryukov, S.S.; et al. Layered and integrated medical countermeasures against Burkholderia pseudomallei infections in C57BL/6 mice. Front. Microbiol. 2022, 14, 965575. [Google Scholar] [CrossRef]
- Brett, P.J.; DeShazer, D.; Woods, D.E. Burkholderia thailandensis sp. nov., a Burkholderia pseudomallei-like species. Int. J. Syst. Bacteriol. 1998, 48, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Sim, B.M.; Chantratita, N.; Ooi, W.F.; Nandi, T.; Tewhey, R.; Wuthiekanun, V.; Thaipadungpanit, J.; Tumapa, S.; Ariyaratne, P.; Sung, W.K.; et al. Genomic acquisition of a capsular polysaccharide virulence cluster by non-pathogenic Burkholderia isolates. Genome Biol. 2010, 11, R89. [Google Scholar] [CrossRef] [PubMed]
- Atkins, T.; Prior, R.G.; Mack, K.; Russell, P.; Nelson, M.; Oyston, P.C.; Dougan, G.; Titball, R.W. A mutant of Burkholderia pseudomallei, auxotrophic in the branched chain amino acid biosynthetic pathway, is attenuated and protective in a murine model of melioidosis. Infect Immun. 2002, 70, 5290–5294. [Google Scholar] [CrossRef]
- Losada, L.; Shea, A.A.; DeShazer, D. A MarR family transcriptional regulator and subinhibitory antibiotics regulate type VI secretion gene clusters in Burkholderia pseudomallei. Microbiology 2018, 164, 1196–1211. [Google Scholar] [CrossRef] [PubMed]
- Simon, R.; Priefer, J.; Puhler, A. A broad host range mobilization system for in vivo genetic engineering: Tranposon mutagenesis in gram negative bacteria. Bio. Technol. 1983, 1, 784–791. [Google Scholar] [CrossRef]
- Barrett, A.R.; Kang, Y.; Inamasu, K.S.; Son, M.S.; Vukovich, J.M.; Hoang, T.T. Genetic tools for allelic replacement in Burkholderia species. Appl. Environ. Microbiol. 2008, 74, 4498–4508. [Google Scholar] [CrossRef] [PubMed]
- Welkos, S.L.; Friedlander, A.M. Pathogenesis and genetic control of resistance to the Sterne strain of Bacillus anthracis. Microb. Pathog. 1988, 4, 53–69. [Google Scholar] [CrossRef]
- Welkos, S.; Cote, C.; Bozue, J.; Twenhafel, N. Pathogenesis and host response to Bacillus anthracis: A mouse model. Salisb. Med. Bull. 1989, 87, 49052. [Google Scholar]
- Welkos, S.L.; Cote, C.K.; Rea, K.M.; Gibbs, P.H. A microtiter fluorometric assay to detect the germination of Bacillus anthracis spores and the germination inhibitory effects of antibodies. J. Microbiol. Methods 2004, 56, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Omotade, T.O.; Heffron, J.D.; Klimko, C.P.; Marchand, C.L.; Miller, L.L.; Halasahoris, S.A.; Bozue, J.A.; Welkos, S.L.; Cote, C.K. D-cycloserine or similar physiochemical compounds may be uniquely suited for use in Bacillus anthracis spore decontamination strategies. J. Appl. Microbiol. 2013, 115, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- Council, N.R. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Welkos, S.; Cote, C.K.; Hahn, U.; Shastak, O.; Jedermann, J.; Bozue, J.; Jung, G.; Ruchala, P.; Pratikhya, P.; Tang, T.; et al. Humanized theta-defensins (retrocyclins) enhance macrophage performance and protect mice from experimental anthrax infections. Antimicrob. Agents Chemother. 2011, 55, 4238–4250. [Google Scholar] [CrossRef] [PubMed]
- Cote, C.K.; Rea, K.M.; Norris, S.L.; van Rooijen, N.; Welkos, S.L. The use of a model of in vivo macrophage depletion to study the role of macrophages during infection with Bacillus anthracis spores. Microb. Pathog. 2004, 37, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Sim, B.K.L.; Li, M.; Osorio, M.; Wu, Y.; Wai, T.T.; Peterson, J.W.; James, E.R.; Chakravarty, S.; Gao, L.; Xu, R.; et al. Protection against inhalation anthrax by immunization with Salmonella enterica serovar Typhi Ty21a stably producing protective antigen of Bacillus anthracis. NPJ Vaccines 2017, 2, 17. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lyons, C.R.; Lovchik, J.; Hutt, J.; Lipscomb, M.F.; Wang, E.; Heninger, S.; Berliba, L.; Garrison, K. Murine model of pulmonary anthrax: Kinetics of dissemination, histopathology, and mouse strain susceptibility. Infect Immun. 2004, 72, 4801–4809. [Google Scholar] [CrossRef] [PubMed]
- Klimko, C.P.; Trevino, S.R.; Moreau, A.M.; Cuadrado, M.J.A.; Meyer, J.R.; Fetterer, D.P.; Welkos, S.L.; Worsham, P.L.; Kreiselmeier, N.; Soffler, C.; et al. The Impact of Age and Sex on Mouse Models of Melioidosis. Pathogens 2020, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Cerrato, V.; Del Prado, G.; Huelves, L.; Naves, P.; Ruiz, V.; Garcia, E.; Ponte, C.; Soriano, F. Comparative efficacy of novobiocin and amoxicillin in experimental sepsis caused by beta-lactam-susceptible and highly resistant pneumococci. Int. J. Antimicrob. Agents 2010, 35, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Marcu, M.G.; Schulte, T.W.; Neckers, L. Novobiocin and related coumarins and depletion of heat shock protein 90-dependent signaling proteins. J. Natl. Cancer Inst. 2000, 92, 242–248. [Google Scholar] [CrossRef] [PubMed]

| Novo Regimen | Mice Infected | Mice Survived | Sterile Mouse Spleens 1 |
|---|---|---|---|
| 2.5 mg 1 h | 10 | 7 | 6 (86%) |
| 2.5 mg 1 h, 8 h | 10 | 8 | 8 (100%) |
| 2.5 mg 1 h, 8 h, 24 h | 10 | 8 | 8 (100%) |
| 1.25 mg 1 h | 10 | 1 | 1 (100%) |
| 1.25 mg 1 h, 8 h | 10 | 7 | 6 (86%) |
| 1.25 mg 1 h, 8 h, 24 h | 10 | 9 | 9 (100%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klimko, C.P.; Welkos, S.L.; Shoe, J.L.; Mou, S.; Hunter, M.; Rill, N.O.; DeShazer, D.; Cote, C.K. Efficacy of Treatment with the Antibiotic Novobiocin against Infection with Bacillus anthracis or Burkholderia pseudomallei. Antibiotics 2022, 11, 1685. https://doi.org/10.3390/antibiotics11121685
Klimko CP, Welkos SL, Shoe JL, Mou S, Hunter M, Rill NO, DeShazer D, Cote CK. Efficacy of Treatment with the Antibiotic Novobiocin against Infection with Bacillus anthracis or Burkholderia pseudomallei. Antibiotics. 2022; 11(12):1685. https://doi.org/10.3390/antibiotics11121685
Chicago/Turabian StyleKlimko, Christopher P., Susan L. Welkos, Jennifer L. Shoe, Sherry Mou, Melissa Hunter, Nathaniel O. Rill, David DeShazer, and Christopher K. Cote. 2022. "Efficacy of Treatment with the Antibiotic Novobiocin against Infection with Bacillus anthracis or Burkholderia pseudomallei" Antibiotics 11, no. 12: 1685. https://doi.org/10.3390/antibiotics11121685
APA StyleKlimko, C. P., Welkos, S. L., Shoe, J. L., Mou, S., Hunter, M., Rill, N. O., DeShazer, D., & Cote, C. K. (2022). Efficacy of Treatment with the Antibiotic Novobiocin against Infection with Bacillus anthracis or Burkholderia pseudomallei. Antibiotics, 11(12), 1685. https://doi.org/10.3390/antibiotics11121685




