Colonization with Carbapenem-Resistant Enterobacteriaceae Contributes to Unfavorable Outcomes in End-Stage Liver Disease Patients
Abstract
1. Introduction
2. Results
2.1. Clinical Characteristics of the Patients
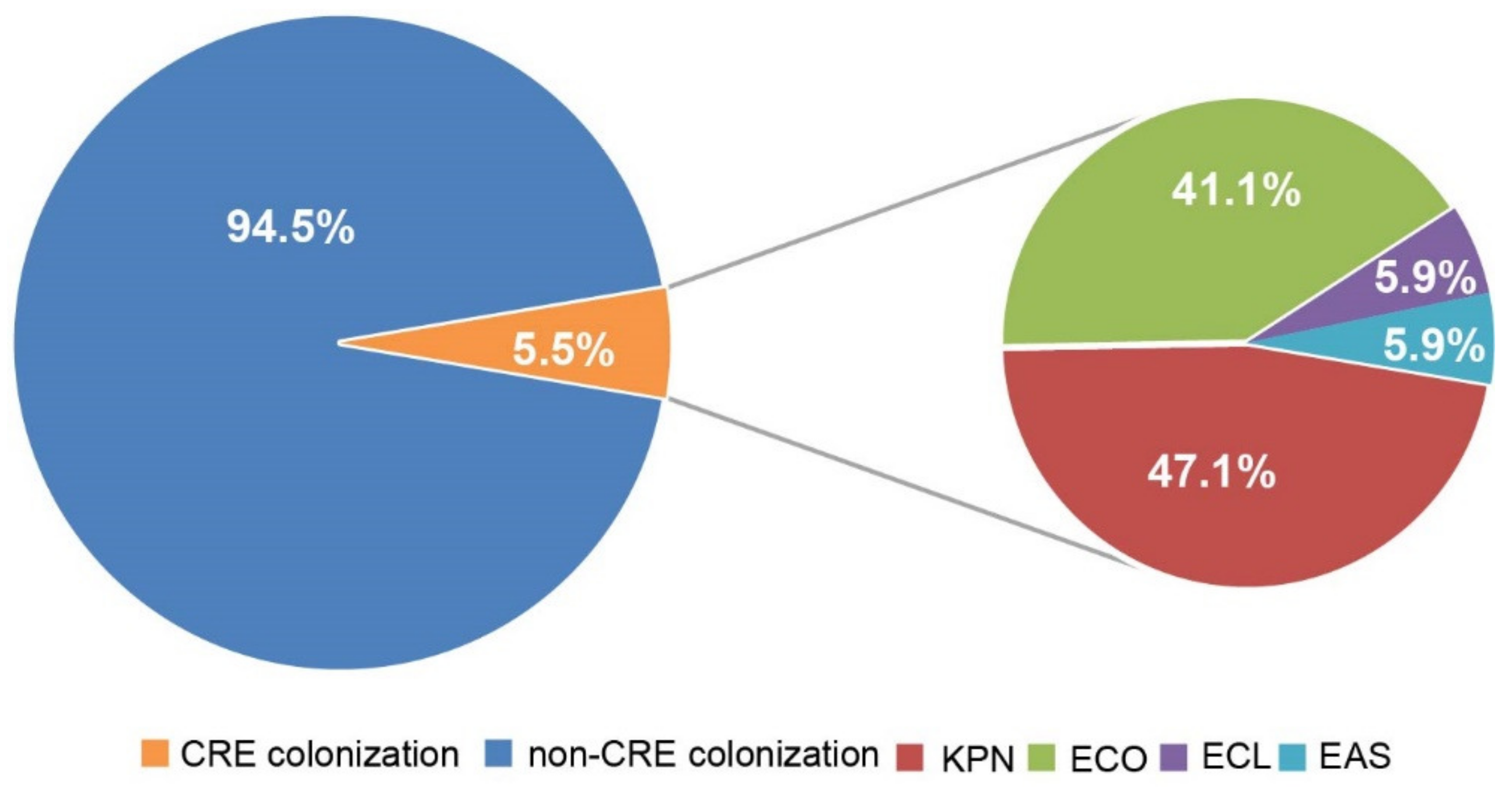
2.2. Risk Factors of CRE Colonization in ESLD
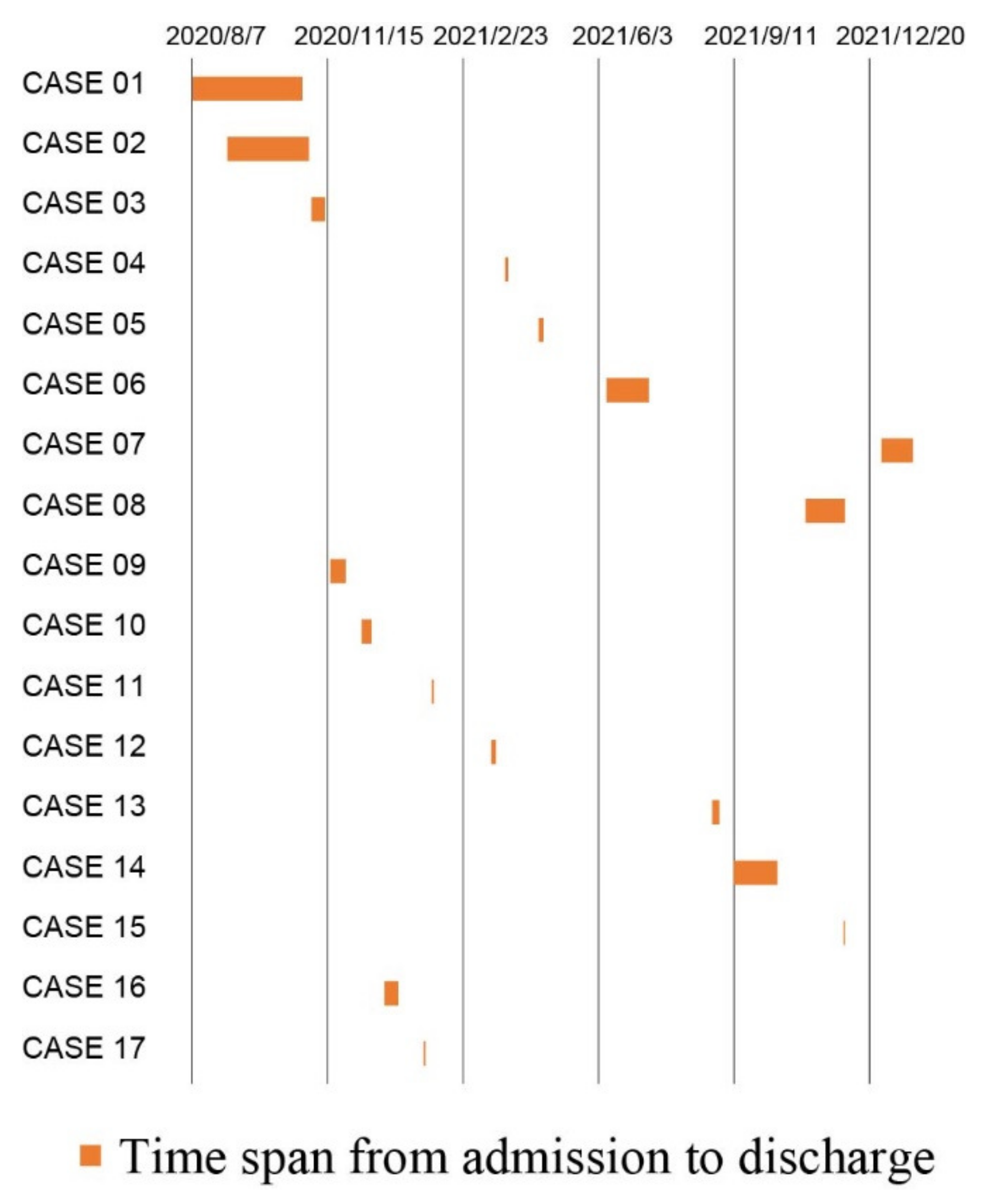
2.3. Timeline Analysis
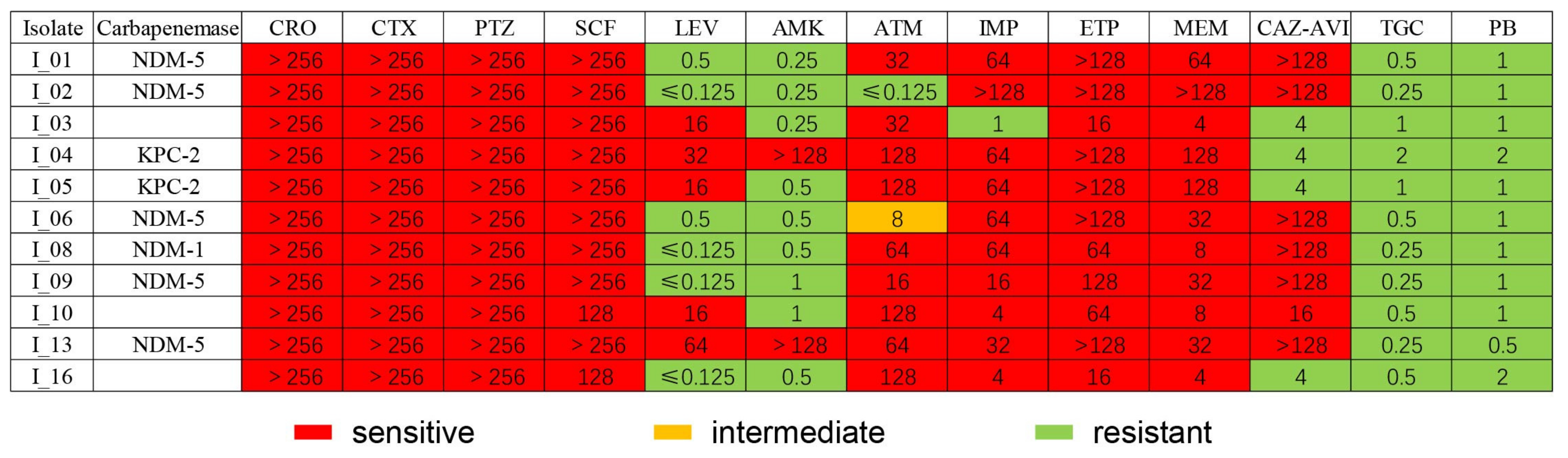
2.4. Antimicrobial Susceptibility Test
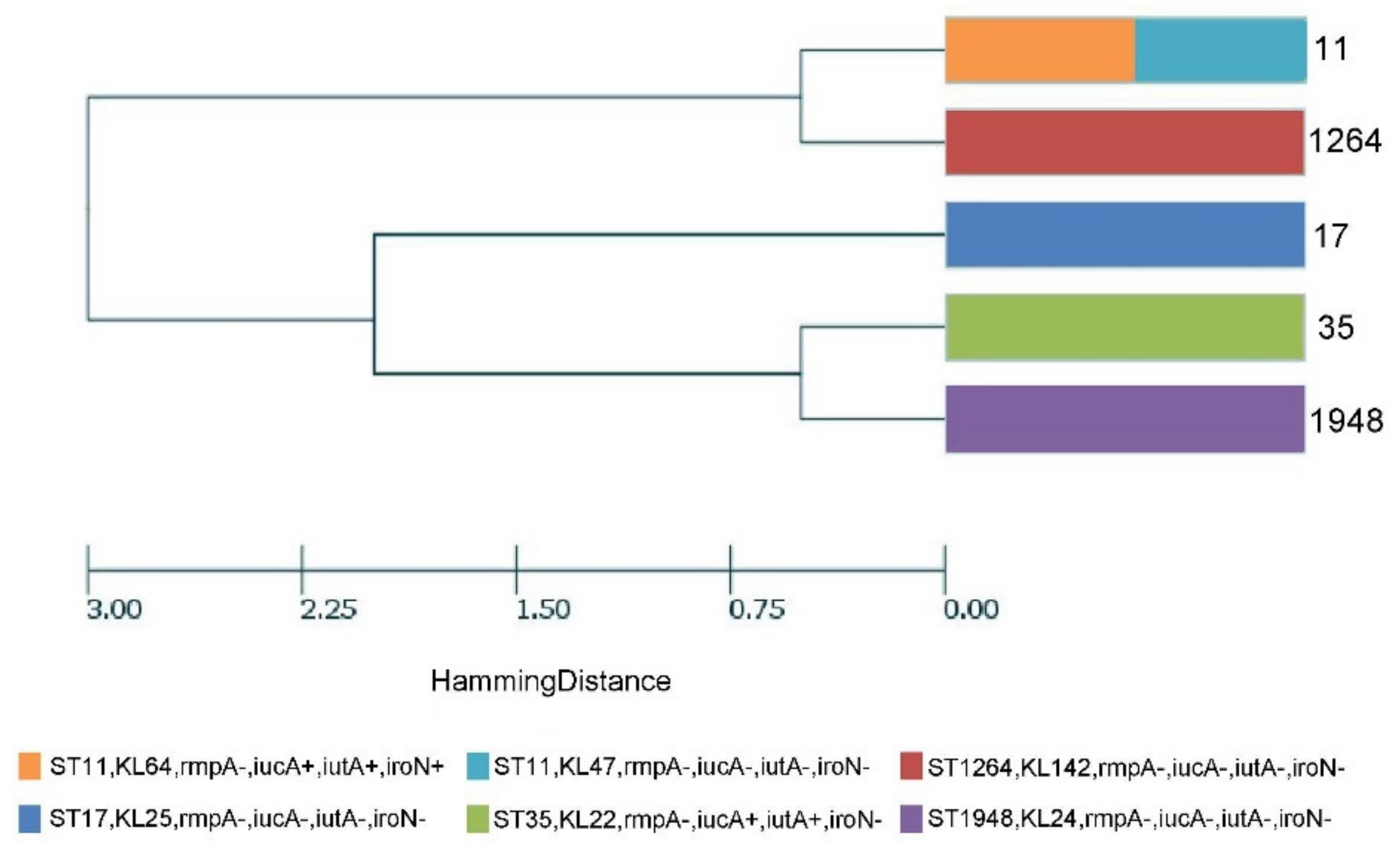
2.5. Molecular Characteristics and Phylogenetic Analysis
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Screening Procedure and Antimicrobial Susceptibility Test
4.3. Molecular Characteristics and Phylogenetic Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albillos, A.; de Gottardi, A.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef]
- Casulleras, M.; Zhang, I.W.; Lopez-Vicario, C.; Claria, J. Leukocytes, Systemic Inflammation and Immunopathology in Acute-on-Chronic Liver Failure. Cells 2020, 9, 2632. [Google Scholar] [CrossRef]
- Arvaniti, V.; D'Amico, G.; Fede, G.; Manousou, P.; Tsochatzis, E.; Pleguezuelo, M.; Burroughs, A.K. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology 2010, 139, 1246–1256, 1256e1–e5. [Google Scholar] [CrossRef]
- Cao, Z.J.; Liu, Y.H.; Zhu, C.W.; Yin, S.; Wang, W.J.; Tang, W.L.; Zhao, G.D.; Xu, Y.M.; Chen, L.; Zhou, T.H.; et al. Bacterial infection triggers and complicates acute-on-chronic liver failure in patients with hepatitis B virus-decompensated cirrhosis: A retrospective cohort study. World. J. Gastroenterol. 2020, 26, 645–656. [Google Scholar] [CrossRef]
- Zhao, R.; Ma, J.; Li, P.; Fang, H.; Sun, S.; Wu, W.; Chen, J.; Zhao, H.; Jin, L.; Shi, Y.; et al. Multidrug-resistant bacterial infections in cirrhotic patients: An epidemiological study. Expert. Rev. Gastroenterol. Hepatol. 2018, 12, 1167–1174. [Google Scholar] [CrossRef]
- Piano, S.; Singh, V.; Caraceni, P.; Maiwall, R.; Alessandria, C.; Fernandez, J.; Soares, E.C.; Kim, D.J.; Kim, S.E.; Marino, M.; et al. Epidemiology and Effects of Bacterial Infections in Patients with Cirrhosis Worldwide. Gastroenterology 2019, 156, 1368–1380, 1380.e1–e10. [Google Scholar] [CrossRef]
- Allaire, M.; Cadranel, J.F.; Nguyen, T.T.N.; Garioud, A.; Zougmore, H.; Heng, R.; Perignon, C.; Ollivier-Hourmand, I.; Dao, T. Management of infections in patients with cirrhosis in the context of increasing therapeutic resistance: A systematic review. Clin. Res. Hepatol. Gastroenterol. 2020, 44, 264–274. [Google Scholar] [CrossRef]
- Yin, Y.; Zhao, C.; Li, H.; Jin, L.; Wang, Q.; Wang, R.; Zhang, Y.; Zhang, J.; Wang, H.; CARES Network. Clinical and microbiological characteristics of adults with hospital-acquired pneumonia: A 10-year prospective observational study in China. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 683–690. [Google Scholar] [CrossRef]
- Hsu, J.Y.; Chuang, Y.C.; Wang, J.T.; Chen, Y.C.; Hsieh, S.M. Healthcare-associated carbapenem-resistant Klebsiella pneumoniae bloodstream infections: Risk factors, mortality, and antimicrobial susceptibility, 2017–2019. J. Med. Assoc. 2021, 120, 1994–2002. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet. Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Thaden, J.T.; Lewis, S.S.; Hazen, K.C.; Huslage, K.; Fowler, V.G., Jr.; Moehring, R.W.; Chen, L.F.; Jones, C.D.; Moore, Z.S.; Sexton, D.J.; et al. Rising rates of carbapenem-resistant enterobacteriaceae in community hospitals: A mixed-methods review of epidemiology and microbiology practices in a network of community hospitals in the southeastern United States. Infect. Control. Hosp. Epidemiol. 2014, 35, 978–983. [Google Scholar] [CrossRef]
- Trecarichi, E.M.; Pagano, L.; Martino, B.; Candoni, A.; Di Blasi, R.; Nadali, G.; Fianchi, L.; Delia, M.; Sica, S.; Perriello, V.; et al. Bloodstream infections caused by Klebsiella pneumoniae in onco-hematological patients: Clinical impact of carbapenem resistance in a multicentre prospective survey. Am. J. Hematol. 2016, 91, 1076–1081. [Google Scholar] [CrossRef]
- Fernandez, J.; Acevedo, J.; Wiest, R.; Gustot, T.; Amoros, A.; Deulofeu, C.; Reverter, E.; Martinez, J.; Saliba, F.; Jalan, R.; et al. Bacterial and fungal infections in acute-on-chronic liver failure: Prevalence, characteristics and impact on prognosis. Gut 2018, 67, 1870–1880. [Google Scholar] [CrossRef]
- Dickstein, Y.; Edelman, R.; Dror, T.; Hussein, K.; Bar-Lavie, Y.; Paul, M. Carbapenem-resistant Enterobacteriaceae colonization and infection in critically ill patients: A retrospective matched cohort comparison with non-carriers. J. Hosp. Infect. 2016, 94, 54–59. [Google Scholar] [CrossRef]
- Pop-Vicas, A.; Strom, J.; Stanley, K.; D'Agata, E.M. Multidrug-resistant gram-negative bacteria among patients who require chronic hemodialysis. Clin. J. Am. Soc. Nephrol. 2008, 3, 752–758. [Google Scholar] [CrossRef]
- Salomao, M.C.; Freire, M.P.; Boszczowski, I.; Raymundo, S.F.; Guedes, A.R.; Levin, A.S. Increased Risk for Carbapenem-Resistant Enterobacteriaceae Colonization in Intensive Care Units after Hospitalization in Emergency Department. Emerg. Infect. Dis. 2020, 26, 1156–1163. [Google Scholar] [CrossRef]
- Zhu, R.; Xu, X.; Lian, S.; Cai, M.; Zhang, H.; Chen, X.; Cao, Y. Intestinal Colonization with Carbapenem-Resistant Enterobacteriaceae in Acute Leukemia Patients: Risk Factors and Molecular Characteristics. Infect. Drug. Resist. 2022, 15, 4275–4283. [Google Scholar] [CrossRef]
- Yang, T.T.; Luo, X.P.; Yang, Q.; Chen, H.C.; Luo, Y.; Zhao, Y.M.; Ye, Y.S.; Lai, X.Y.; Yu, J.; Tan, Y.M.; et al. Different screening frequencies of carbapenem-resistant Enterobacteriaceae in patients undergoing hematopoietic stem cell transplantation: Which one is better? Antimicrob. Resist. Infect. Control 2020, 9, 49. [Google Scholar] [CrossRef]
- Ferstl, P.G.; Filmann, N.; Heilgenthal, E.M.; Schnitzbauer, A.A.; Bechstein, W.O.; Kempf, V.A.J.; Villinger, D.; Schultze, T.G.; Hogardt, M.; Stephan, C.; et al. Colonization with multidrug-resistant organisms is associated with in increased mortality in liver transplant candidates. PLoS ONE 2021, 16, e0245091. [Google Scholar] [CrossRef]
- Kumar, A.; Mohapatra, S.; Bir, R.; Tyagi, S.; Bakhshi, S.; Mahapatra, M.; Gautam, H.; Sood, S.; Das, B.K.; Kapil, A. Intestinal Colonization Due to Carbapenem-Resistant Enterobacteriaceae Among Hematological Malignancy Patients in India: Prevalence and Molecular Charecterisation. Indian. J. Hematol. Blood. Transfus. 2022, 38, 1–7. [Google Scholar] [CrossRef]
- van Duin, D.; Arias, C.A.; Komarow, L.; Chen, L.; Hanson, B.M.; Weston, G.; Cober, E.; Garner, O.B.; Jacob, J.T.; Satlin, M.J.; et al. Molecular and clinical epidemiology of carbapenem-resistant Enterobacterales in the USA (CRACKLE-2): A prospective cohort study. Lancet. Infect. Dis. 2020, 20, 731–741. [Google Scholar] [CrossRef]
- Debby, B.D.; Ganor, O.; Yasmin, M.; David, L.; Nathan, K.; Ilana, T.; Dalit, S.; Smollan, G.; Galia, R. Epidemiology of carbapenem resistant Klebsiella pneumoniae colonization in an intensive care unit. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1811–1817. [Google Scholar] [CrossRef]
- Qin, X.; Wu, S.; Hao, M.; Zhu, J.; Ding, B.; Yang, Y.; Xu, X.; Wang, M.; Yang, F.; Hu, F. The Colonization of Carbapenem-Resistant Klebsiella pneumoniae: Epidemiology, Resistance Mechanisms, and Risk Factors in Patients Admitted to Intensive Care Units in China. J. Infect. Dis. 2020, 221, S206–S214. [Google Scholar] [CrossRef]
- Schultze, T.G.; Ferstl, P.G.; Villinger, D.; Hogardt, M.; Bechstein, W.O.; Gottig, S.; Wichelhaus, T.A.; Zeuzem, S.; Trebicka, J.; Waidmann, O.; et al. Molecular Surveillance of Carbapenem-Resistant Gram-Negative Bacteria in Liver Transplant Candidates. Front. Microbiol. 2021, 12, 791574. [Google Scholar] [CrossRef]
- Wang, M.; Earley, M.; Chen, L.; Hanson, B.M.; Yu, Y.; Liu, Z.; Salcedo, S.; Cober, E.; Li, L.; Kanj, S.S.; et al. Clinical outcomes and bacterial characteristics of carbapenem-resistant Klebsiella pneumoniae complex among patients from different global regions (CRACKLE-2): A prospective, multicentre, cohort study. Lancet. Infect. Dis. 2022, 22, 401–412. [Google Scholar] [CrossRef]
- Cano, A.; Gutierrez-Gutierrez, B.; Machuca, I.; Gracia-Ahufinger, I.; Perez-Nadales, E.; Causse, M.; Caston, J.J.; Guzman-Puche, J.; Torre-Gimenez, J.; Kindelan, L.; et al. Risks of Infection and Mortality Among Patients Colonized with Klebsiella pneumoniae Carbapenemase-Producing K. pneumoniae: Validation of Scores and Proposal for Management. Clin. Infect. Dis. 2018, 66, 1204–1210. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Burns, K.; Rodriguez-Bano, J.; Borg, M.; Daikos, G.; Dumpis, U.; Lucet, J.C.; Moro, M.L.; Tacconelli, E.; Simonsen, G.S.; et al. Infection prevention and control measures and tools for the prevention of entry of carbapenem-resistant Enterobacteriaceae into healthcare settings: Guidance from the European Centre for Disease Prevention and Control. Antimicrob. Resist. 2017, 6, 113. [Google Scholar] [CrossRef]
- Gay, M.; Pares, A.; Carrascal, M.; Bosch-i-Crespo, P.; Gorga, M.; Mas, A.; Abian, J. Proteomic analysis of polypeptides captured from blood during extracorporeal albumin dialysis in patients with cholestasis and resistant pruritus. PLoS ONE 2011, 6, e21850. [Google Scholar] [CrossRef]
- Garcia Martinez, J.J.; Bendjelid, K. Artificial liver support systems: What is new over the last decade? Ann. Intensive. Care 2018, 8, 109. [Google Scholar] [CrossRef]
- Severe Liver Disease and Artificial Liver Group Chinese Society of Hepatology Chinese Medical Association. Expert consensus on clinical application of artificial liver and blood purification (2022 edition). J. Clin. Hepatol. 2022, 38, 767–775. [Google Scholar] [CrossRef]
- Wilson, G.M.; Suda, K.J.; Fitzpatrick, M.A.; Bartle, B.; Pfeiffer, C.D.; Jones, M.; Rubin, M.A.; Perencevich, E.; Evans, M.; Evans, C.T.; et al. Risk Factors Associated with Carbapenemase-Producing Carbapenem-Resistant Enterobacteriaceae Positive Cultures in a Cohort of US Veterans. Clin. Infect. Dis. 2021, 73, 1370–1378. [Google Scholar] [CrossRef]
- Falcone, M.; Tiseo, G.; Galfo, V.; Giordano, C.; Leonildi, A.; Marciano, E.; De Simone, P.; Biancofiore, G.; Boggi, U.; Barnini, S.; et al. Bloodstream infections in patients with rectal colonization by Klebsiella pneumoniae producing different type of carbapenemases: A prospective, cohort study (CHIMERA study). Clin. Microbiol. Infect. 2022, 28, 298.e1–298.e7. [Google Scholar] [CrossRef]
- Paul, M.; Carrara, E.; Retamar, P.; Tangden, T.; Bitterman, R.; Bonomo, R.A.; de Waele, J.; Daikos, G.L.; Akova, M.; Harbarth, S.; et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine). Clin. Microbiol. Infec. 2022, 28, 521–547. [Google Scholar] [CrossRef]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America 2022 Guidance on the Treatment of Extended-Spectrum beta-lactamase Producing Enterobacterales (ESBL-E), Carbapenem-Resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with Difficult-to-Treat Resistance (DTR-P. aeruginosa). Clin. Infect. Dis. 2022, 75, 187–212. [Google Scholar] [CrossRef]
- Chinese Society of Infectious Diseases; Chinese Medical Association. Expert consensus on diagnosis and treatment of end-stage liver disease complicated infection (2021 version). Chin. J. Hepatol. 2022, 30, 168–178. [Google Scholar] [CrossRef]
- Brisse, S.; Passet, V.; Haugaard, A.B.; Babosan, A.; Kassis-Chikhani, N.; Struve, C.; Decre, D. wzi Gene sequencing, a rapid method for determination of capsular type for Klebsiella strains. J Clin Microbiol. 2013, 51, 4073–4078. [Google Scholar] [CrossRef]
| Variables | Total | Non-CRE Colonization | CRE Colonization | p-Value |
|---|---|---|---|---|
| Patients | 311 | 294 | 17 | NA |
| Age (years) | 51.08 ± 12.10 | 51.27 ± 12.12 | 47.82 ± 11.56 | 0.255 |
| Male | 255 (81.99%) | 240 (81.63%) | 15 (88.24%) | 0.491 |
| Hepatitis B or C | 256 (82.32%) | 242 (82.31%) | 14 (82.35%) | 0.997 |
| Cirrhosis | 205 (65.92%) | 196 (66.67%) | 9 (52.94%) | 0.246 |
| Charlson comorbidity index | 3.00 (3.00–4.00) | 3.00 (3.00–5.00) | 3.00 (3.00–3.00) | 0.096 |
| Diabetes | 45 (14.47%) | 45 (15.30%) | 0 | 0.146 |
| Tumor | 61 (19.61%) | 60 (20.41%) | 1 (5.88%) | 0.143 |
| Admitted from hospital | 295 (94.86) | 279 (94.90) | 16 (94.12) | 0.887 |
| Length of hospital stay | 7.00 (2.00–13.00) | 7.00 (2.00–13.00) | 10 (2.50–30.00) | 0.305 |
| WBC (*e9/L) | 5.41 (3.61–8.17) | 5.42 (3.60–8.21) | 5.30 (3.75–8.63) | 0.667 |
| Hb (g/L) | 98.00 (81.00–115.00) | 98.00 (81.75–115.00) | 101.00 (80.50–109.50) | 0.795 |
| PLT (*e9/L) | 85.00 (56.00–131.00) | 85 (56.00–131.00) | 81 (50.50–118.00) | 0.496 |
| ALT (U/L) | 41.00 (23.00–95.00) | 41.00 (22.75–95.00) | 35.00 (27.00–75.00) | 0.886 |
| AST (U/L) | 68.00(38.00–114.00) | 67.5 (37.00–114.00) | 87 (53.00–119.50) | 0.364 |
| ALB (g/L) | 34.49 ± 5.29 | 34.56 ± 5.28 | 33.25 ± 5.37 | 0.320 |
| TB (umol/L) | 154.40 (42.40–331.00) | 150.85 (39.53–327.85) | 198.00 (80.50–525.00) | 0.146 |
| CHE (U/L) | 2968.00 (1928.00–4191.00) | 2974.25 (1927.25–4140.25) | 2571 (1921.50–4462.00) | 0.907 |
| HDL (mmol/L) | 0.24 (0.12–0.55) | 0.24 (0.13–0.59) | 0.14 (0.12–0.26) | 0.055 |
| LDL (mmol/L) | 1.47 (0.99–2.05) | 1.47 (1.00–2.05) | 1.1 (0.80–1.83) | 0.141 |
| Cr (umol/L) | 67.00 (55.00–85.00) | 67.00 (55.00–85.25) | 60.00 (48.75–88.00) | 0.445 |
| INR | 1.67 (1.32–2.22) | 1.67 (1.32–2.21) | 2.10 (1.35–2.40) | 0.432 |
| MELD score | 22.00 (13.45–32.31) | 21.53 (13.30–31.97) | 27.37 (14.22–39.51) | 0.240 |
| Child–Pugh score | 9.00 (8.00–11.00) | 9.00 (8.00–11.00) | 10.00 (9.00–11.50) | 0.182 |
| ICU admission | 10 (3.22%) | 10 (3.40%) | 0 | 1.000 |
| Gastrointestinal bleeding | 27 (8.68%) | 26 (8.84%) | 1 (5.88%) | 0.673 |
| Blood transfusion | 272 (87.46%) | 256 (87.07%) | 16 (94.12%) | 0.394 |
| Parenteral nutrition | 20 (6.43%) | 19 (6.46%) | 1 (5.88%) | 0.924 |
| Immunosuppressive or antitumor therapy | 44 (14.15%) | 40 (13.61%) | 4 (23.53%) | 0.254 |
| Invasive procedures | 179 (57.56%) | 165 (56.12%) | 14 (82.35%) | 0.033 |
| Central venous catheterization | 133 (42.77%) | 123 (41.84%) | 10 (58.82%) | 0.169 |
| Artificial liver support | 53 (17.04%) | 45 (15.31%) | 8 (47.06%) | 0.001 |
| Antibiotic therapy | 238 (76.53%) | 221 (75.17%) | 17 (100.00%) | 0.019 |
| Carbapenems | 85 (27.33%) | 79 (26.87%) | 6 (35.29%) | 0.449 |
| Penicillins | 4 (1.29%) | 4 (1.36%) | 0 | 1.000 |
| Cephalosporins | 44 (14.15%) | 39 (13.27%) | 5 (29.41%) | 0.063 |
| Comprised β-lactamases antibiotics | 184 (59.16%) | 172 (58.50%) | 12 (70.59%) | 0.324 |
| Quinolones | 26 (8.36%) | 26 (8.84%) | 0 | 0.378 |
| CRE infection | 2 (0.64%) | 1 (0.34%) | 1 (5.88%) | 0.005 |
| Unfavorable outcome | 79 (25.40%) | 71 (24.15%) | 8 (47.06%) | 0.035 |
| Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| p-Value | Hazard Ratio | 95% CI | p-Value | Hazard Ratio | 95% CI | |
| Age (years) | 0.256 | 0.977 | 0.938–1.017 | |||
| Gender | 0.496 | 1.687 | 0.375–7.598 | |||
| Hepatitis B or C | 0.997 | 1.003 | 0.278–3.615 | |||
| Cirrhosis | 0.251 | 0.563 | 0.211–1.503 | |||
| Charlson comorbidity index | 0.380 | 0.825 | 0.538–1.267 | |||
| Admitted from hospital | 0.887 | 0.860 | 0.107–6.927 | |||
| Length of hospital stay | 0.032 | 1.026 | 1.002–1.051 | 0.200 | 1.018 | 0.991–1.045 |
| WBC (*e9/L) | 0.603 | 1.030 | 0.921–1.153 | |||
| ALT(U/L) | 0.394 | 0.997 | 0.990–1.004 | |||
| TB (umol/L) | 0.105 | 1.002 | 1.000–1.004 | |||
| Cr(umol/L) | 0.524 | 0.995 | 0.979–1.011 | |||
| INR | 0.855 | 0.986 | 0.849–1.145 | |||
| MELD score | 0.233 | 1.025 | 0.984–1.067 | |||
| Child–Pugh score | 0.125 | 1.236 | 0.943–1.621 | |||
| ICU admission | 0.999 | 0 | NA | |||
| Gastrointestinal bleeding | 0.676 | 0.644 | 0.082–5.055 | |||
| Blood transfusion | 0.408 | 2.375 | 0.306–18.427 | |||
| Parenteral nutrition | 0.924 | 0.905 | 0.114–7.191 | |||
| Immunosuppressive or antitumor therapy | 0.262 | 1.954 | 0.607–6.290 | |||
| Invasive procedures | 0.045 | 3.648 | 1.027–12.966 | |||
| Central venous catheterization | 0.176 | 1.986 | 0.736–5.363 | |||
| Artificial liver support | 0.002 | 4.919 | 1.802–13.422 | 0.009 | 4.071 | 1.427–11.618 |
| Surgery | 0.254 | 3.612 | 0.398–32.775 | |||
| Antibiotic therapy | 0.997 | 124267258 | NA | |||
| Carbapenems | 0.451 | 1.484 | 0.531–4.148 | |||
| Penicillins | 0.999 | 0 | NA | |||
| Cephalosporins | 0.073 | 2.724 | 0.910–8.155 | |||
| Comprised β-lactamases antibiotics | 0.329 | 1.702 | 0.585–4.957 | |||
| Quinolones | 0.998 | 0 | NA | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, G.; Pang, Y.; Zheng, J.; Zhuo, C.; Guo, Y.; Liang, J.; Li, X.; Lei, Z.; Zhu, J.; Xu, L.; et al. Colonization with Carbapenem-Resistant Enterobacteriaceae Contributes to Unfavorable Outcomes in End-Stage Liver Disease Patients. Antibiotics 2022, 11, 1667. https://doi.org/10.3390/antibiotics11111667
Zeng G, Pang Y, Zheng J, Zhuo C, Guo Y, Liang J, Li X, Lei Z, Zhu J, Xu L, et al. Colonization with Carbapenem-Resistant Enterobacteriaceae Contributes to Unfavorable Outcomes in End-Stage Liver Disease Patients. Antibiotics. 2022; 11(11):1667. https://doi.org/10.3390/antibiotics11111667
Chicago/Turabian StyleZeng, Guofen, Yihua Pang, Jiaxin Zheng, Chuyue Zhuo, Yingyi Guo, Jiayin Liang, Xiaojie Li, Ziying Lei, Jianyun Zhu, Lejia Xu, and et al. 2022. "Colonization with Carbapenem-Resistant Enterobacteriaceae Contributes to Unfavorable Outcomes in End-Stage Liver Disease Patients" Antibiotics 11, no. 11: 1667. https://doi.org/10.3390/antibiotics11111667
APA StyleZeng, G., Pang, Y., Zheng, J., Zhuo, C., Guo, Y., Liang, J., Li, X., Lei, Z., Zhu, J., Xu, L., Gao, Z., Zhuo, C., & Liu, J. (2022). Colonization with Carbapenem-Resistant Enterobacteriaceae Contributes to Unfavorable Outcomes in End-Stage Liver Disease Patients. Antibiotics, 11(11), 1667. https://doi.org/10.3390/antibiotics11111667







