Novel Polyhydroquinoline-Hydrazide-Linked Schiff’s Base Derivatives: Multistep Synthesis, Antimicrobial, and Calcium-Channel-Blocking Activities
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Antibacterial Bioassay
2.3. In Vitro Calcium-Channel-Blocking Study in Isolated Aorta from SD Rats
3. Conclusions
4. Experimental
4.1. General
4.2. Animals
4.3. Synthesis of Ethyl-2-(2-ethoxy-4-formylphenoxy) Acetate
4.4. Synthesis of Polyhydroquinoline (1)
4.5. Synthesis of Polyhydroquinoline Hydrazide (2)
4.6. General Procedure for the Synthesis of Schiff’s Base Derivatives of Polyhydroquinoline (3–27)
4.7. Spectral Interpretation of the Synthesized Compounds (Figure S3–S29)
- Ethyl 4-(3-Ethoxy-4-(2-ethoxy-2-oxoethoxy)phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (1)
- Ethyl 4-(3-ethoxy-4-(2-(hydrazinyloxy)-2-oxoethoxy)phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (2)
- Ethyl 4-(3-ethoxy-4-(2-(2-(4-nitrobenzylidene)hydrazinyl)-2-oxoethoxy)phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (3)
- Ethyl 4-(3-ethoxy-4-(2-(2-(3-nitrobenzylidene)hydrazinyl)-2-oxoethoxy)phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (4)
- Ethyl 4-(4-(2-(2-(2,4-dichlorobenzylidene)hydrazinyl)-2-oxoethoxy)-3-ethoxyphen yl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (5)
- Ethyl 4-(3-ethoxy-4-(2-(2-(4-hydroxybenzylidene)hydrazinyl)-2-oxoethoxy)phen yl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (6)
- Ethyl 4-(3-ethoxy-4-(2-(2-(3-hydroxybenzylidene)hydrazinyl)-2-oxoethoxy)phen yl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (7)
- Ethyl 4-(4-(2-(2-(3,5-dibromo-4-hydroxybenzylidene)hydrazinyl)-2-oxoethoxy)-3-ethoxyphenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (8)
- Ethyl 4-(4-(2-(2-(2,4-dihydroxybenzylidene)hydrazinyl)-2-oxoethoxy)-3-ethoxy phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (9)
- Ethyl 4-(3-ethoxy-4-(2-(2-(2-hydroxy-3-methoxybenzylidene)hydrazinyl)-2-oxoe thoxy)phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (10)
- Ethyl 4-(4-(2-(2-(2,4-dimethoxybenzylidene)hydrazinyl)-2-oxoethoxy)-3-ethoxy phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (11)
- Ethyl 4-(4-(2-(2-(4-(diethylamino)benzylidene)hydrazinyl)-2-oxoethoxy)-3-ethoxy phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (12)
- Ethyl 4-(4-(2-(2-(4-bromo-2-fluorobenzylidene)hydrazinyl)-2-oxoethoxy)-3-ethoxy phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (13)
- Ethyl 4-(3-ethoxy-4-(2-oxo-2-(2-(3,4,5-trimethoxybenzylidene)hydrazinyl)ethoxy) phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (14)
- Ethyl 4-(3-ethoxy-4-(2-(2-((2-hydroxynaphthalen-1-yl)methylene)hydrazinyl)-2-oxoethoxy)phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (15)
- Ethyl 4-(3-ethoxy-4-(2-(2-(2-methoxybenzylidene)hydrazinyl)-2-oxoethoxy) phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (16)
- Ethyl 4-(4-(2-(2-(2-chlorobenzylidene)hydrazinyl)-2-oxoethoxy)-3-ethoxyphen yl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (17)
- Ethyl 4-(4-(2-(2-(3,4-dichlorobenzylidene)hydrazinyl)-2-oxoethoxy)-3-ethoxy phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (18)
- Ethyl 4-(4-(2-(2-(3,4-dimethoxybenzylidene)hydrazinyl)-2-oxoethoxy)-3-ethoxy phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (19)
- Ethyl 4-(4′-(9′-(11′-(2″-hydroxy-3″-methoxybenzylidene)hydrazinyl)-9′-oxoe thoxy)-3′-ethoxyphenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (20)
- Ethyl 4-(3-ethoxy-4-(2-(2-((5-methylfuran-2-yl)methylene)hydrazinyl)-2-oxoethoxy)phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (21)
- Ethyl 4-(3-ethoxy-4-(2-(2-(3-methoxybenzylidene)hydrazinyl)-2-oxoethoxy)phen yl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (22)
- Ethyl 4-(3-ethoxy-4-(2-(2-(4-methoxybenzylidene)hydrazinyl)-2-oxoethoxy)phen yl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (23)
- Ethyl 4-(3-ethoxy-4-(2-(2-(4-fluorobenzylidene)hydrazinyl)-2-oxoethoxy)phen yl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (24)
- Ethyl 4-(3-ethoxy-4-(2-(2-(2-hydroxybenzylidene)hydrazinyl)-2-oxoethoxy)phen yl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (25)
- Ethyl 4-(3-ethoxy-4-(2-(2-(naphthalen-1-ylmethylene)hydrazinyl)-2-oxoethoxy)phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (26)
- Ethyl 4-(4-(2-(2-butylidenehydrazinyl)-2-oxoethoxy)-3-ethoxyphenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (27)
4.8. Antibacterial Bioassay
4.9. In Vitro Calcium-Channel-Blocking Study in Isolated Aorta from SD Rats
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, V.K.; Singh, S.K. Synthesis, utility and medicinal importance of 1,2- and 1,4-dihydropyridines. RSC Adv. 2017, 7, 2682–2732. [Google Scholar] [CrossRef] [Green Version]
- Davarpanah, J.; Ghahremani, M.; Najafi, O. Synthesis of 1, 4-dihydropyridine and polyhydroquinoline derivatives via Hantzsch reaction using nicotinic acid as a green and reusable catalyst. J. Mol. Struct. 2019, 1177, 525–535. [Google Scholar]
- Farsi, R.; Mohammadi, M.K.; Saghanezhad, S.J. Sulfonamide-functionalized covalent organic framework (COF-SO3H): An efficient heterogeneous acidic catalyst for the one-pot preparation of polyhydroquinoline and 1,4-dihydropyridine derivatives. Res. Chem. Intermed. 2021, 47, 1161–1179. [Google Scholar] [CrossRef]
- Sausins, A.; Duburs, G. Synthesis of 1,4-dihydropyridines by cyclocondensation reactions. Heterocycles 1988, 27, 269–289. [Google Scholar] [CrossRef]
- Gordeev, M.F.; Patel, D.V.; Gordon, E.M. Approaches to combinatorial synthesis of heterocycles: A solid-phase synthesis of 1,4-dihydropyridines. J. Org. Chem. 1996, 61, 924–928. [Google Scholar] [CrossRef]
- Ma, Z.P.; Liao, J.C.; Zhao, C.; Cai, D.Z. Effects of the 1,4-dihydropyridine L-type calcium channel blocker benidipine on bone marrow stromal cells. Cell Tissue Res. 2015, 361, 467–476. [Google Scholar] [CrossRef]
- Sirisha, K.; Achaiah, G.; Reddy, V.M. Facile Synthesis and antibacterial, antitubercular, and anticancer Activities of Novel 1,4-Dihydropyridines. Arch. Pharm. 2010, 343, 342–352. [Google Scholar] [CrossRef]
- Godfraind, T.; Miller, R.; Wibo, M. Calcium antagonism and calcium entry blockade. Pharmacol. Rev. 1986, 38, 321–416. [Google Scholar]
- Krauze, A.; Ģērmane, S.; Eberlin, O.; Šturms, I.; Klusā, V.; Duburs, G. Derivatives of 3-cyano-6-phenyl-4-(3-pyriyl)-pyridine-2 (1H)-thione and their neurotropic activity. Eur. J. Med. Chem. 1999, 34, 301–310. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, S.; Tripathi, V.D.; Maurya, R.A.; Srivastava, S.P.; Bhatia, G.; Tamrakar, A.; Srivastava, A.K. Design and synthesis of 2,4-disubstituted polyhydroquinolines as prospective antihyperglycemic and lipid modulating agents. Bioorg. Med. Chem. 2010, 18, 4138–4148. [Google Scholar] [CrossRef]
- Nikookar, H.; Mohammadi-Khanaposhtani, M.; Imanparast, S.; Faramarzi, M.A.; Ranjbar, P.R.; Mahdavi, M.; Larijani, B. Design, synthesis and in vitro α-glucosidase inhibition of novel dihydropyrano [3,2-c] quinoline derivatives as potential anti-diabetic agents. Bioorg. Chem. 2018, 77, 280–286. [Google Scholar] [CrossRef]
- Ogawa, A.K.; Willoughby, C.A.; Bergeron, R.; Ellsworth, K.P.; Geissler, W.M.; Myer, R.W.; Yao, J.; Harris, G.; Chapman, K.T. Glucose-lowering in a db/db mouse model by dihydropyridine diacid glycogen phosphorylase inhibitors. Bioorg. Med. Chem. Lett. 2003, 13, 3405–3408. [Google Scholar] [CrossRef]
- Kumar Gupta, S.; Mishra, A. Synthesis, characterization & screening for anti-inflammatory & analgesic activity of quinoline derivatives bearing azetidinones scaffolds. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2016, 15, 31–43. [Google Scholar]
- Bretzel, R.G.; Bollen, C.C.; Maeser, E.; Federlin, K.F. Nephroprotective effects of nitrendipine in hypertensive tune I and type II diabetic patients. Am. J. Kidney Dis. 1993, 21, S53–S64. [Google Scholar] [CrossRef]
- Klusa, V. Cerebrocrast. Neuroprotectant, cognition enhancer. Drugs Future 1995, 20, 135–138. [Google Scholar] [CrossRef]
- Oskuie, E.F.; Azizi, S.; Ghasemi, Z.; Pirouzmand, M.; Kojanag, B.N.; Soleymani, J. Zn/MCM-41-catalyzed unsymmetrical Hantzsch reaction and the evaluation of optical properties and anti-cancer activities of the polyhydroquinoline products. Monatsh. Chem. 2020, 151, 243–249. [Google Scholar] [CrossRef]
- Teng, P.; Li, C.; Peng, Z.; Marie, V.A.; Nimmagadda, A.; Su, M.; Li, Y.; Sun, X.; Cai, J. Facilely accessible quinoline derivatives as potent antibacterial agents. Bioorg. Med. Chem. 2018, 26, 3573–3579. [Google Scholar] [CrossRef] [PubMed]
- Mirani Nezhad, S.; Nazarzadeh Zare, E.; Davarpanah, A.; Pourmousavi, S.A.; Ashrafizadeh, M.; Kumar, A.P. Ionic liquid-assisted fabrication of bioactive heterogeneous magnetic nanocatalyst with antioxidant and antibacterial activities for the synthesis of polyhydroquinoline derivatives. Molecules 2022, 27, 1748. [Google Scholar] [CrossRef]
- Nishiya, Y.; Kosaka, N.; Uchii, M.; Sugimoto, S. A potent 1,4-dihydropyridine L-type calcium channel blocker, benidipine, promotes osteoblast differentiation. Calcif. Tissue Int. 2002, 70, 30–39. [Google Scholar] [CrossRef]
- Yasar, S.; Corrada, M.; Brookmeyer, R.; Kawas, C. Calcium channel blockers and risk of AD: The Baltimore Longitudinal Study of Aging. Neurobiol. Aging. 2005, 26, 157–163. [Google Scholar] [CrossRef] [Green Version]
- Jadhvar, S.C.; Kasraliker, H.M.; Goswami, S.V.; Chakrawar, A.V.; Bhusare, S.R. One-pot synthesis and evaluation of anticancer activity of polyhydroquinoline derivatives catalyzed by [Msim] Cl. Res. Chem. Intermed. 2017, 43, 7211–7221. [Google Scholar] [CrossRef]
- Coates, A.; Hu, Y.; Bax, R.; Page, C. The future challenges facing the development of new antimicrobial drugs. Nat. Rev. Drug Discov. 2002, 1, 895–910. [Google Scholar] [CrossRef] [PubMed]
- Jampilek, J. Design and discovery of new antibacterial agents: Advances, perspectives, challenges. Curr. Med. Chem. 2018, 25, 4972–5006. [Google Scholar] [CrossRef] [PubMed]
- Heta, S.; Robo, I. The side effects of the most commonly used group of antibiotics in periodontal treatments. Med. Sci. 2018, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Gootz, T.D. Discovery and development of new antimicrobial agents. Clin. Microbiol. Rev. 1990, 3, 13–31. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [Green Version]
- Venkatapathy, K.; Magesh, C.J.; Lavanya, G.; Perumal, P.T.; Prema, S. Design, synthesis, molecular docking, and spectral studies of new class of carbazolyl polyhydroquinoline derivatives as promising antibacterial agents with noncytotoxicity towards human mononuclear cells from peripheral blood. J. Heterocycl. Chem. 2020, 57, 1936–1955. [Google Scholar] [CrossRef]
- Committee, G. European Society of Hypertension–European Society of Cardiology guidelines for the management of arterial hypertension. J. Hypertens. 2003, 21, 1011–1053. [Google Scholar]
- Ozer, E.K.; Gunduz, M.G.; El-Khouly, A.; Sara, Y.; Simsek, R.; Iskit, A.B.; Safak, C. Synthesis of fused 1,4-dihydropyridines as potential calcium channel blockers. Turk. J. Biochem. 2018, 43, 578–586. [Google Scholar] [CrossRef]
- Schleifer, K.J. Stereoselective characterization of the 1,4-dihydropyridine binding site at L-type calcium channels in the resting state and the opened/inactivated state. J. Med. Chem. 1999, 42, 2204–2211. [Google Scholar] [CrossRef]
- Gad, S. Cadmium. In Encyclopedia of Toxicology; Wexler, P., Ed.; Academic Press: San Diego, CA, USA, 2014. [Google Scholar]
- Edraki, N.; Mehdipour, A.R.; Khoshneviszadeh, M.; Miri, R. Dihydropyridines: Evaluation of their current and future pharmacological applications. Drug Discov. 2009, 14, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, M.J.; Brox, A.; Bestawros, A.N. Calcium channel blockers: An update. Am. J. Med. 2004, 116, 35–43. [Google Scholar] [CrossRef]
- Opie, L.H. Calcium channel antagonists in the treatment of coronary artery disease: Fundamental pharmacological properties relevant to clinical use. Prog. Cardiovasc. Dis. 1996, 38, 273–290. [Google Scholar] [CrossRef]
- Babich, M.F.; Kalin, M.L.; Mcleod, D.C. Calcium-channel blockers in acute myocardial infarction. Drug Intell. Clin. Pharm. 1989, 23, 538–547. [Google Scholar] [CrossRef]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T., Jr. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. J. Am. Med. Assoc. 2003, 289, 2560–2571. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.; Abdullah, M.I.; Ahmed, E.; Sharif, A.; Irfan, A.; Masood, S. Anti-HIV cytotoxicity enzyme inhibition and molecular docking studies of quinoline based chalcones as potential non-nucleoside reverse transcriptase inhibitors (NNRT). Bioorg. Chem. 2016, 65, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.D.; Nagakalyan, S.; Prasad, G. Solvent-free synthesis of polyhydroquinoline derivatives employing mesoporous vanadium ion doped titania nanoparticles as a robust heterogeneous catalyst via the Hantzsch reaction. RSC Adv. 2017, 7, 3611–3616. [Google Scholar]
- Karaki, H.; Urakawa, N.; Kutsky, P. Potassium-induced contraction in smooth muscle. J. Smooth Muscle Res. 1984, 20, 427–444. [Google Scholar] [CrossRef] [Green Version]
- Karaki, H.; Weiss, G.B. Calcium release in smooth muscle. Life Sci. 1988, 42, 111–122. [Google Scholar] [CrossRef]
- Godfraind, T. EDRF and cyclic GMP control gating of receptor-operated calcium channels in vascular smooth muscle. Eur. J. Pharmacol. 1986, 126, 341–343. [Google Scholar] [CrossRef]
- Shan, L.-M.; Wang, H. Pharmacological characteristics of the endothelial target for acetylcholine induced vascular relaxation. Life Sci. 2002, 70, 1285–1298. [Google Scholar] [CrossRef]
- Kumar, A.; Maurya, R.A. Synthesis of polyhydroquinoline derivatives through unsymmetric Hantzsch reaction using organocatalysts. Tetrahedron 2007, 63, 1946–1952. [Google Scholar] [CrossRef]
- Said, M.; Ahmad, J.; Rehman, W.; Badshah, A.; Khan, H.; Khan, M.; Rahim, F.; Spasyuk, D.M. Synthesis, structural characterization and antibacterial studies of trisubstituted guanidines and their copper (II) complexes. Inorganica Chim. Acta 2015, 434, 7–13. [Google Scholar] [CrossRef]
- Khalil, A.T.; Khan, I.; Ahmad, K.; Khan, Y.A.; Khan, M.; Khan, M.J. Synergistic antibacterial effect of honey and Herba Ocimi Basilici against some bacterial pathogens. J. Tradit. Chin. Med. 2013, 33, 810–814. [Google Scholar] [CrossRef]
- Spelbrink, R.G.; Dilmac, N.; Allen, A.; Smith, T.J.; Shah, D.M.; Hockerman, G.H. Differential antifungal and calcium channel-blocking activity among structurally related plant defensins. Plant Physiol. 2004, 135, 2055–2067. [Google Scholar] [CrossRef]
- Vuorela, H.; Vuorela, P.; Törnquist, K.; Alaranta, S. Calcium channel blocking activity: Screening methods for plant derived compounds. Phytomedicine 1997, 4, 167–180. [Google Scholar] [CrossRef]
- Kumar, P.P.; Stotz, S.C.; Paramashivappa, R.; Beedle, A.M.; Zamponi, G.W.; Rao, A.S. Synthesis and evaluation of a new class of nifedipine analogs with T-type calcium channel blocking activity. Mol. Pharmacol 2002, 61, 649–658. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.L.; Wang, Y.Y.; Cheng, J.; Zhao, Y.Y. Calcium channel blocking activity of calycosin, a major active component of Astragali Radix, on rat aorta. Acta Pharmacol. Sin. 2006, 27, 1007–1012. [Google Scholar] [CrossRef]


| Compounds | R | Yield | Compounds | R | Yield |
|---|---|---|---|---|---|
| 3 |  | 81 | 16 |  | 65 |
| 4 |  | 75 | 17 |  | 73 |
| 5 |  | 69 | 18 |  | 79 |
| 6 |  | 71 | 19 |  | 72 |
| 7 |  | 72 | 20 |  | 78 |
| 8 |  | 81 | 21 |  | 76 |
| 9 |  | 74 | 22 |  | 69 |
| 10 |  | 68 | 23 |  | 70 |
| 11 | 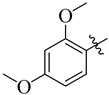 | 82 | 24 |  | 87 |
| 12 |  | 70 | 25 |  | 73 |
| 13 |  | 67 | 26 |  | 80 |
| 14 |  | 66 | 27 |  | 82 |
| 15 |  | 81 |
| Code | Escherichia coli (mm) | Enterococcus faecalis (mm) | ||||
|---|---|---|---|---|---|---|
| 2 µg/mL | 1 µg/mL | 0.5 µg/mL | 2 µg/mL | 1 µg/mL | 0.5 µg/mL | |
| 17 | 18 | 16 | 14 | 18 | 16 | 12 |
| 21 | 18 | 16 | 14 | 18 | 16 | 14 |
| 22 | 18 | 16 | 14 | 18 | 15 | 13 |
| 1 | 18 | 12 | 9 | 18 | 14 | 12 |
| 16 | 16 | 14 | 10 | 18 | 12 | 10 |
| 8 | 16 | 14 | 12 | 18 | 16 | 14 |
| 2 | 16 | 10 | 7 | 16 | 14 | 10 |
| 26 | 14 | 12 | 8 | 16 | 14 | 10 |
| 13 | 14 | 10 | 6 | 16 | 10 | 8 |
| 3 | 12 | 10 | 8 | 14 | 10 | 8 |
| 12 | 12 | 10 | 8 | 14 | 12 | 10 |
| 9 | 10 | 8 | 4 | 10 | 8 | 4 |
| 18 | 10 | 8 | 6 | 10 | 8 | 6 |
| 5 | 10 | 6 | 4 | 10 | 6 | 4 |
| 27 | 8 | 6 | 4 | 10 | 8 | 4 |
| Amoxicillin | 30 | 24 | 22 | 32 | 28 | 24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zainab; Yu, H.; Rehman, N.U.; Ali, M.; Alam, A.; Latif, A.; Shahab, N.; Amir Khan, I.; Jabbar Shah, A.; Khan, M.; et al. Novel Polyhydroquinoline-Hydrazide-Linked Schiff’s Base Derivatives: Multistep Synthesis, Antimicrobial, and Calcium-Channel-Blocking Activities. Antibiotics 2022, 11, 1568. https://doi.org/10.3390/antibiotics11111568
Zainab, Yu H, Rehman NU, Ali M, Alam A, Latif A, Shahab N, Amir Khan I, Jabbar Shah A, Khan M, et al. Novel Polyhydroquinoline-Hydrazide-Linked Schiff’s Base Derivatives: Multistep Synthesis, Antimicrobial, and Calcium-Channel-Blocking Activities. Antibiotics. 2022; 11(11):1568. https://doi.org/10.3390/antibiotics11111568
Chicago/Turabian StyleZainab, Haitao Yu, Najeeb Ur Rehman, Mumtaz Ali, Aftab Alam, Abdul Latif, Nazish Shahab, Irfan Amir Khan, Abdul Jabbar Shah, Momin Khan, and et al. 2022. "Novel Polyhydroquinoline-Hydrazide-Linked Schiff’s Base Derivatives: Multistep Synthesis, Antimicrobial, and Calcium-Channel-Blocking Activities" Antibiotics 11, no. 11: 1568. https://doi.org/10.3390/antibiotics11111568
APA StyleZainab, Yu, H., Rehman, N. U., Ali, M., Alam, A., Latif, A., Shahab, N., Amir Khan, I., Jabbar Shah, A., Khan, M., Al-Ghafri, A., Al-Harrasi, A., & Ahmad, M. (2022). Novel Polyhydroquinoline-Hydrazide-Linked Schiff’s Base Derivatives: Multistep Synthesis, Antimicrobial, and Calcium-Channel-Blocking Activities. Antibiotics, 11(11), 1568. https://doi.org/10.3390/antibiotics11111568









