Outstanding Enrofloxacin Removal Using an Unmodified Low-Cost Sorbent Prepared from the Leaves of Pyracantha koidzumii
Abstract
:1. Introduction
2. Results and Discussion
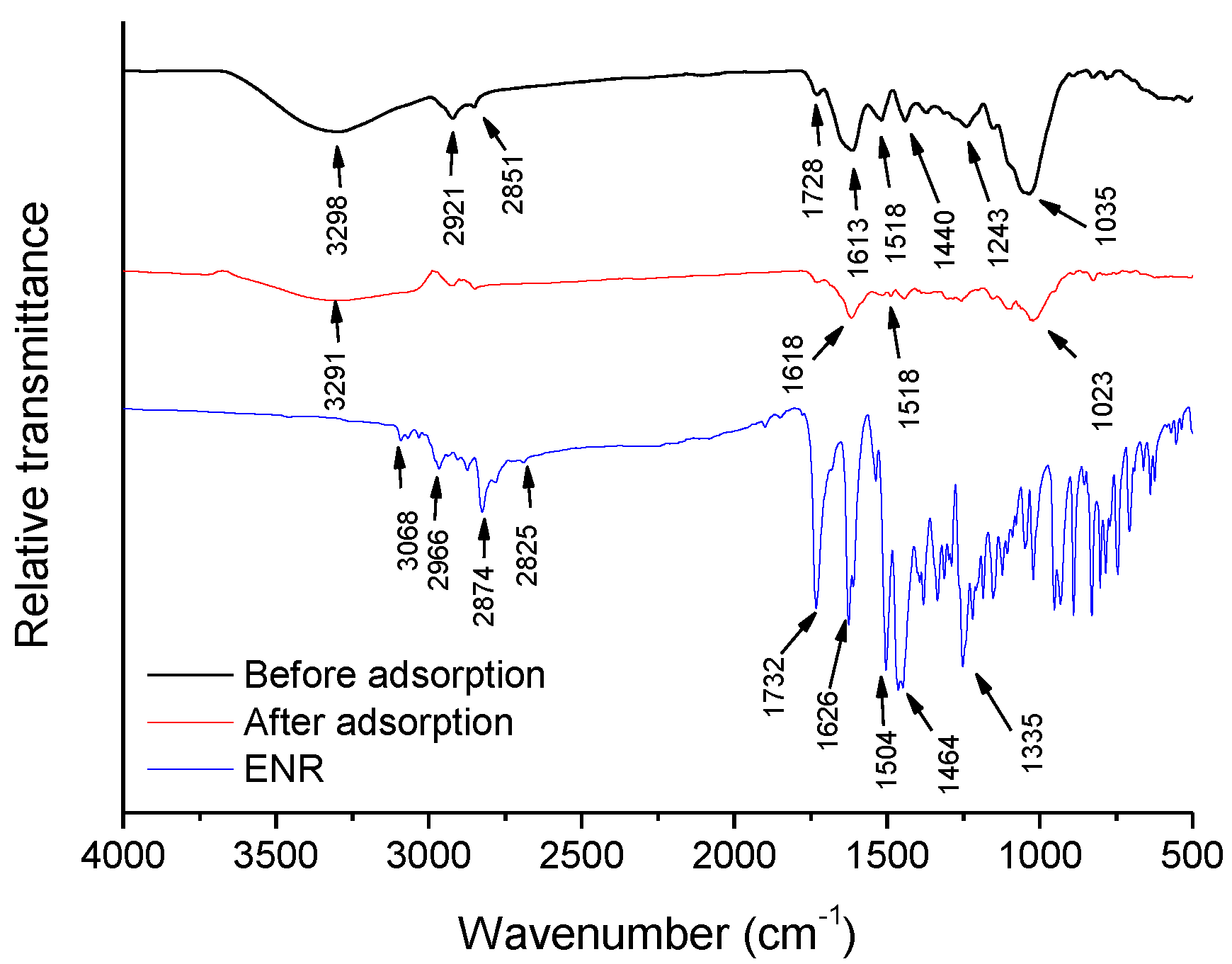
2.1. Characterization
2.2. Adsorption Studies
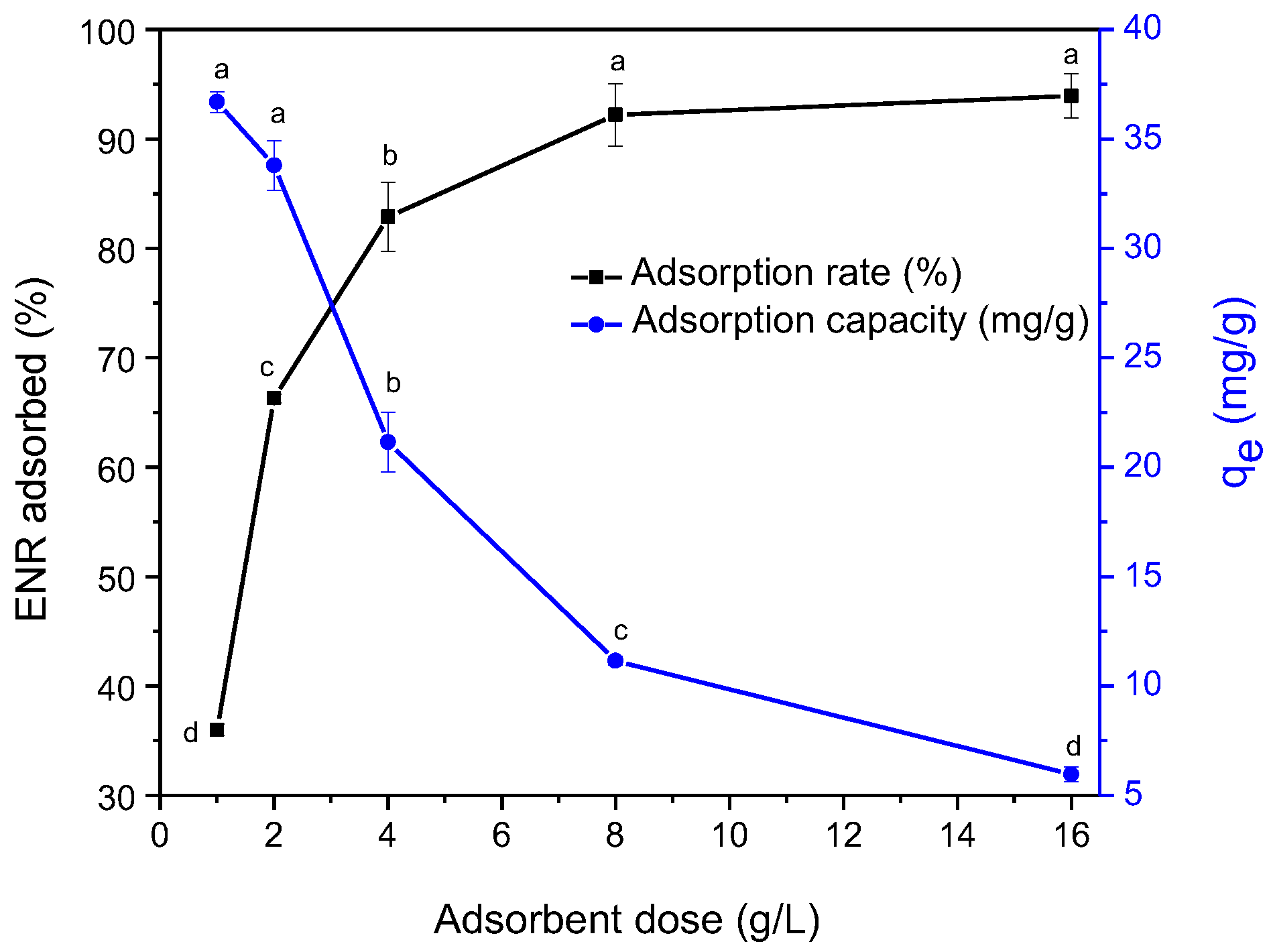
2.2.1. Optimal Adsorbent Dosage
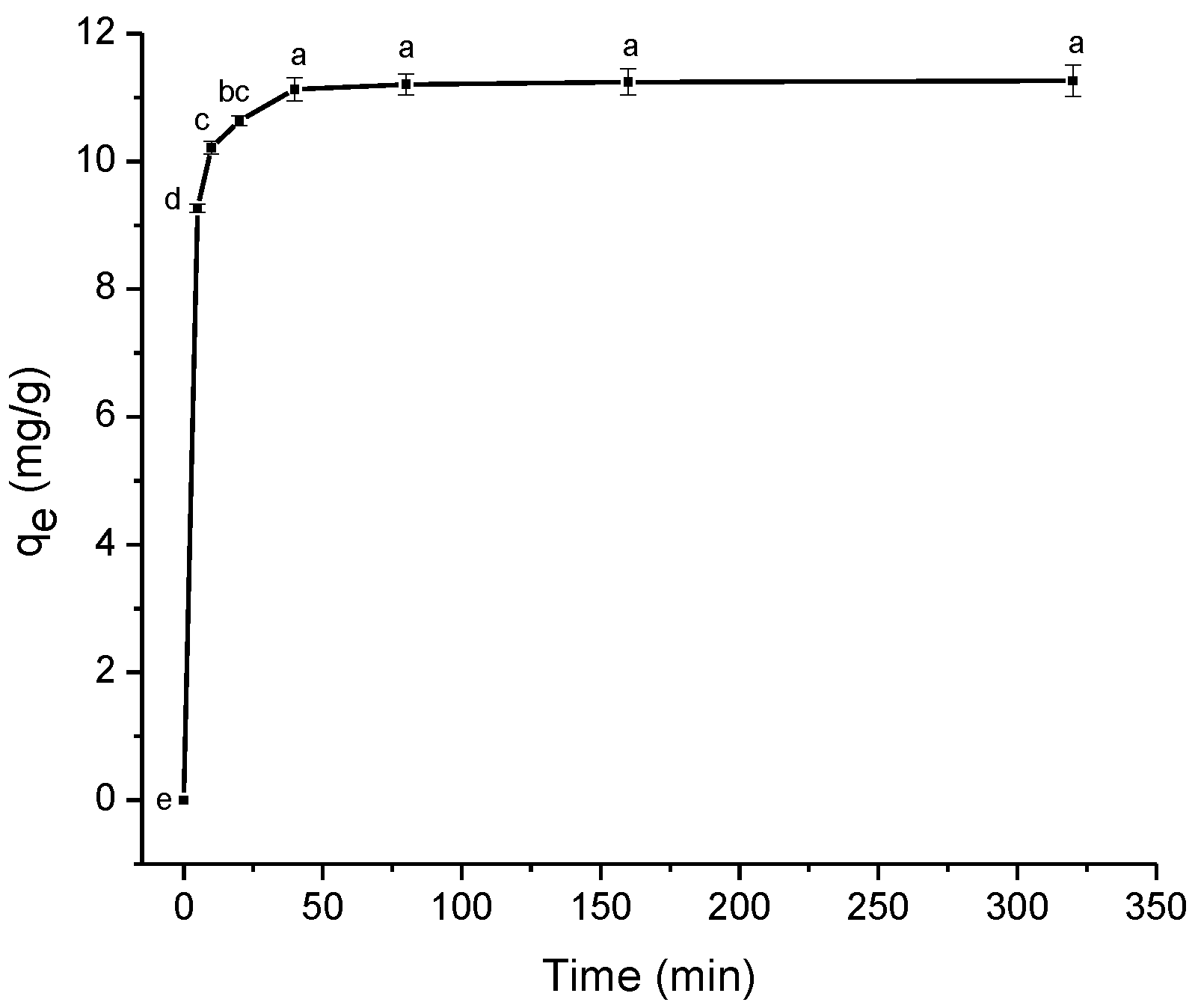
2.2.2. Effect of Contact Time
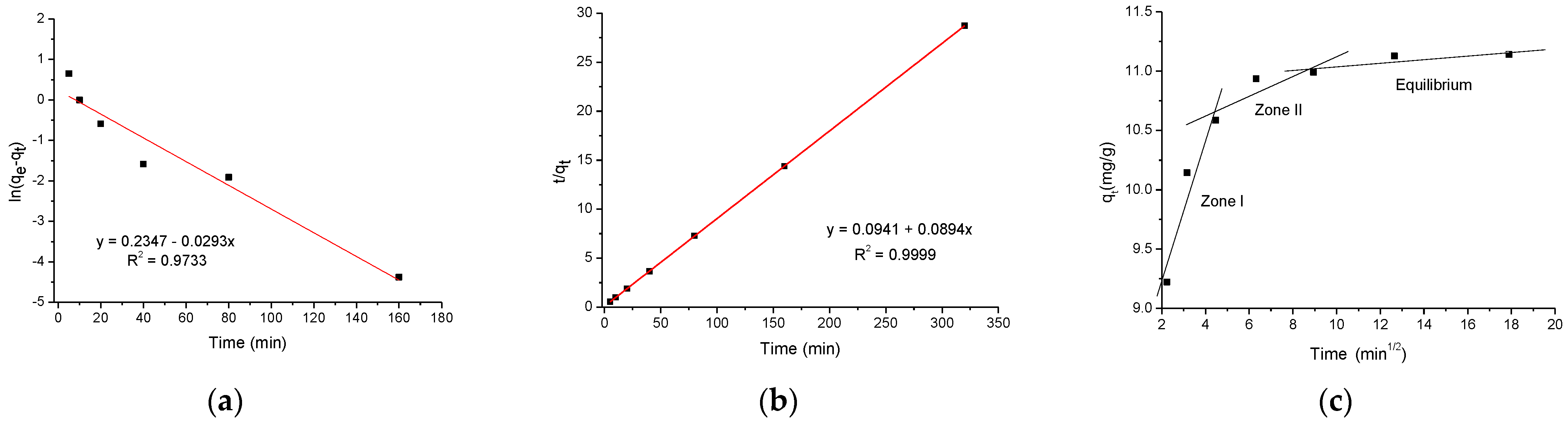
2.2.3. Kinetic Studies
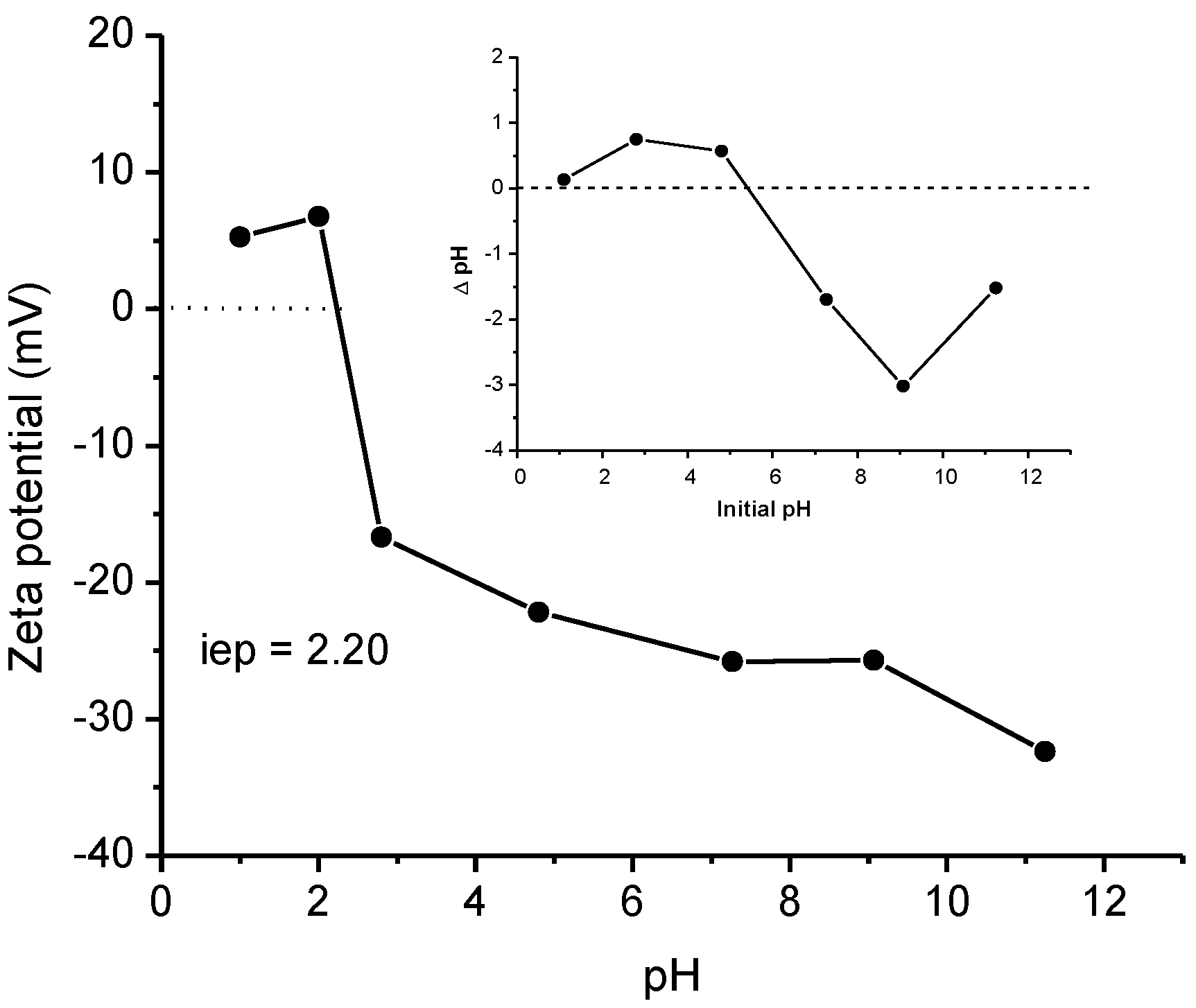
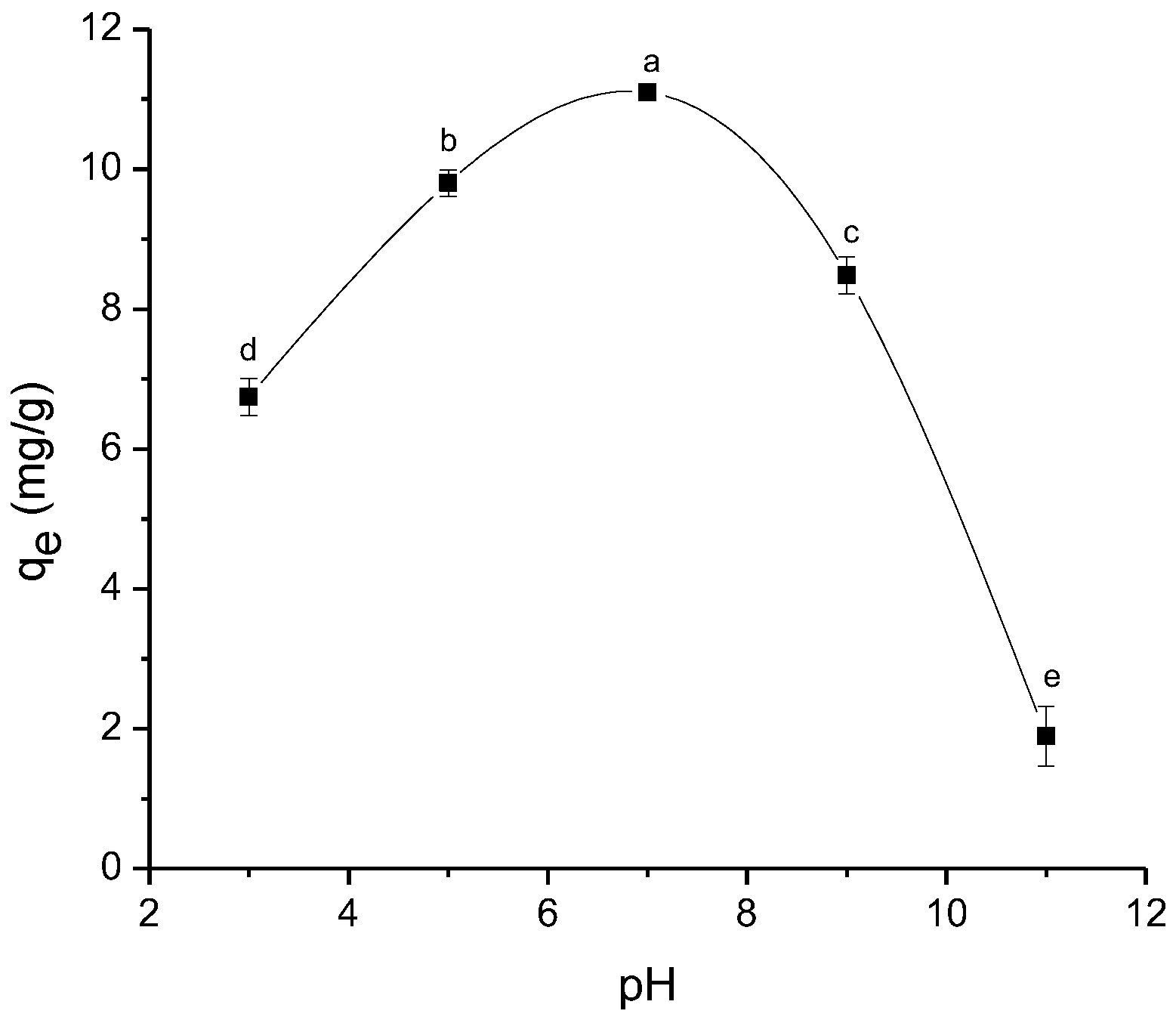
2.2.4. Effect of Solution pH
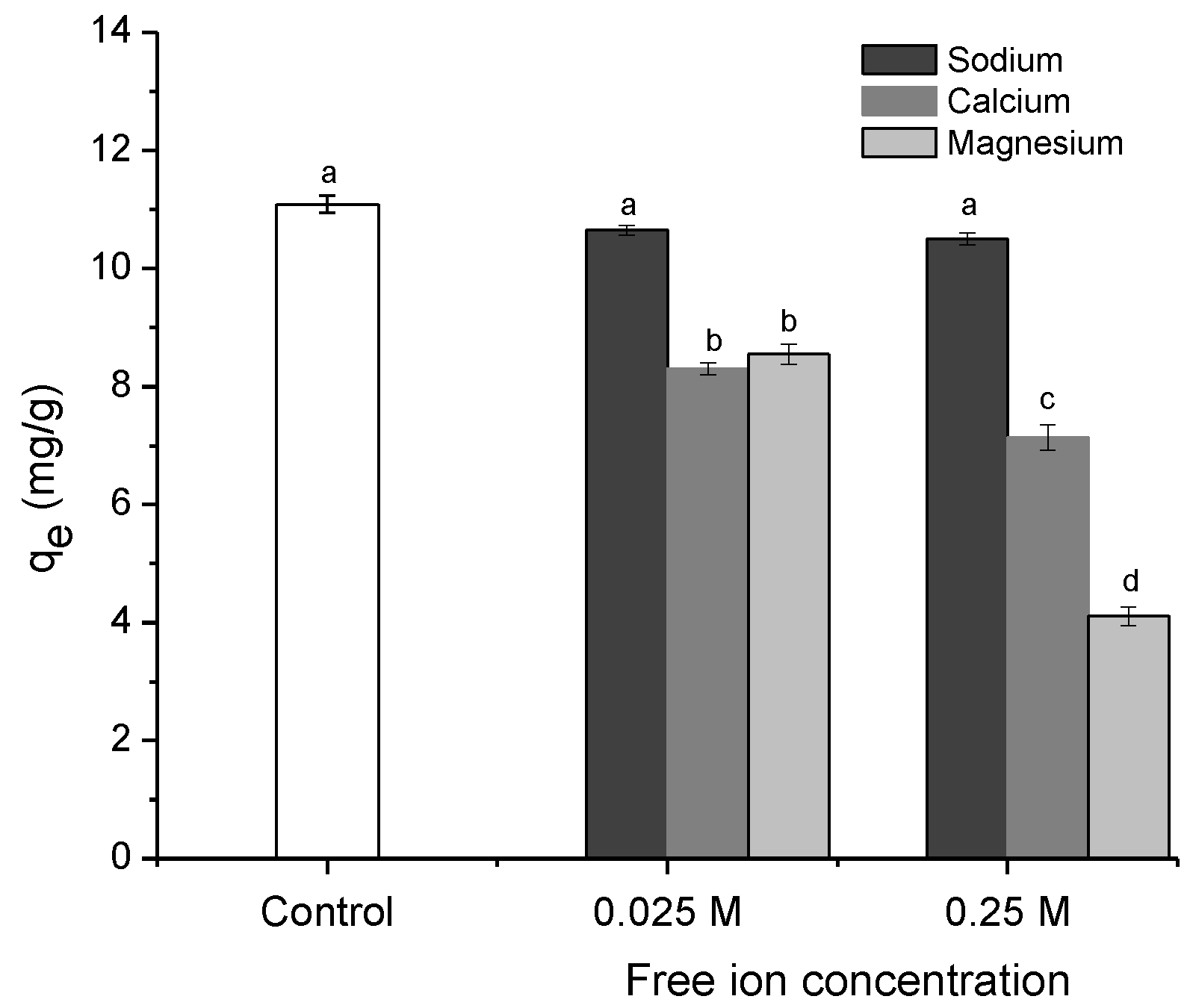
2.2.5. Effect of Solution Ionic Strength
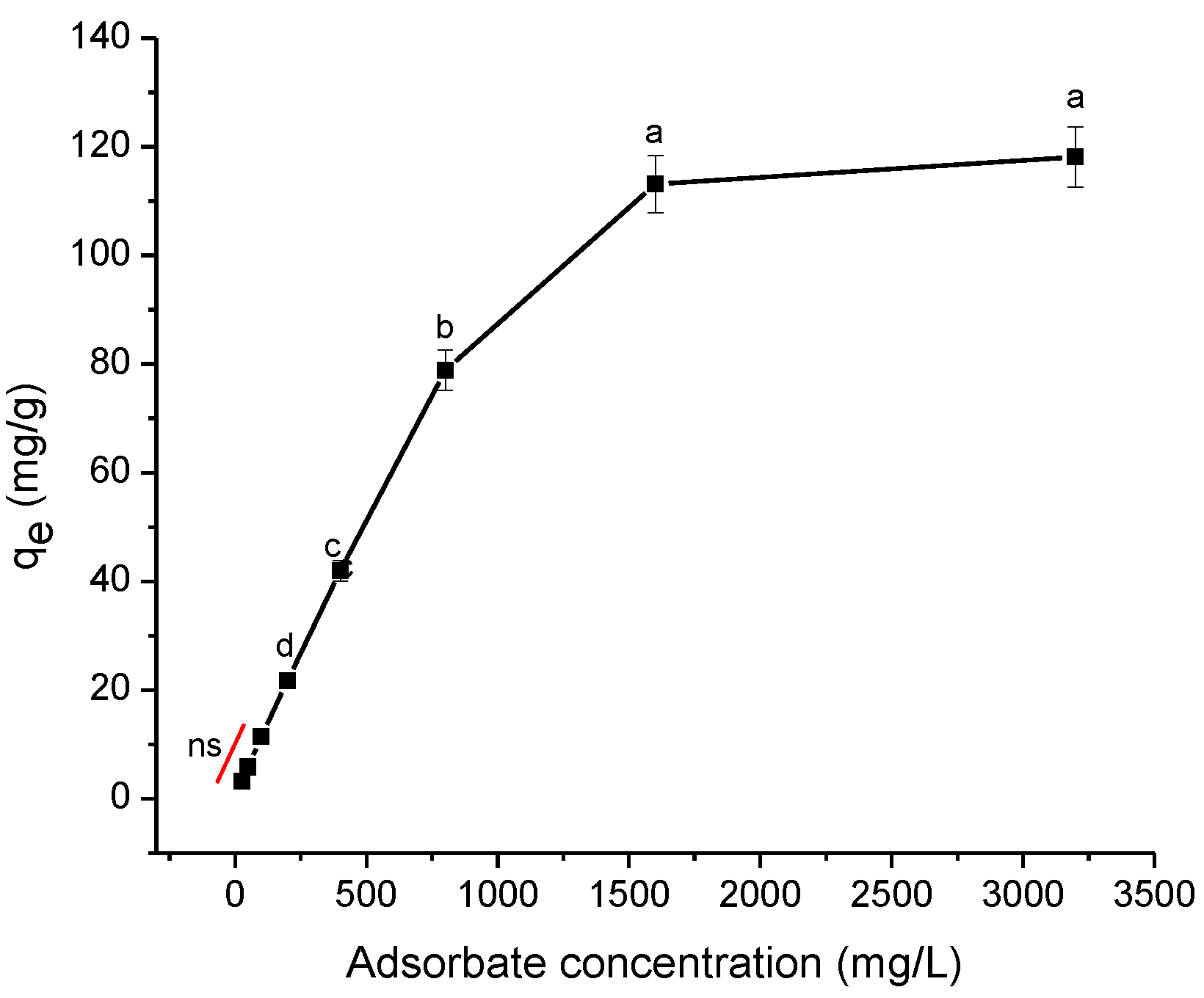
2.2.6. Effect of Adsorbate Concentration
2.2.7. Effect of Photosynthetic Pigments
2.2.8. Equilibrium Studies
2.2.9. Thermodynamics
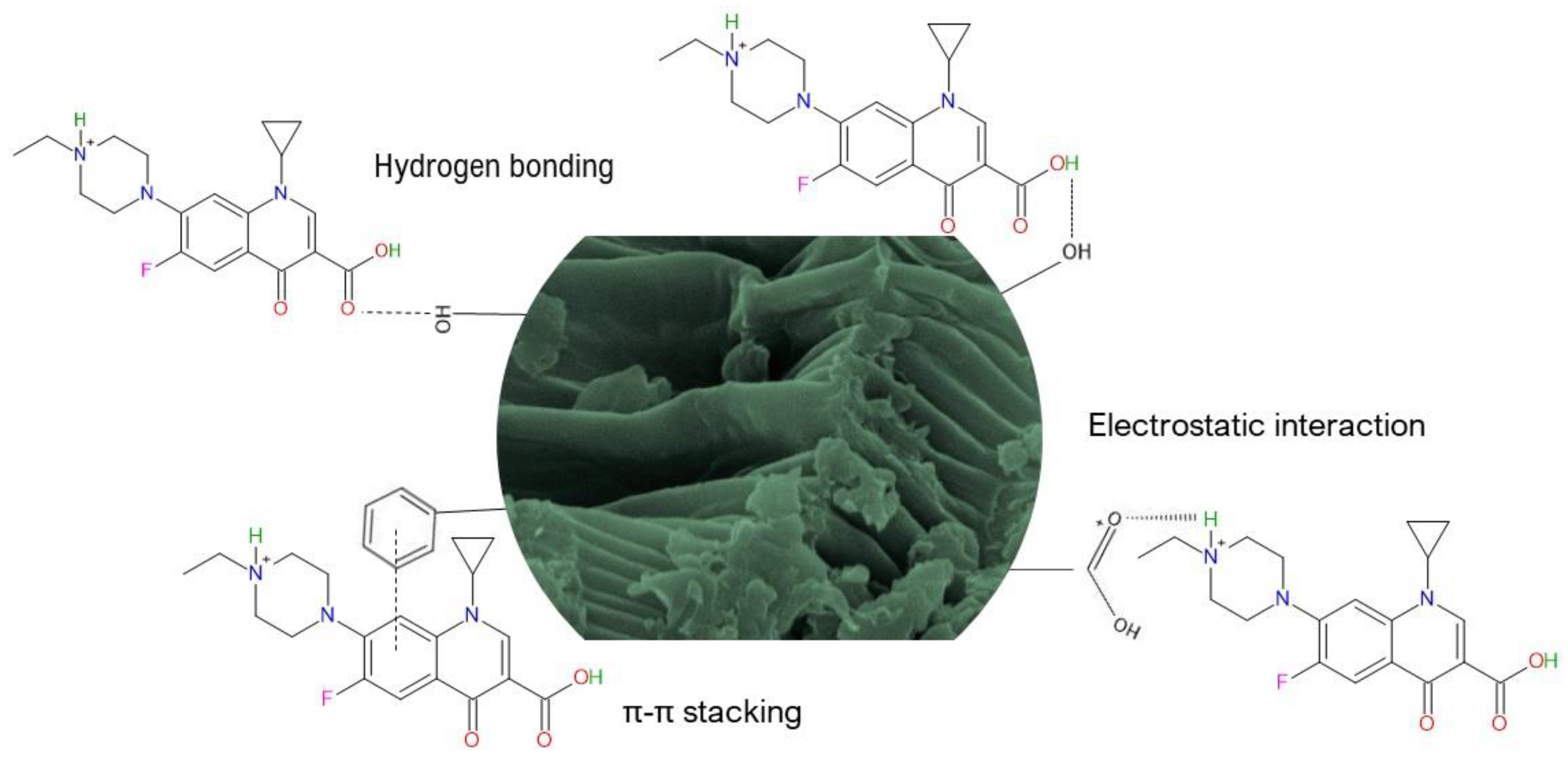
2.2.10. The Proposed Adsorption Mechanism
- Electrostatic interactions: the oxygen-containing functional groups were involved in ENR adsorption through electrostatic interactions. These functional groups on the sorbent surface could electrostatically bind to the amino group of the ENR molecule. Consequently, the electrostatic interactions may be the most important mechanism of the adsorption of ENR onto the P. koidzumii sorbent;
- Hydrogen bonding interactions: in the FTIR spectrum of the P. koidzumii sorbent after its interaction with ENR (Figure 1), the band at 3298 cm−1 shifted to 3291 cm−1, and the band at 1035 cm−1 shifted to 1023 cm−1. These frequency shifts may be attributed to the interaction between ENR and both the hydroxyl and ether groups, respectively. Consequently, hydrogen bonding can also play a dominant role in ENR adsorption;
- π-π orbital interactions: the significant reduction in intensity and shift to larger wavenumbers of the band at 1613 cm−1 confirmed the participation of aromatics present on the surface of the adsorbent on ENR adsorption. Thus, π-π orbital interactions are also involved in the ENR adsorption mechanism.
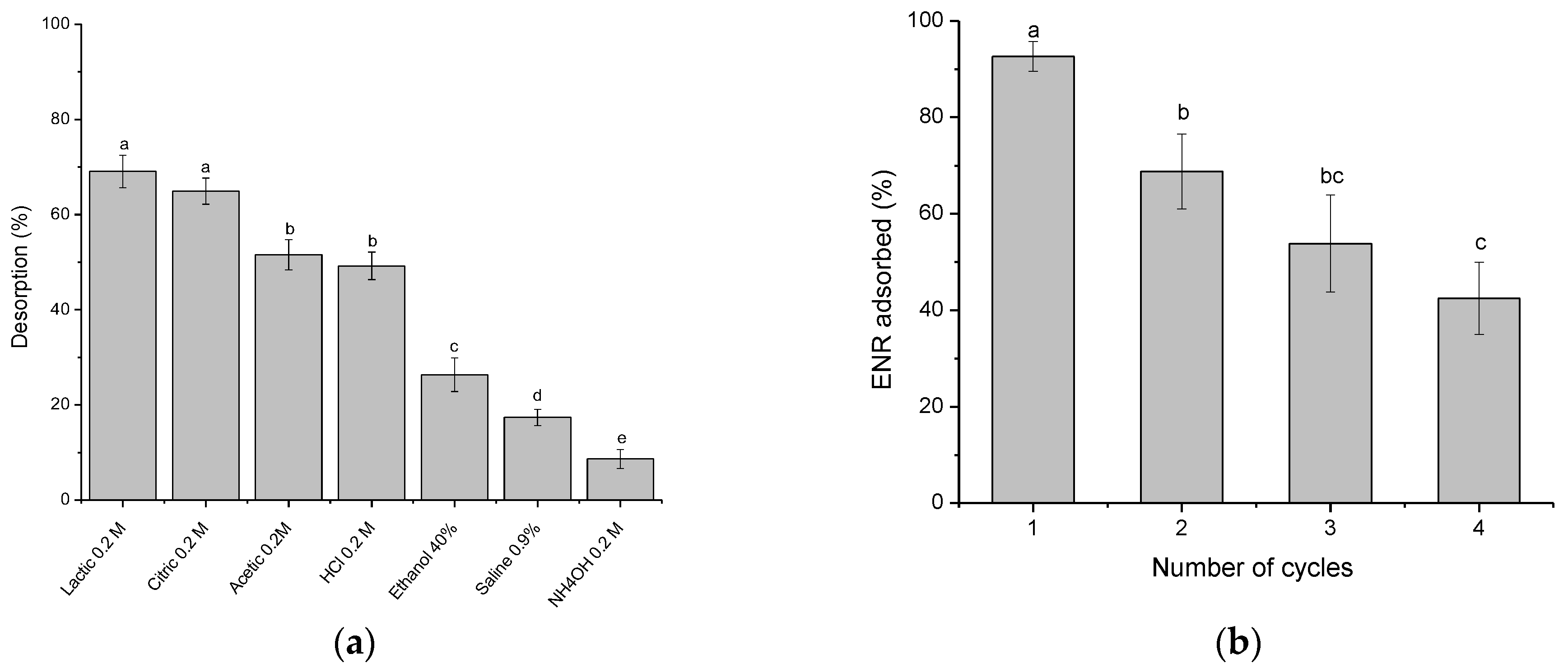
2.2.11. Reusability of Adsorbent
3. Materials and Methods
3.1. Adsorbate
3.2. Adsorbent
3.3. Characterization
3.4. Adsorption Studies
3.4.1. The Effect of Different Factors on the Sorption of Enrofloxacin
3.4.2. Recycling and Regeneration Experiments
3.5. Experimental Design and Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martinez, M.; McDermott, P.; Walker, R. Pharmacology of the fluoroquinolones: A perspective for the use in domestic animals. Vet. J. 2006, 172, 10–28. [Google Scholar] [CrossRef] [PubMed]
- Teglia, C.M.; Perez, F.A.; Michlig, N.; Repetti, M.R.; Goicoechea, H.C.; Culzoni, M.J. Occurrence, distribution, and ecological risk of fluoroquinolones in rivers and wastewaters. Environ. Toxicol. Chem. 2019, 38, 2305–2313. [Google Scholar] [CrossRef] [PubMed]
- Focazio, M.J.; Kolpin, D.W.; Barnes, K.K.; Furlong, E.T.; Meyer, M.T.; Zaugg, S.D.; Barber, L.B.; Thurman, M.E. A national reconnaissance for pharmaceuticals and other organic wastewater contaminants in the United States—II) Untreated drinking water sources. Sci. Total Environ. 2008, 402, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-J.; Mo, C.-H.; Li, Y.-W.; Gao, P.; Tai, Y.-P.; Zhang, Y.; Ruan, Z.-L.; Xu, J.-W. Determination of four fluoroquinolone antibiotics in tap water in Guangzhou and Macao. Environ. Pollut. 2010, 158, 2350–2358. [Google Scholar] [CrossRef]
- Viana, P.; Meisel, L.; Lopes, A.; De Jesus, R.; Sarmento, G.; Duarte, S.; Sepodes, B.; Fernandes, A.; Dos Santos, M.M.C.; Almeida, A.; et al. Identification of Antibiotics in Surface-Groundwater. A Tool towards the Ecopharmacovigilance Approach: A Portuguese Case-Study. Antibiotics 2021, 10, 888. [Google Scholar] [CrossRef] [PubMed]
- Balcıoğlu, I.A.; Ötker, M. Treatment of pharmaceutical wastewater containing antibiotics by O3 and O3/H2O2 processes. Chemosphere 2003, 50, 85–95. [Google Scholar] [CrossRef]
- Košutić, K.; Dolar, D.; Ašperger, D.; Kunst, B. Removal of antibiotics from a model wastewater by RO/NF membranes. Sep. Purif. Technol. 2007, 53, 244–249. [Google Scholar] [CrossRef]
- Guinea, E.; Brillas, E.; Centellas, F.; Cañizares, P.; Rodrigo, M.A.; Sáez, C. Oxidation of enrofloxacin with conductive-diamond electrochemical oxidation, ozonation and Fenton oxidation. A comparison. Water Res. 2009, 43, 2131–2138. [Google Scholar] [CrossRef]
- Guinea, E.; Garrido, J.A.; Rodríguez, R.M.; Cabot, P.-L.; Arias, C.; Centellas, F.; Brillas, E. Degradation of the fluoroquinolone enrofloxacin by electrochemical advanced oxidation processes based on hydrogen peroxide electrogeneration. Electrochim. Acta 2010, 55, 2101–2115. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, M.; Xin, Y.; Chai, C.; Chen, Q. Construction of immobilized CuS/TiO2 nanobelts heterojunction photocatalyst for photocatalytic degradation of enrofloxacin: Synthesis, characterization, influencing factors and mechanism insight. J. Chem. Technol. Biotechnol. 2019, 94, 2219–2228. [Google Scholar] [CrossRef]
- Peng, G.; Li, T.; Ai, B.; Yang, S.; Fu, J.; He, Q.; Yu, G.; Deng, S. Highly efficient removal of enrofloxacin by magnetic montmorillonite via adsorption and persulfate oxidation. Chem. Eng. J. 2019, 360, 1119–1127. [Google Scholar] [CrossRef]
- Wang, W.; Ma, X.; Sun, J.; Chen, J.; Zhang, J.; Wang, Y.; Wang, J.; Zhang, H. Adsorption of enrofloxacin on acid/alkali-modified corn stalk biochar. Spectr. Lett. 2019, 52, 367–375. [Google Scholar] [CrossRef]
- Yan, W.; Zhang, J.; Jing, C. Adsorption of Enrofloxacin on montmorillonite: Two-dimensional correlation ATR/FTIR spectroscopy study. J. Colloid Interface Sci. 2013, 390, 196–203. [Google Scholar] [CrossRef]
- Duan, W.; Li, M.; Xiao, W.; Wang, N.; Niu, B.; Zhou, L.; Zheng, Y. Enhanced adsorption of three fluoroquinolone antibiotics using polypyrrole functionalized Calotropis gigantea fiber. Colloid. Surf. A-Physicochem. Eng. Asp. 2019, 574, 178–187. [Google Scholar] [CrossRef]
- Liakos, E.V.; Rekos, K.; Giannakoudakis, D.A.; Mitropoulos, A.C.; Fu, J.; Kyzas, G.Z. Activated Porous Carbon Derived from Tea and Plane Tree Leaves Biomass for the Removal of Pharmaceutical Compounds from Wastewaters. Antibiotics 2021, 10, 65. [Google Scholar] [CrossRef]
- Crespo-Alonso, M.; Nurchi, V.M.; Biesuz, R.; Alberti, G.; Spano, N.; Pilo, M.I.; Sanna, G. Biomass against emerging pollution in wastewater: Ability of cork for the removal of ofloxacin from aqueous solutions at different pH. J. Environ. Chem. Eng. 2013, 1, 1199–1204. [Google Scholar] [CrossRef]
- Nurchi, V.M.; Crespo-Alonso, M.; Pilo, M.I.; Spano, N.; Sanna, G.; Toniolo, R. Sorption of ofloxacin and chrysoidine by grape stalk. A representative case of biomass removal of emerging pollutants from wastewater. Arab. J. Chem. 2019, 12, 1141–1147. [Google Scholar] [CrossRef]
- Qureshi, T.; Memon, N.; Memon, S.Q.; Ashraf, M.A. Decontamination of ofloxacin: Optimization of removal process onto sawdust using response surface methodology. Desalin. Water Treat. 2016, 57, 221–229. [Google Scholar] [CrossRef]
- Wuana, R.A.; Sha’Ato, R.; Iorhen, S. Aqueous phase removal of ofloxacin using adsorbents from Moringa oleifera pod husks. Adv. Environ. Res. 2015, 4, 49–68. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, W.; Shi, J.; Zhu, S.; Yan, Y. Enhanced levofloxacin removal from water using zirconium (IV) loaded corn bracts. Environ. Sci. Pollut. Res. 2017, 24, 10685–10694. [Google Scholar] [CrossRef]
- Mekhamer, W.; Al-Tamimi, S. Removal of ciprofloxacin from simulated wastewater by pomegranate peels. Environ. Sci. Pollut. Res. 2019, 26, 2297–2304. [Google Scholar] [CrossRef] [PubMed]
- Peñafiel, M.E.; Vanegas, E.; Bermejo, D.; Matesanz, J.M.; Ormad, M.P. Organic residues as adsorbent for the removal of ciprofloxacin from aqueous solution. Hyperfine Interact. 2019, 240, 71. [Google Scholar] [CrossRef]
- Tolić, K.; Mutavdžić Pavlović, D.; Stankir, N.; Runje, M. Biosorbents from Tomato, Tangerine, and Maple Leaves for the Removal of Ciprofloxacin from Aqueous Media. Water Air Soil Pollut. 2021, 232, 218. [Google Scholar] [CrossRef]
- Paredes-Laverde, M.; Silva-Agredo, J.; Torres-Palma, R.A. Removal of norfloxacin in deionized, municipal water and urine using rice (Oryza sativa) and coffee (Coffea arabica) husk wastes as natural adsorbents. J. Environ. Manage. 2018, 213, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, W.; Liu, J.; Huang, K.; Wu, C.; Shao, H.; Chen, H.; Liu, X. Modification of garlic peel by nitric acid and its application as a novel adsorbent for solid-phase extraction of quinolone antibiotics. Chem. Eng. J. 2017, 326, 745–755. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, W.; Liu, Z.; Liu, J.; Zhu, L.; Liu, X.; Huang, K. Renewable Tb/Eu-loaded garlic peels for enhanced adsorption of enrofloxacin: Kinetics, isotherms, thermodynamics, and mechanism. ACS Sustain. Chem. Eng. 2018, 6, 15264–15272. [Google Scholar] [CrossRef]
- Duan, W.; Xiao, W.; Wang, N.; Niu, B.; Zheng, Y. Removal of three fluoroquinolone antibiotics by NaClO2-modified biosorbent from fruit fiber of C. Procera. J. Nat. Fibers 2020, 17, 1594–1604. [Google Scholar] [CrossRef]
- Sayen, S.; Ortenbach-Lopez, M.; Guillon, E. Sorptive removal of enrofloxacin antibiotic from aqueous solution using a ligno-cellulosic substrate from wheat bran. J. Environ. Chem. Eng. 2018, 6, 5820–5829. [Google Scholar] [CrossRef]
- The United Nations Environment Programme. Converting Waste Agricultural Biomass into a Resource; UNEP: Osaka, Japan; Shiga, Japan, 2009. [Google Scholar]
- Ramales-Valderrama, R.A.; Vázquez-Durán, A.; Méndez-Albores, A. Biosorption of B-aflatoxins using biomasses obtained from formosa firethorn [Pyracantha koidzumii (Hayata) Rehder]. Toxins 2016, 8, 218. [Google Scholar] [CrossRef]
- Taşar, Ş.; Kaya, F.; Özer, A. Biosorption of lead (II) ions from aqueous solution by peanut shells: Equilibrium, thermodynamic and kinetic studies. J. Environ. Chem. Eng. 2014, 2, 1018–1026. [Google Scholar] [CrossRef]
- Kumar, G.P.; Phani, A.; Prasad, R.; Sanganal, J.S.; Manali, N.; Gupta, R.; Rashmi, N.; Prabhakara, G.; Salins, C.P.; Sandeep, K. Polyvinylpyrrolidone oral films of enrofloxacin: Film characterization and drug release. Int. J. Pharm. 2014, 471, 146–152. [Google Scholar] [CrossRef]
- Martinez, Y.; Piñuel, L.; Castro, G.; Breccia, J. Polyvinyl alcohol–pectin cryogel films for controlled release of enrofloxacin. Appl. Biochem. Biotechnol. 2012, 167, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Vilvanathan, S.; Shanthakumar, S. Removal of Ni (II) and Co (II) ions from aqueous solution using teak (Tectona grandis) leaves powder: Adsorption kinetics, equilibrium and thermodynamics study. Desalin. Water Treat. 2016, 57, 3995–4007. [Google Scholar] [CrossRef]
- Nava-Ramírez, M.J.; Salazar, A.M.; Sordo, M.; López-Coello, C.; Téllez-Isaías, G.; Méndez-Albores, A.; Vázquez-Durán, A. Ability of low contents of biosorbents to bind the food carcinogen aflatoxin B1 in vitro. Food Chem. 2021, 345, 128863. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Muneer, M.; Jamal, M.A.; Maqbool, T.; Tahir, T. Biosorption of metribuzin pesticide by Cucumber (Cucumis sativus) peels-zinc oxide nanoparticles composite. Sci. Rep. 2022, 12, 5840. [Google Scholar] [CrossRef]
- Chokejaroenrat, C.; Sakulthaew, C.; Satchasataporn, K.; Snow, D.D.; Ali, T.E.; Assiri, M.A.; Watcharenwong, A.; Imman, S.; Suriyachai, N.; Kreetachat, T. Enrofloxacin and Sulfamethoxazole Sorption on Carbonized Leonardite: Kinetics, Isotherms, Influential Effects, and Antibacterial Activity toward S. aureus ATCC 25923. Antibiotics 2022, 11, 1261. [Google Scholar] [CrossRef]
- Lizondo, M.; Pons, M.; Gallardo, M.; Estelrich, J. Physicochemical properties of enrofloxacin. J. Pharm. Biomed. Anal. 1997, 15, 1845–1849. [Google Scholar] [CrossRef]
- Vázquez-Durán, A.; Nava-Ramírez, M.d.J.; Hernández-Patlán, D.; Solís-Cruz, B.; Hernández-Gómez, V.; Téllez-Isaías, G.; Méndez-Albores, A. Potential of kale and lettuce residues as natural adsorbents of the carcinogen aflatoxin B1 in a dynamic gastrointestinal tract-simulated model. Toxins 2021, 13, 771. [Google Scholar] [CrossRef]
- Li, N.; Gao, B.; Yang, R.; Yang, H. Simple fabrication of carboxymethyl cellulose and κ-carrageenan composite aerogel with efficient performance in removal of fluoroquinolone antibiotics from water. Front. Environ. Sci. Eng. 2022, 16, 133. [Google Scholar] [CrossRef]
- Wintermans, J.; De Mots, A. Spectrophotometric characteristics of chlorophylls a and b and their phenophytins in ethanol. Biochim. Biophys. Acta 1965, 109, 448–453. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Hayward, D.O.; Trapnell, B.M.W. Chemisorption, 2nd ed.; Butterworth: London, UK, 1964; p. 323. [Google Scholar]
- Mahmoud, D.K.; Salleh, M.A.M.; Karim, W.A.W.A.; Idris, A.; Abidin, Z.Z. Batch adsorption of basic dye using acid treated kenaf fibre char: Equilibrium, kinetic and thermodynamic studies. Chem. Eng. J. 2012, 181, 449–457. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis of AOAC International; AOAC International: Rockville, MD, USA, 2005. [Google Scholar]

| Component | % |
|---|---|
| Moisture content | 6.29 ± 0.01 |
| Crude protein | 16.00 ± 0.08 |
| Crude fat | 2.02 ± 0.09 |
| Total ash | 5.08 ± 0.07 |
| Carbohydrate * | 70.61 ± 0.08 |
| Kinetic Model | Parameter qe (mg/g) | Enrofloxacin 11.12 | |
|---|---|---|---|
| Pseudo-first-order | qe (mg/g) | 1.27 | |
| k1 (1/min) | 0.029 | ||
| R2 | 0.9733 | ||
| Pseudo-second-order | qe (mg/g) | 11.18 | |
| k2 (g/mg min) | 0.085 | ||
| R2 | 0.9999 | ||
| Intraparticle diffusion | kp (mg/g min1/2) | 0.592 | |
| Zone 1 | C (mg/g) | 8.04 | |
| R2 | 0.9131 | ||
| kp (mg/g min1/2) | 0.086 | ||
| Zone 2 | C (mg/g) | 10.27 | |
| R2 | 0.7765 | ||
| Equilibrium | qe (mg/g) | 11.01 |
| Isotherm | Equation | Parameter | Temperature (K) | ||
|---|---|---|---|---|---|
| 288 | 298 | 308 | |||
| Langmuir | qm (mg/g) | 116.28 | 120.48 | 138.89 | |
| b (L/mg) | 0.011 | 0.008 | 0.005 | ||
| R2 | 0.9998 | 0.9990 | 0.9889 | ||
| Freundlich | Kf (L/g) | 4.377 | 3.382 | 2.890 | |
| n | 2.107 | 1.946 | 1.856 | ||
| R2 | 0.9144 | 0.9304 | 0.9242 | ||
| Dubinin–Radushkevich | qm (mg/g) | 52.29 | 52.99 | 50.91 | |
| E (kJ/mol) | 0.278 | 0.204 | 0.174 | ||
| R2 | 0.6027 | 0.6212 | 0.5661 | ||
| Adsorbent | Modification | pH | Isotherm Model | Maximum Adsorption Capacity (mg/g) | Reference |
|---|---|---|---|---|---|
| Garlic peel (GP) | None | NR | Langmuir | 0.65 | [25] |
| HNO3 | 9.89 | ||||
| Garlic peel (GP) | HNO3-GP | 7 | Langmuir | 29.8 | [26] |
| Tb@GP | 580 | ||||
| Eu@GP | 421 | ||||
| Tb/Eu@GP | 769 | ||||
| Calotropis gigantea fiber | NaClO2/acetic acid | 6 | Langmuir | 62.93 | [27] |
| Wheat bran | Acid-basic treatments | 6 | Sips | 91.5 | [28] |
| P. koidzumii leaves | None | 7 | Langmuir | 138.89 | This research |
| Temperature (K) | (KJ/mol) | (KJ/mol) | ∆S° (J/mol K) |
|---|---|---|---|
| 288 | 7.435 | 9.605 | 7.408 |
| 298 | 7.361 | ||
| 308 | 7.287 |
| Molecular Formula | Chemical Structure | Molecular Weight (g/mol) | pKa1 (Carboxyl) | pKa2 (Piperazinyl) | Fluorescence (nm) λex/λem |
|---|---|---|---|---|---|
| C19H22FN3O3 | 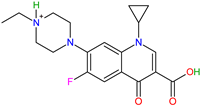 | 359.39 | 5.95 | 8.70 | 272/430 |
| Item | Experimental Run | Enrofloxacin Concentration (mg/L) | Adsorbent Dose (g/L) | Contact Time (min) | Solution pH | Temperature (K) | Solution Ion Strength (M) |
|---|---|---|---|---|---|---|---|
| 1 | Sorbent dose | 100 | 1–16 | 1440 | 7 | 298 | - |
| 2 | Contact time | 100 | 8 | 0–320 | 7 | 298 | - |
| 3 | Solution pH | 100 | 8 | 40 | 3–11 | 298 | - |
| 4 | Ion strength | 100 | 8 | 40 | 7 | 298 | 0.025 and 0.25 |
| 5 | Adsorbate concentration | 50–3200 | 8 | 40 | 7 | 298 | - |
| 6 | Temperature | 100 | 8 | 40 | 7 | 288–308 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Escutia, R.; Méndez-Albores, A.; Vázquez-Durán, A. Outstanding Enrofloxacin Removal Using an Unmodified Low-Cost Sorbent Prepared from the Leaves of Pyracantha koidzumii. Antibiotics 2022, 11, 1563. https://doi.org/10.3390/antibiotics11111563
Martínez-Escutia R, Méndez-Albores A, Vázquez-Durán A. Outstanding Enrofloxacin Removal Using an Unmodified Low-Cost Sorbent Prepared from the Leaves of Pyracantha koidzumii. Antibiotics. 2022; 11(11):1563. https://doi.org/10.3390/antibiotics11111563
Chicago/Turabian StyleMartínez-Escutia, Rubén, Abraham Méndez-Albores, and Alma Vázquez-Durán. 2022. "Outstanding Enrofloxacin Removal Using an Unmodified Low-Cost Sorbent Prepared from the Leaves of Pyracantha koidzumii" Antibiotics 11, no. 11: 1563. https://doi.org/10.3390/antibiotics11111563
APA StyleMartínez-Escutia, R., Méndez-Albores, A., & Vázquez-Durán, A. (2022). Outstanding Enrofloxacin Removal Using an Unmodified Low-Cost Sorbent Prepared from the Leaves of Pyracantha koidzumii. Antibiotics, 11(11), 1563. https://doi.org/10.3390/antibiotics11111563








