Physician-Targeted Interventions in Antibiotic Prescribing for Urinary Tract Infections in General Practice: A Systematic Review
Abstract
:1. Introduction
2. Results
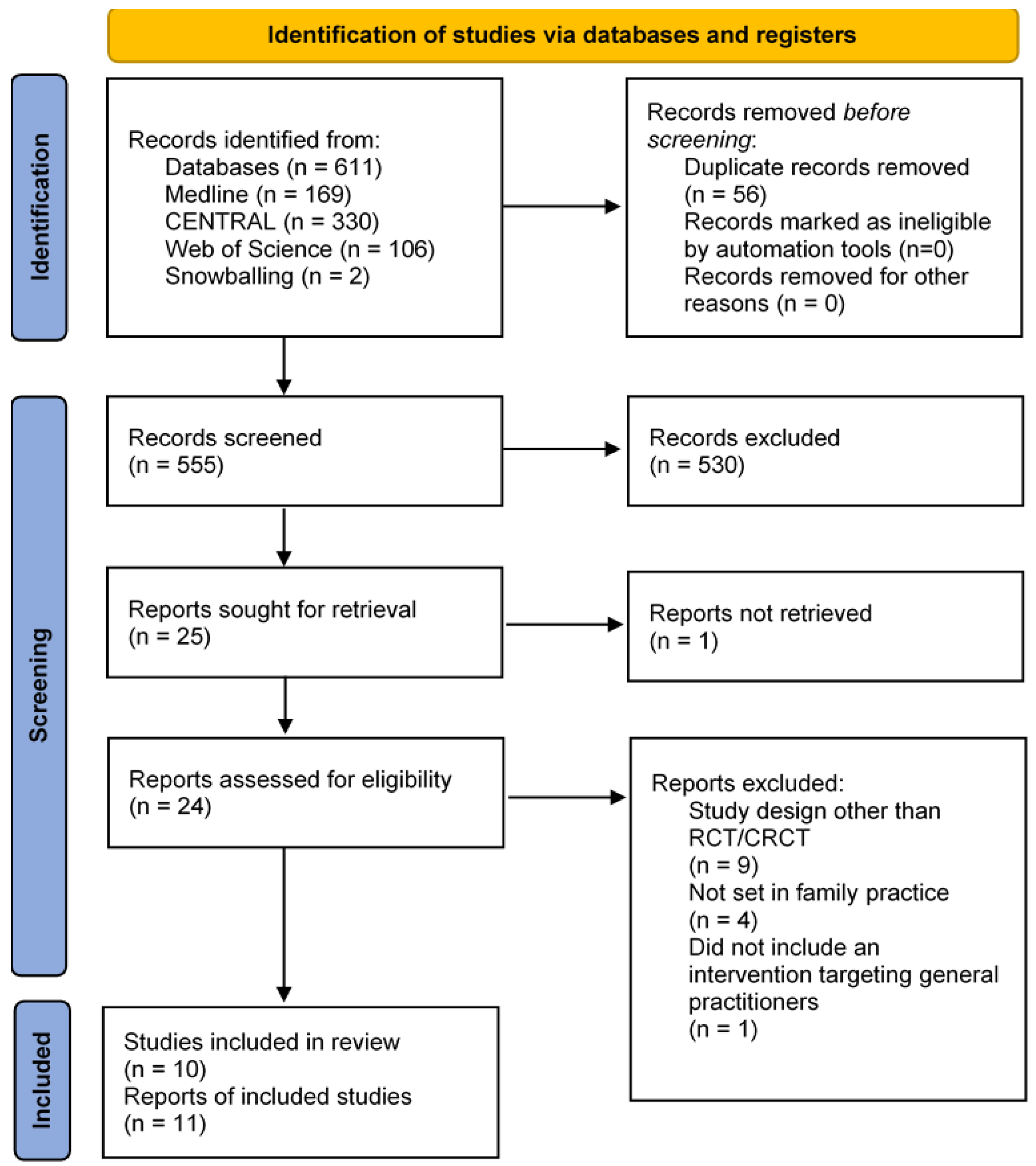
2.1. Study Selection
2.2. Study Characteristics
2.3. Study Quality
2.4. Primary Outcomes
2.4.1. Effect of the Interventions on the Number of Antibiotic Prescriptions
2.4.2. Effect of the Interventions on the Appropriateness of Antibiotic Prescriptions
2.4.3. Effect of the Interventions on Broad-Spectrum Antibiotic Prescriptions
2.5. Additional Outcomes
3. Discussion
3.1. Summary
3.2. Explanation of Results
3.3. Strengths and Limitations
3.4. Implications for Practice
4. Materials and Methods
4.1. Search Strategy
4.2. Inclusion & Exclusion Criteria
4.3. Study Selection
4.4. Quality Assessment
4.5. Data Analysis
4.6. Reporting of Results
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Registration and Protocol
Appendix A
| ID | Search | Hits |
| #1 | MeSH descriptor: [Randomised Controlled Trials as Topic] explode all trees | 15180 |
| #2 | MeSH descriptor: [Non-Randomised Controlled Trials as Topic] explode all trees | 88 |
| #3 | MeSH descriptor: [Controlled Before-After Studies] explode all trees | 85 |
| #4 | MeSH descriptor: [Interrupted Time Series Analysis] explode all trees | 67 |
| #5 | random* control* trial* OR RCT OR random* trial OR non-random* trial OR controlled before-after stud* OR interrupted time serie* OR interrupted time serie* stud* OR repeated measure* OR repeated measures stud* | 1327639 |
| #6 | (#1 OR #2 OR #3 OR #4 OR #5) | 1327654 |
| #7 | MeSH descriptor: [Family Practice] explode all trees | 1981 |
| #8 | MeSH descriptor: [General Practice] explode all trees | 2504 |
| #9 | MeSH descriptor: [General Practitioners] explode all trees | 336 |
| #10 | MeSH descriptor: [Primary Health Care] explode all trees | 8364 |
| #11 | MeSH descriptor: [Ambulatory Care] explode all trees | 3753 |
| #12 | MeSH descriptor: [Outpatients] explode all trees | 1379 |
| #13 | (primary AND (care OR health care OR healthcare)) OR primary care provider* OR primary care physician* OR family medicine OR family practice OR general practitioner* OR family doctor* general practice OR ambulatory care OR outpatient OR outpatient care | 163256 |
| #14 | (#7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13) | 166331 |
| #15 | (audit AND feedback) OR communit* of practice OR educational game* OR educational meeting* OR educational meeting* educational material* OR educational outreach visit* OR academic detailing OR inter-professional education OR interprofessional education OR local consensus process* OR local opinion leader* OR patient-mediated intervention* OR patientmediated intervention* OR patient mediated intervention* OR reminder* OR delayed prescription* OR delayed antibiotic prescription* OR tailored intervention* OR financial incentive* OR intervention* OR program* | 555221 |
| #16 | MeSH descriptor: [Learning Health System] explode all trees | 2 |
| #17 | MeSH descriptor: [Educational Technology] explode all trees | 3991 |
| #18 | MeSH descriptor: [Academic Performance] explode all trees | 116 |
| #19 | MeSH descriptor: [Reminder Systems] explode all trees | 1016 |
| #20 | MeSH descriptor: [Quality Assurance, Health Care] explode all trees | 3426 |
| #21 | MeSH descriptor: [Education, Medical, Continuing] explode all trees | 747 |
| #22 | (#15 OR #17 OR #18 OR #19 OR #20 OR #21) | 558815 |
| #23 | MeSH descriptor: [Anti-Bacterial Agents] explode all trees | 13002 |
| #24 | antibiotic* OR antimicrobial* OR anti-microbial* OR antibacterial* OR anti-bacterial* | 49434 |
| #25 | (#23 OR #24) | 50346 |
| #26 | (prescr* OR recipe* OR course* OR dispens*) | 120534 |
| #27 | (#25 AND #26) | 8598 |
| #28 | MeSH descriptor: [Urinary Tract Infections] explode all trees | 2645 |
| #29 | MeSH descriptor: [Cystitis] explode all trees | 470 |
| #30 | urinary tract infection* OR UTI OR UTIs OR cystitis OR pyelonephritis OR prostatitis | 13518 |
| #31 | (#28 OR #29 OR #30) | 13765 |
| #32 | #6 AND #14 AND #22 AND #27 AND #31 | 331 |
| #1 | TS = (random* control* trial* OR random* trial* OR clinical trial* OR non-clinical trial* OR non clinical trial* OR non-random* trial OR non-random* trial OR controlled before-after stud* OR controlled before & after stud* OR controlled before and after stud* OR interrupted time serie* OR time serie* OR repeated measure* OR repeated measure* stud*) | 1903206 |
| #2 | TS = (family practice* OR general practice* OR general practitioner* OR primary health care OR primary care physician* OR (primary AND (care OR health care OR healthcare)) OR primary care provider* OR family medicine OR family practice OR general practitioner* OR family doctor* OR ambulatory care OR outpatient*) | 777538 |
| #3 | TS = ((audit AND feedback) OR communit* of practice OR learning health system* OR educational technology* OR academic performance* OR reminder system* OR quality assurance* OR continuing medical education OR educational game* OR educational meeting* OR educational material* OR educational outreach visit* OR academic detailing OR inter-professional education OR interprofessional education OR local consensus process* OR local opinion leader* OR patient-mediated intervention* OR patient mediated intervention* OR reminder* OR tailored intervention* OR financial incentive* OR intervention* OR program* OR algorithm* OR one-on-one session* OR one on one session*) | 5774938 |
| #4 | TS = ((antibiotic* OR antimicrobial* OR anti-microbial* OR antibacterial* OR anti-bacterial*) AND (prescri* OR recipe* OR course* OR dispens*)) | 40255 |
| #5 | TS = (urinary tract infection* OR UTI OR UTIs OR cystitis OR bacteriuria OR pyuria OR pyelonephritis OR prostatitis) | 69020 |
| #6 | #1 AND #2 AND #3 AND #4 AND #5 | 106 |
Appendix B
| Intervention | Definition |
|---|---|
| Audit & feedback | A summary of health workers’ performance over a specified period of time, given to them in a written, electronic, or verbal format. The summary may include recommendations for clinical action. |
| Communities of practice | Groups of people with a common interest who deepen their knowledge and expertise in this area by interacting on an ongoing basis. |
| Educational games | The use of games as an educational strategy to improve standards of care. |
| Educational materials | Distribution to individuals, or groups, of educational materials to support clinical care, i.e., any intervention in which knowledge is distributed. For example, this may be facilitated by the internet, learning critical appraisal skills; skills for electronic retrieval of information, diagnostic formulation; question formulation. |
| Educational meetings | Courses, workshops, conferences, or other educational meetings. |
| Educational outreaching, academic detailing | Personal visits by a trained person to health workers in their own settings, to provide information with the aim of changing practice. |
| Interprofessional education | Continuing education for health professionals that involves more than one profession in joint, interactive learning. |
| Local consensus processes | Formal or informal local consensus processes, for example agreeing a clinical protocol to manage a patient group, adapting a guideline for a local health system, or promoting the implementation of guidelines. |
| Local opinion leaders | The identification and use of identifiable local opinion leaders to promote good clinical practice. |
| Patient-mediated interventions | Any intervention aimed at changing the performance of healthcare professionals through interactions with patients, or information provided by or to patients. |
| Reminders | Manual or computerized interventions that prompt health workers to perform an action during a consultation with a patient, for example computer decision support systems. |
| Tailored interventions | Interventions to change practice that are selected based on an assessment of barriers to change, for example through interviews or surveys. |
References
- Nielen, M.; Hek, K.; Korevaar, J.; van Dijk, L.; Weesie, Y. Cijfers Huisartsen-Gezondheidsproblemen. 2021. Available online: https://www.nivel.nl/nl/nivel-zorgregistraties-eerste-lijn/gezondheidsproblemen (accessed on 21 June 2021).
- Christiaens, T.C.M.; de Meyere, M.; Verschraegen, G.; Peersman, W.; Heytens, S.; de Maeseneer, J.M. Randomised controlled trial of nitrofurantoin versus placebo in the treatment of uncomplicated urinary tract infection in adult women. Br. J. Gen. Pract. 2002, 52, 729–734. [Google Scholar] [PubMed]
- Ferry, S.A.; Holm, S.E.; Stenlund, H.; Lundholm, R.; Monsen, T.J. The natural course of uncomplicated lower urinary tract infection in women illustrated by a randomised placebo controlled study. Scand. J. Infect. Dis. 2004, 36, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Butler, C.C.; Hawking, M.K.D.; Quigley, A.; McNulty, C.A.M. Incidence, severity, help seeking, and management of uncomplicated urinary tract infection: A population-based survey. Br. J. Gen. Pract. 2015, 65, e702–e707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butler, C.C.; Francis, N.; Thomas-Jones, E.; Llor, C.; Bongard, E.; Moore, M.; Little, P.; Bates, J.; Lau, M.; Pickles, T.; et al. Variations in presentation, management, and patient outcomes of urinary tract infection: A prospective four-country primary care observational cohort study. Br. J. Gen. Pract. 2017, 67, e830–e841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emmerson, A.M.; Jones, A.M. The quinolones: Decades of development and use. J. Antimicrob. Chemother. 2003, 51, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Mitscher, L.A. Bacterial topoisomerase inhibitors: Quinolone and pyridone antibacterial agents. Chem. Rev. 2005, 105, 559–592. [Google Scholar] [CrossRef]
- Andriole, V.T. The quinolones: Past, present, and future. Clin. Infect. Dis. 2005, 41, S113–S119. [Google Scholar] [CrossRef] [Green Version]
- Aldred, K.J.; Kerns, R.J.; Osheroff, N. Mechanism of Quinolone Action and Resistance. Biochemistry 2014, 53, 1565–1574. [Google Scholar] [CrossRef]
- Grigoryan, L.; Nash, S.; Zoorob, R.; Germanos, G.J.; Horsfield, M.S.; Khan, F.M.; Martin, L.; Trautner, B.W. Qualitative Analysis of Primary Care Provider Prescribing Decisions for Urinary Tract Infections. Antibiotics 2019, 8, 84. [Google Scholar] [CrossRef] [Green Version]
- Drekonja, D.M.; Grigoryan, L.; Lichtenberger, P.; Graber, C.J.; Patel, P.K.; Van, J.N.; Dillon, L.M.; Wang, Y.; Gauthier, T.P.; Wiseman, S.W.; et al. Teamwork and safety climate affect antimicrobial stewardship for asymptomatic bacteriuria. Epidemiology 2019, 40, 963–967. [Google Scholar] [CrossRef]
- Duane, S.; Domegan, C.; Callan, A.; Galvin, S.; Cormican, M.; Bennett, K.; Murphy, A.W.; Vellinga, A. Using qualitative insights to change practice: Exploring the culture of antibiotic prescribing and consumption for urinary tract infections. BMJ Open 2016, 6, e008894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lecky, D.M.; Howdle, J.; Butler, C.C.; McNulty, C.A.M. Optimising management of UTIs in primary care: A qualitative study of patient and GP perspectives to inform the development of an evidence-based, shared decision-making resource. Br. J. Gen. Pract. 2020, 70, e330–e338. [Google Scholar] [CrossRef] [PubMed]
- Duane, S.; Beatty, P.; Murphy, A.W.; Vellinga, A. Exploring experiences of delayed prescribing and symptomatic treatment for urinary tract infections among general practitioners and patients in ambulatory care: A qualitative study. Antibiotics 2016, 5, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmes, A.H.; Moore, L.S.P.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J.V. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016, 387, 176–187. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Sridhar, D.; Blaser, M.; Wang, M.; Woolhouse, M. Achieving global targets for antimicrobial resistance. Science 2016, 353, 874–875. [Google Scholar] [CrossRef] [Green Version]
- Ranji, S.R.; Steinman, M.A.; Shojania, K.G.; Gonzales, R. Interventions to Reduce Unnecessary Antibiotic Prescribing: A Systematic Review and Quantitative Analysis. Med. Care 2008, 46, 847–862. [Google Scholar] [CrossRef]
- Drekonja, D.M.; Filice, G.A.; Greer, N.; Olson, A.; Macdonald, R.; Rutks, I.; Wilt, T.J. Antimicrobial Stewardship in Outpatient Settings: A Systematic Review. Infect. Control Hosp. Epidemiol. 2014, 36, 142–152. [Google Scholar] [CrossRef]
- Lundborg, C.S.; Wahlström, R.; Oke, T.; Tomson, G.; Diwan, V.K. Influencing prescribing for urinary tract infection and asthma in primary care in Sweden: A randomised controlled trial of an interactive educational intervention. J. Clin. Epidemiol. 1999, 52, 801–812. [Google Scholar] [CrossRef]
- Lagerløv, P.; Loeb, M.; Andrew, M.; Hjortdahl, P. Improving doctors’ prescribing behaviour through reflection on guidelines and prescription feedback: A randomised controlled study. QHC 2000, 9, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Ilett, K.F.; Johnson, S.; Greenhill, G.; Mullen, L.; Brockis, J.; Golledge, C.L.; Reid, D.B. Modification of general practitioner prescribing of antibiotics by use of a therapeutics adviser (academic detailer). Br. J. Clin. Pharmacol. 2000, 49, 168–173. [Google Scholar] [CrossRef]
- Veninga, C.; Denig, P.; Zwaagstra, R.; Haaijer-Ruskamp, F.M. Improving drug treatment in general practice. J. Clin. Epidemiol. 2000, 53, 762–772. [Google Scholar] [CrossRef]
- Flottorp, S.; Oxman, A.D.; Håvelsrud, K.; Treweek, S.; Herrin, J. Cluster randomised controlled trial of tailored interventions to improve the management of urinary tract infections in women and sore throat. BMJ 2002, 325, 367–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martens, J.D.; van der Weijden, T.; Severens, J.L.; de Clercq, P.A.; de Bruijn, D.P.; Kester, A.D.M.; Winkens, R.A.G. The effect of computer reminders on GPs’ prescribing behaviour: A cluster-randomised trial. Int. J. Med. Inform. 2007, 76, S403–S416. [Google Scholar] [CrossRef]
- Vellinga, A.; Galvin, S.; Duane, S.; Callan, A.; Benneth, K.; Cormican, M.; Domegan, C.; Murphy, A.W. Intervention to improve the quality of antimicrobial prescribing for urinary tract infection: A cluster randomised trial. Can. Med. Assoc. J. 2015, 188, 108–115. [Google Scholar] [CrossRef] [Green Version]
- Hürlimann, D.; Limacher, A.; Schabel, M.; Zanetti, G.; Berger, C.; Mühlemann, K.; Kronenberg, A. Improvement of antibiotic prescription in outpatient care: A cluster-randomised intervention study using a sentinel surveillance network of physicians. J. Antimicrob. J. Antimicrob. Chemother. 2014, 70, 602–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNulty, C.; Hawking, M.; Lecky, D.; Jones, L.; Owens, R.; Charlett, A.; Butler, C.; Moore, P.; Francis, N. Effects of primary care antimicrobial stewardship outreach on antibiotic use by general practice staff: Pragmatic randomised controlled trial of the TARGET antibiotics workshop. J. Antimicrob. Chemother. 2018, 73, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- McIsaac, W.; Kukan, S.; Huszti, E.; Szadkowski, L.; O’Neill, B.; Virani, S.; Ivers, N.; Lall, R.; Toor, N.; Shah, M.; et al. A pragmatic randomised trial of a primary care antimicrobial stewardship intervention in Ontario, Canada. BMC Fam. Pract. 2021, 22, 185. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Syn. Meth. 2020, 12, 55–61. [Google Scholar] [CrossRef]
- Arnold, S.R.; Straus, S.E. Interventions to improve antibiotic prescribing practices in ambulatory care. Cochrane Database Syst. Rev. 2005, 2005, CD003539. [Google Scholar] [CrossRef]
- Grimshaw, J.M.; Shirran, L.; Thomas, R.; Mowatt, G.; Fraser, C.; Bero, L.; Grilli, R.; Harvey, E.; Oxman, A.; O’Brien, M.A. Changing provider behaviour: An overview of systematic reviews of interventions. Med. Care 2001, 39, II-2–II-45. [Google Scholar] [CrossRef]
- van Braak, M.; Visser, M.; Holtrop, M.; Statius Muller, I.; Bont, J.; van Dijk, N. What motivates general practitioners to change practice behaviour? A qualitative study of audit and feedback group sessions in Dutch general practice. BMJ Open 2019, 9, e025286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, J.W.; Glasziou, P.P. Computerised reminders and feedback in medication management: A systematic review of randomised controlled trials. Med. J. Aust. 2003, 178, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Van Driel, A. Antibiotic resistance of uropathogenic Escherichia coli and ESBL prevalence in general practice patients over 10 years. Br. J. Gen. Pract. 2020, 70, bjgp20X711533. [Google Scholar] [CrossRef]
- van der Zande, R.L.; Bouvy, M.; Teichert, M. Adherence to guideline recommendations for urinary tract infections in adult women: A cross-sectional study. Prim. Health Care Res. Dev. 2021, 22, e11. [Google Scholar] [CrossRef] [PubMed]
- Bouma, M.; Geerlings, S.E.; Klinkhamer, S.; Knottnerus, B.J.; Platteel, T.N.; Reuland, E.A.; Visser, H.S.; Wolters, R.J. NHG Standaard Urineweginfecties. NHG Website April 2020. Available online: https://richtlijnen.nhg.org/standaarden/urineweginfecties (accessed on 25 October 2022).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Effective Practice and Organisation of Care (EPOC). EPOC Taxonomy. 2015. Available online: https://epoc.cochrane.org/epoc-taxonomy (accessed on 4 October 2022).

| Author (Year) | Design | Country | Population | Intervention | Comparator | Primary Outcome(S) | Disease/Drug Target | First Choice Antibiotic |
|---|---|---|---|---|---|---|---|---|
| Lundborg (1999) [19] | CRCT | Sweden | GP groups (n = 36) | audit & feedback, educational meetings | Multifaceted intervention for asthma | Number of antibiotic prescriptions for UTIs, number of prescriptions for asthma | UTI, asthma | trimethoprim, pivmecillinam, nitrofurantoin |
| Lagerløv (2000) [20] | CRCT | Norway | GP groups (n = 32) | audit & feedback, educational meetings, local consensus processes | Multifaceted intervention for asthma | The difference in proportions of short and long treatments for UTIs and asthma before and after the intervention | UTI, asthma | NR |
| Ilett (2000) [21] | RCT | Australia | GPs (n = 112) | educational materials, academic detailing | Standard care | Number of antibiotic prescriptions for upper and lower RTI and UTIs | UTI, RTI | trimethoprim |
| Veninga (2000) [22] | CRCT | The Netherlands | GP groups (n = 84) | audit & feedback, educational materials, educational meetings | Intervention for increasing corticosteroids prescribing for asthma | The proportion of first-choice drugs dispensed of all prescribed UTIs drugs, the average duration of treatment for first- choice UTI drugs. | UTI, asthma | trimethoprim, nitrofurantoin, sulfamethizol |
| Flottorp (2002) [23] | CRCT | Norway | GP practices (n = 142) | educational materials, educational meetings, patient-mediated interventions, reminders | Multifaceted intervention for sore throat. | Change in antibiotic prescription rate | UTI, sore throat | NR |
| Martens (2007) [24] | CRCT | The Netherlands | GP practices (n = 23) | reminders | Computer reminder system for cholesterol lowering drugs | Prescription according to the guideline recommendation as a percentage of total prescriptions | Bacterial infections, asthma, COPD, high cholesterol | trimethoprim, nitrofurantoin |
| Vellinga A (2015) [25] | CRCT | Ireland | GP practices (n = 30) | audit & feedback, educational meetings, patient-mediated interventions, reminders | Standard care | Proportion of prescriptions for recommended first-line antimicrobials. | UTI | trimethoprim, nitrofurantoin |
| Vellinga B (2015) [25] | CRCT | Ireland | GP practices (n = 30) | audit & feedback, educational meetings, patient-mediated interventions, reminders | Standard care | Proportion of prescriptions for recommended first-line antimicrobials | UTI | trimethoprim, nitrofurantoin |
| Hürlimann (2015) [26] | CRCT | Switzerland | GP practices (n = 140) | audit & feedback, educational materials | Standard care | Percentage of co-trimoxazole prescriptions for UTI, percentage of penicillin prescriptions for RTI | UTI, RTI, COPD | trimethoprim/ sulfamethoxazole |
| McNulty (2018) [27] | CRCT | UK | GP practices (n = 150) | audit & feedback, educational materials, educational meetings, patient-mediated interventions | Standard care | Total oral antibiotics dispensed (per 1000 practice patients, excluding anti-tuberculosis and minocycline) | UTI, RTI | nitrofurantoin |
| McIsaac (2021) [28] | CRCT | Canada | Primary care clinics (n = 6) | audit & feedback, educational materials, academic detailing, patient-mediated interventions | Standard care | Total antibiotic prescriptions for URI, sore throat presentations, acute sinusitis, acute bronchitis, and acute uncomplicated cystitis | Uncomplicated cystitis, acute sinusitis, URI, sore throat, acute bronchitis | NR |
| Study | Intervention (%) | Control (%) | OR | 95% CI |
|---|---|---|---|---|
| Lundborg | 1857/205836 (0.9) 1 | 1880/191673 (1.0) | 0.92 | 0.86–0.98 |
| Ilett | 503/7262 (6.9) 2 | 546/9654 (5.7) | 1.19 | 1.17–1.21 |
| Flottorp | 1167/2522 (46.3) 3 | 1285/2961 (43.4) | 1.12 | 1.01–1.25 |
| Vellinga A | 584/743 (78.6) 3 | 521/783 (66.5) | 1.85 | 1.47–2.32 |
| Vellinga B | 559/738 (75.8) 3 | 521/783 (66.5) | 1.57 | 1.25–1.97 |
| Hürlimann | 3217/15625 (20.6) 2 | 2744/13327 (20.6) | 1.00 | 0.94–1.06 |
| McNulty | 67850/343892 (19.7) 2 | 67210/372427 (18.0) | 0.99 * | 0.95–1.03 |
| McIsaac | 128/161 (79.5) 3 | 92/119 (77.3) | 1.12 * | 0.71–1.79 |
| Study | Intervention (%) | Control (%) | OR | 95% CI |
|---|---|---|---|---|
| Lundborg | 1227/1857 (66.1) 1 | 1018/1880 (54.1) | 1.65 | 1.37–1.98 |
| Ilett | 261/7262 (3.6) 2 | 291/9654 (3.0) | 1.20 | 1.01–1.42 |
| Veninga | 2456/2760 (89.0) 1 | 2412/2838 (85.0) | 1.43 | 1.22–1.67 |
| Martens | NR (73) | NR (57) | p < 0.05 | |
| Vellinga A | 507/743 (68.2) 3 | 345/783 (44.1) | 2.7 * | 1.8–4.1 |
| Vellinga B | 491/738 (66.5) 3 | 345/783 (44.1) | 2.0 * | 1.3–3.0 |
| Hürlimann | 1129/3217 (35.1) 1 | 516/2744 (18.8) | 2.16 * | 1.19–3.91 |
| McNulty | 24394/343892 (7.1) 2 | 23164/372427 (6.2) | 1.07 * | 1.00–1.15 |
| McIsaac | 238/258 (92.2) 3 | 197/232 (84.9) | 1.41 * | 0.66–3.01 |
| Study | Antibiotic | Intervention (%) 1 | Control (%) 1 | OR | 95% CI |
|---|---|---|---|---|---|
| Lundborg | Quinolones | 590/1857 (31.8) | 829/1880 (44.1) | 0.59 | 0.52–0.67 |
| Ilett | Amoxicillin | 1447/7262 (19.9) | 2151/9654 (22.3) | 0.87 | 0.81–0.94 |
| Amoxicillin + CA | 249/7262 (3.4) | 333/9654 (3.4) | 0.99 | 0.84–1.17 | |
| Vellinga A | Amoxicillin + CA | 37/743 (4.9) | 81/783 (10.3) | 0.4 * | 0.3–0.7 |
| Quinolones | 19/743 (2.6) | 52/783 (6.6) | 0.6 * | 0.3–1.00 | |
| Vellinga B | Amoxicillin + CA | 24/743 (3.2) | 81/783 (10.3) | 0.3 * | 0.2–0.6 |
| Quinolones | 16/738 (2.2) | 52/783 (6.6) | 0.7 * | 0.3–1.4 | |
| Hürlimann | Penicillins | 161/3217 (5.0) | 99/2744 (3.6) | 1.41 | 1.09–1.82 |
| Quinolones | 1174/3217 (36.5) | 1430/2744 (52.1) | 0.53 | 0.48–0.59 | |
| McNulty | All | 42837/343892 (12.5) | 49735/372427 (13.4) | 0.99 * | 0.93–1.05 |
| Amoxicillin + CA | 24017/343892 (7.0) | 28784/372427 (7.7) | 0.97 * | 0.89–1.05 | |
| Cephalosporins | 8551/343892 (2.5) | 10727/372472 (2.9) | 1.00 * | 0.87–1.16 | |
| Quinolones | 10269/343892 (3.0) | 10244/372427 (2.8) | 1.04 * | 0.95–1.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cox, S.; Lo-A-Foe, K.; van Hoof, M.; Dinant, G.-J.; Oudhuis, G.; Savelkoul, P.; Cals, J.; de Bont, E. Physician-Targeted Interventions in Antibiotic Prescribing for Urinary Tract Infections in General Practice: A Systematic Review. Antibiotics 2022, 11, 1560. https://doi.org/10.3390/antibiotics11111560
Cox S, Lo-A-Foe K, van Hoof M, Dinant G-J, Oudhuis G, Savelkoul P, Cals J, de Bont E. Physician-Targeted Interventions in Antibiotic Prescribing for Urinary Tract Infections in General Practice: A Systematic Review. Antibiotics. 2022; 11(11):1560. https://doi.org/10.3390/antibiotics11111560
Chicago/Turabian StyleCox, Stefan, Kelly Lo-A-Foe, Minke van Hoof, Geert-Jan Dinant, Guy Oudhuis, Paul Savelkoul, Jochen Cals, and Eefje de Bont. 2022. "Physician-Targeted Interventions in Antibiotic Prescribing for Urinary Tract Infections in General Practice: A Systematic Review" Antibiotics 11, no. 11: 1560. https://doi.org/10.3390/antibiotics11111560
APA StyleCox, S., Lo-A-Foe, K., van Hoof, M., Dinant, G.-J., Oudhuis, G., Savelkoul, P., Cals, J., & de Bont, E. (2022). Physician-Targeted Interventions in Antibiotic Prescribing for Urinary Tract Infections in General Practice: A Systematic Review. Antibiotics, 11(11), 1560. https://doi.org/10.3390/antibiotics11111560







