Antifungal Activity of Avocado Seed Recombinant GASA/Snakin PaSn
Abstract
:1. Introduction
2. Results
2.1. E. coli Avocado PaSn Recombinant Proteint Expression and Purification
2.2. Identification of Heterologous Avocado PaSn Protein
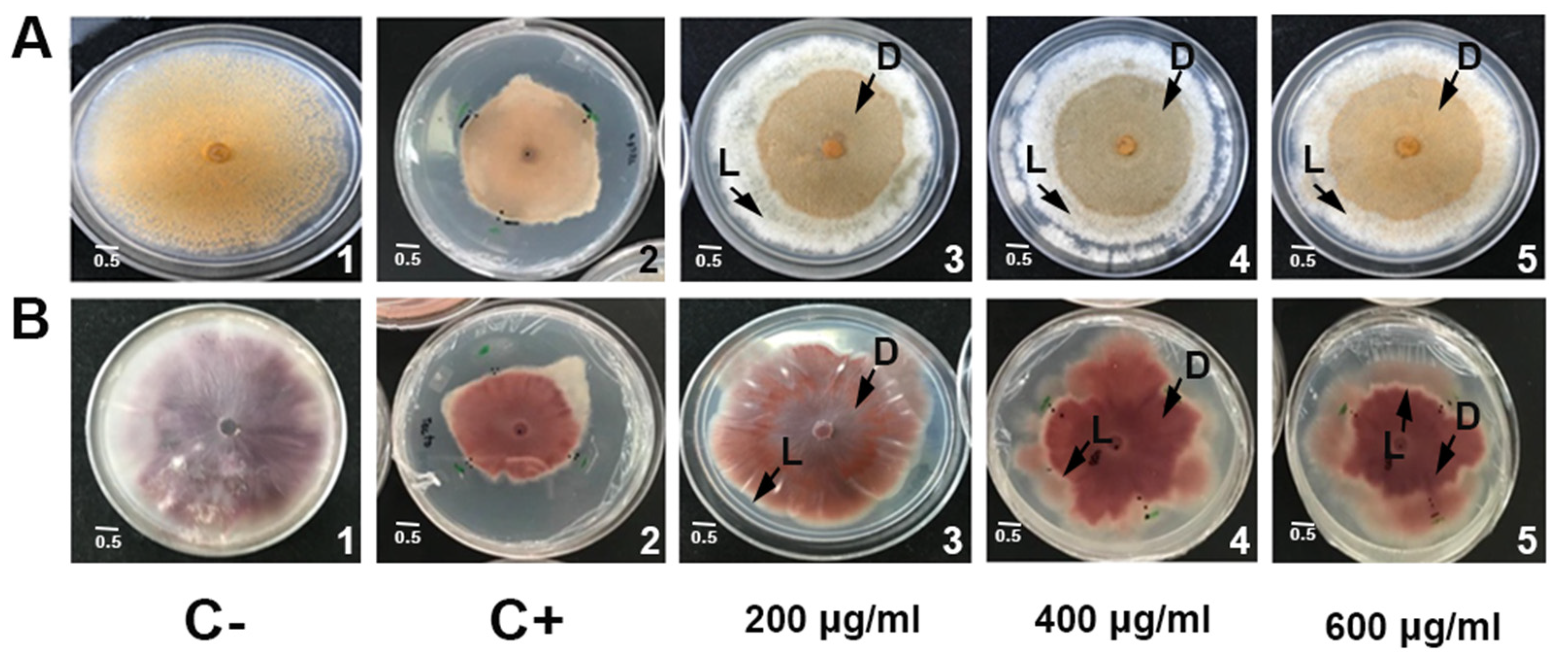
2.3. Growth Inhibitory Activity of PaSn against Phytopathogen Filamentous Fungi
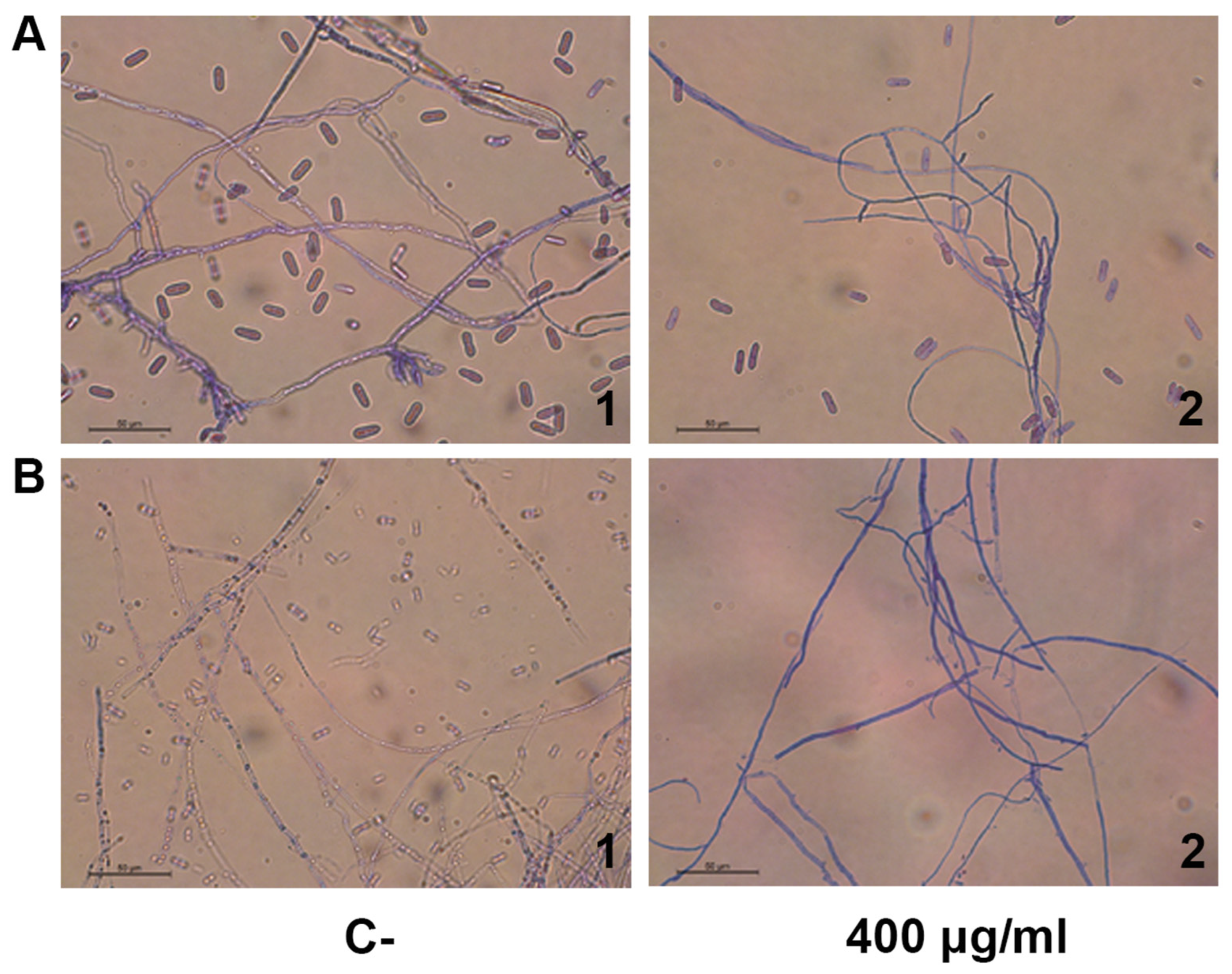
2.4. Permeabilization of Phytopathogen Membrane
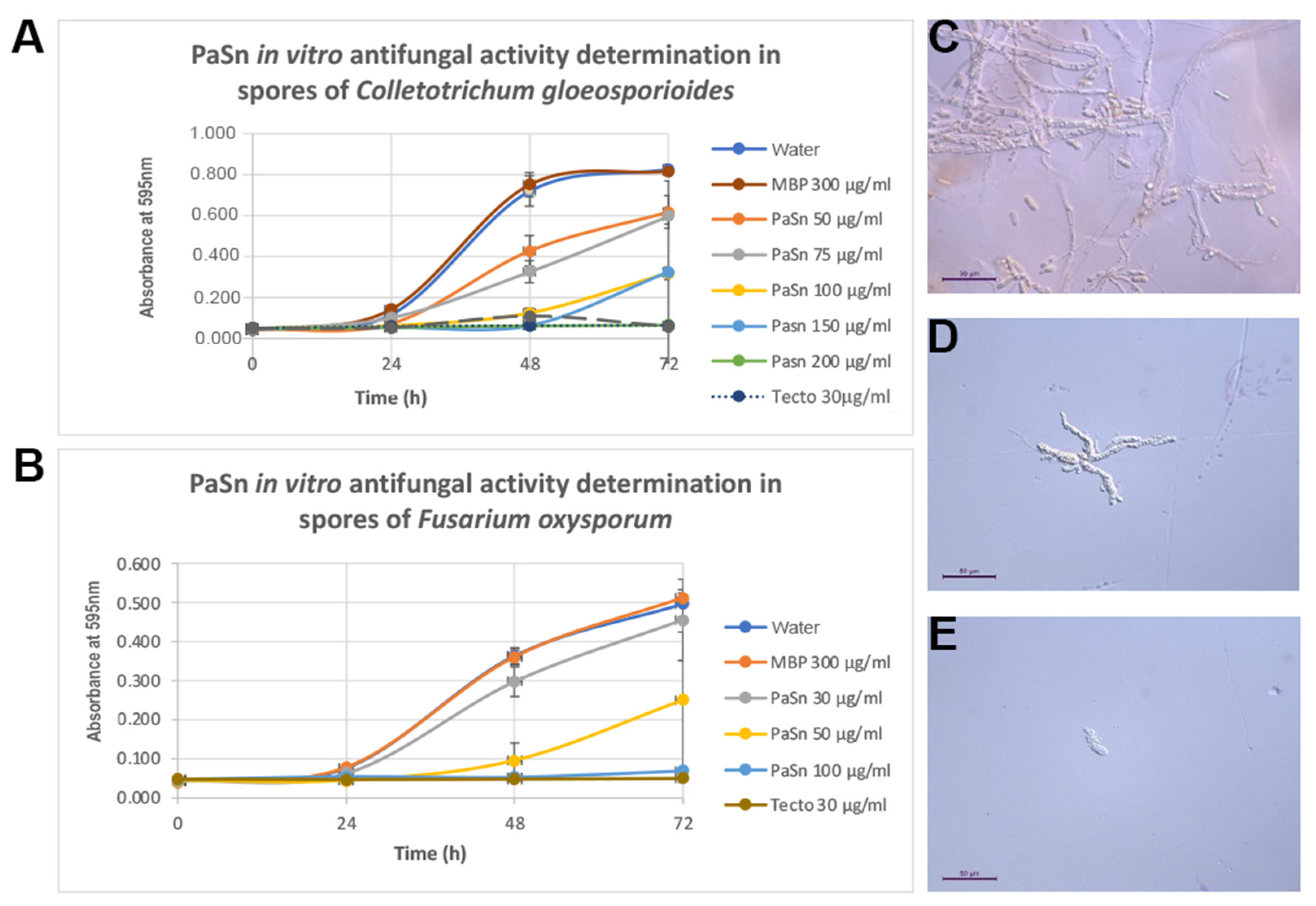
2.5. Phytopathogen Fungal Inhibition Spore Germination
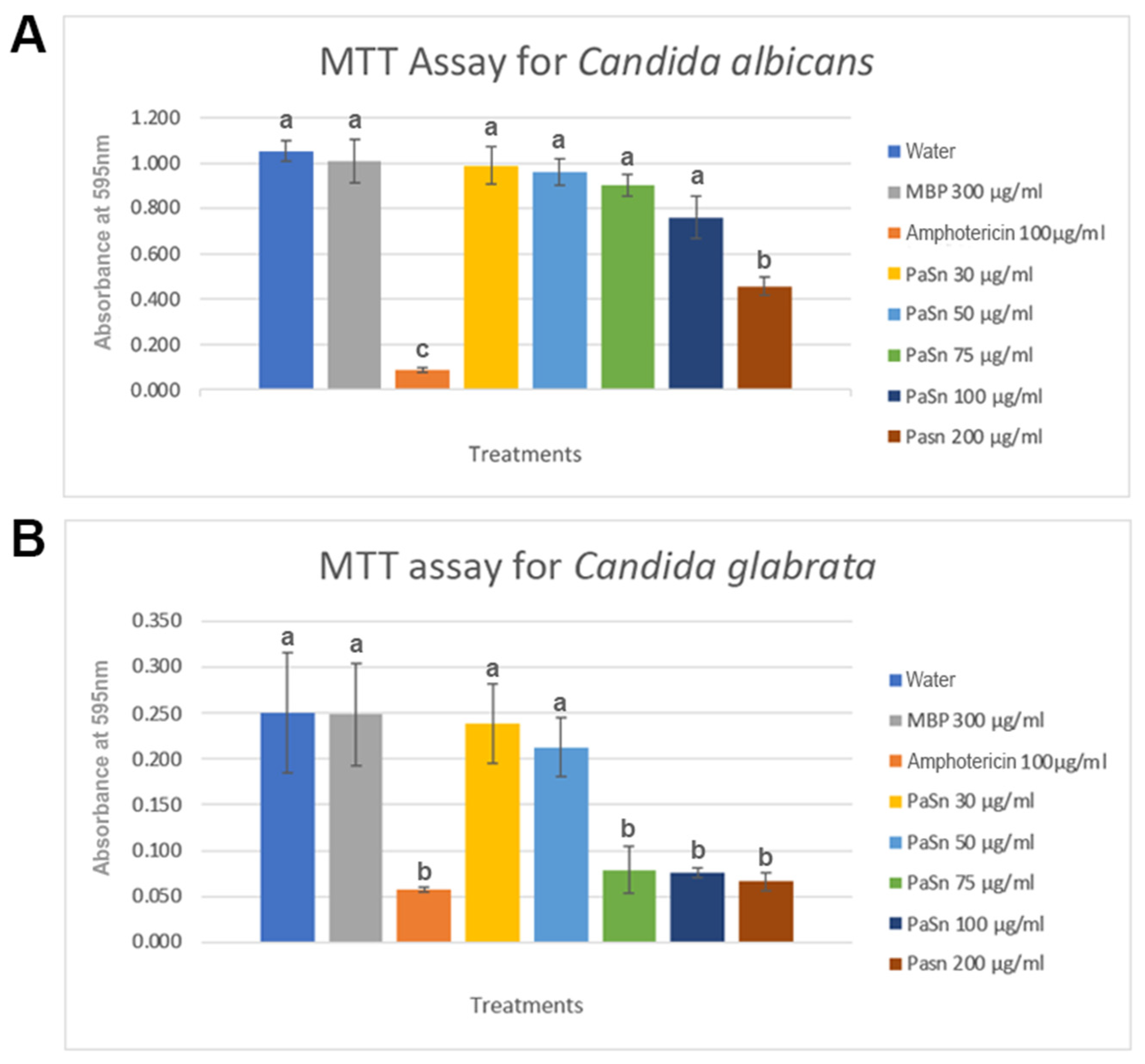
2.6. PaSn Activity against Human Pathogen Fungal
3. Discussion and Conclusions
4. Materials and Methods
4.1. Fungal Strains
4.2. Construction of Recombinant Plasmid, Expression, and Purification of Recombinant PaSn
4.3. Identification of Recombinant Avocado PaSn by Mass Spectrometry
4.4. In vitro Antifungal Activity against Phytopathogens and Membrane Permeabilization
4.5. Fungal Spore Germination Inhibition Assay
4.6. Human Pathogen Fungal Growth Inhibition Assay by MTT
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dabas, D.; Shegog, R.M.; Ziegler, G.R.; Lambert, J.D. Avocado (Persea americana) seed as a source of bioactive phytochemicals. Curr. Pharm. Des. 2013, 19, 6133–6140. [Google Scholar] [CrossRef] [PubMed]
- Cid-Pérez, T.S.; Hernández-Carranza, P.; Ochoa-Velasco, C.E.; Ruiz-López, I.I.; Nevárez-Moorillón, G.V.; Ávila-Sosa, R. Avocado seeds (Persea americana cv. Criollo sp.): Lipophilic compounds profile and biological activities. Saudi J. Biol. Sci. 2021, 28, 3384–3390. [Google Scholar] [CrossRef]
- Bahru, T.B.; Tadele, Z.H.; Ajebe, E.G. A review on avocado seed: Functionality, composition, antioxidant and antimicrobial properties. Chem. Sci. Int. J. 2019, 27, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Leite, J.J.G.; Brito, É.H.S.; Cordeiro, R.A.; Brilhante, R.S.N.; Sidrim, J.J.C.; Bertini, L.M.; Morais, S.M.d.; Rocha, M.F.G. Chemical composition, toxicity and larvicidal and antifungal activities of Persea americana (avocado) seed extracts. Rev. Soc. Bras. Med. Trop. 2009, 42, 110–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segura, A.; Moreno, M.; Madueno, F.; Molina, A.; García-Olmedo, F. Snakin-1, a peptide from potato that is active against plant pathogens. Mol. Plant Microbe Interact. 1999, 12, 16–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nahirñak, V.; Almasia, N.I.; Hopp, H.E.; Vazquez-Rovere, C. Snakin/GASA proteins: Involvement in hormone crosstalk and redox homeostasis. Plant Signal. Behav. 2012, 7, 1004–1008. [Google Scholar] [CrossRef] [Green Version]
- Guzmán-Rodríguez, J.; Ibarra-Laclette, E.; Suárez-Rodríguez, L.M.; Herrera-Estrella, L.; Ochoa-Zarzosa, A.; Rodríguez-Zapata, L.C.; Salgado-Garciglia, R.; Jimenez-Moraila, B.; López-Meza, J.E.; López-Gómez, R. Analysis of expressed sequence tags (ESTs) of avocado seed (Persea americana var. drymifolia) reveals abundant expression of the antimicrobial peptide snakin gene. Plant Physiol. Biochem. 2013, 70, 318–324. [Google Scholar] [CrossRef]
- Suárez-Rodríguez, L.M.; Sánchez-Albarrán, F.; León-Corona, H.; López-Gómez, R. Transcriptome (ESTs) of Avocado “Native” Mexicano Early Seed Development Shows Abundance of Regulatory, Antioxidant and Defense Genes; Jimenez-Lopez, J.C., Ed.; Advances in Seed Biology; INTECH: Rijeka, Croatia, 2017; pp. 45–63. ISBN 978-953-51-3621-7. [Google Scholar] [CrossRef] [Green Version]
- Ochoa-Zarzosa, A.; Báez-Magaña, M.; Guzmán-Rodríguez, J.; Flores-Alvarez, L.J.; Lara-Márquez, M.; Zavala-Guerrero, B.; Salgado-Garciglia, R.; López-Gómez, R.; López-Meza, J.E. Bioactive molecules from native mexican avocado fruit (Persea americana var. drymifolia): A Review. Plant Foods Hum. Nutr. 2021, 76, 133–142. [Google Scholar] [CrossRef]
- Rodríguez-López, E.S.; González-Prieto, J.M.; Mayek-Pérez, N. La Infección de Colletotrichum gloeosporioides (Penz.) Penz. y Sacc. en Aguacatero (Persea americana Mill.): Aspectos Bioquímicos y Genéticos. Rev. Mex. Fitopatol. 2009, 27, 53–63, ISSN: 0185-3309. [Google Scholar]
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Routenbach, M.; Trozkie, A.M.; Vosloo, J.A. Antifungal peptides: To be or not to be membrane active. Biochimie 2016, 130, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3.B.1–A3.B.3. [Google Scholar] [CrossRef] [PubMed]
- Boonpa, K.; Tantong, S.; Weerawanich, K.; Panpetch, P.; Pringsulaka, O.; Yingchutrakul, Y.; Roytrakul, S.; Sirikantaramas, S. Heterologous expression and antimicrobial activity of OsGASR3 from rice (Oryza sativa L.). J. Plant Physiol. 2018, 224–225, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Kovalskaya, N.; Hammond, R.W. Expression and functional characterization of the plant antimicrobial snakin-1 and defensin recombinant proteins. Protein Expr. Purif. 2009, 63, 12–17. [Google Scholar] [CrossRef] [Green Version]
- Kuddus, M.R.; Rumi, F.; Tsutsumi, M.; Takahashi, R.; Yamano, M.; Kamiya, M.; Kikukawa, T.; Demura, M.; Aizawa, T. Expression, purification, and characterization of the recombinant cysteine-rich antimicrobial peptide snakin-1 in Pichia pastoris. Protein Expr. Purif. 2016, 122, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Herbel, V.; Schäfer, H.; Wink, M. Recombinant production of Snakin-2 (an antimicrobial peptide from tomato) in E. coli and analysis of its bioactivity. Molecules 2015, 20, 14889–14901. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Velivelli, S.L.S.; Shah, D.M. Antifungal potency and modes of action of a novel olive tree defensin against closely related ascomycete fungal pathogens. Mol. Plant Microbe Interact. 2019, 32, 1649–1664. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Han, M.; Cao, D.; Xu, M. Molecular and biological properties of snakins: The foremost cysteine-rich plant host defense peptides. J. Fungi 2020, 6, 220. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of micrograms quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Díaz-Murillo, V.; Medina-Estrada, I.; López-Meza, J.E.; Ochoa-Zarzosa, A. Defensin γ-thionin from Capsicum chinense has immunomodulatory effects on bovine mammary epithelial cells during Staphylococcus aureus internalization. Peptides 2016, 78, 109–118. [Google Scholar] [CrossRef]
- Shevchenko, A.; Wilm, M.; Vorm, O.; Mann, M. Mass spectrometric sequencing of proteins from silver-stained polyacrylamide gels. Anal. Chem. 1996, 68, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Ncube, N.S.; Afolayan, A.J.; Okoh, A.I. Assessment techniques of antimicrobial properties of natural compounds of plant origin: Current methods and future trends. Afr. J. Biotechnol. 2008, 7, 1797–1806. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Martínez, M.A.; Suárez-Rodríguez, L.M.; López-Meza, J.E.; Ochoa-Zarzosa, A.; Salgado-Garciglia, R.; Fernández-Pavia, S.P.; López-Gómez, R. Antifungal Activity of Avocado Seed Recombinant GASA/Snakin PaSn. Antibiotics 2022, 11, 1558. https://doi.org/10.3390/antibiotics11111558
Hernández-Martínez MA, Suárez-Rodríguez LM, López-Meza JE, Ochoa-Zarzosa A, Salgado-Garciglia R, Fernández-Pavia SP, López-Gómez R. Antifungal Activity of Avocado Seed Recombinant GASA/Snakin PaSn. Antibiotics. 2022; 11(11):1558. https://doi.org/10.3390/antibiotics11111558
Chicago/Turabian StyleHernández-Martínez, Marco Antonio, Luis María Suárez-Rodríguez, Joel Edmundo López-Meza, Alejandra Ochoa-Zarzosa, Rafael Salgado-Garciglia, Silvia Patricia Fernández-Pavia, and Rodolfo López-Gómez. 2022. "Antifungal Activity of Avocado Seed Recombinant GASA/Snakin PaSn" Antibiotics 11, no. 11: 1558. https://doi.org/10.3390/antibiotics11111558






