Systematic Surveillance and Meta-Analysis of Antimicrobial Resistance and Food Sources from China and the USA
Abstract
Highlights
- Systematic analyzation to assess the spread of AMR bacteria prevalence in retail food products and the subsequent exposure to antibiotic resistance.
- Out of 13,018 food samples, 5000 samples were contaminated.
- Meat shows high to medium potential of AMR exposure for Gram-positive and Gram-negative foodborne pathogens.
- Salmonella and Staphylococcus aureus were two predominant bacteria seen in China and the USA, respectively.
- Multidrug resistance was detected in most of the food samples from both countries.
- Food samples were more resistant to β-lactams and tetracyclines.
- Government bodies were formed to tackle AMR from food.
Abstract
1. Introduction
2. Materials and Methods
2.1. The Explication of the Field of Research
2.1.1. China
2.1.2. USA
2.2. Food Categories
2.3. Data Extraction
2.4. Search Strategy
2.5. Screening and Data Extraction Process
2.6. Data Analysis
3. Results
3.1. Descriptive Analysis of All Included Studies: General Findings
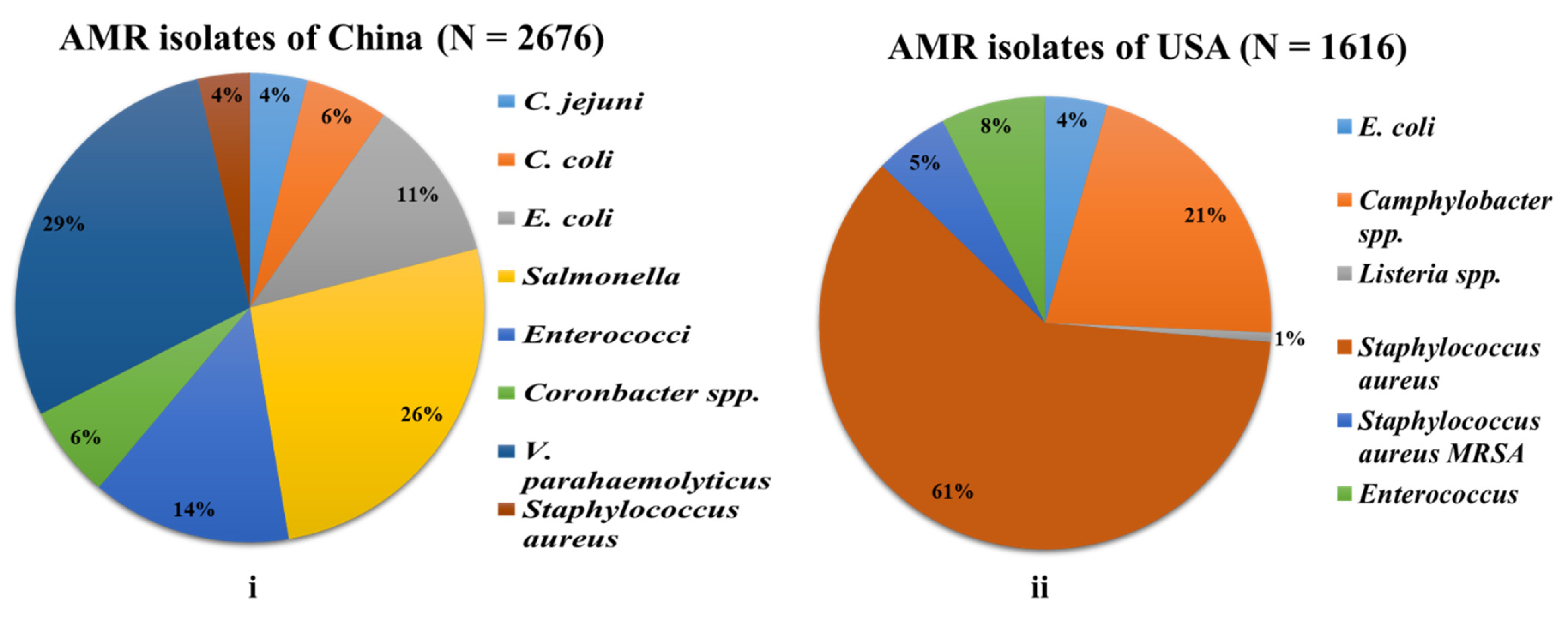
3.1.1. China
3.1.2. USA
3.2. Major Bacteria Groups and Their Relevant Food Product Categories with AMR
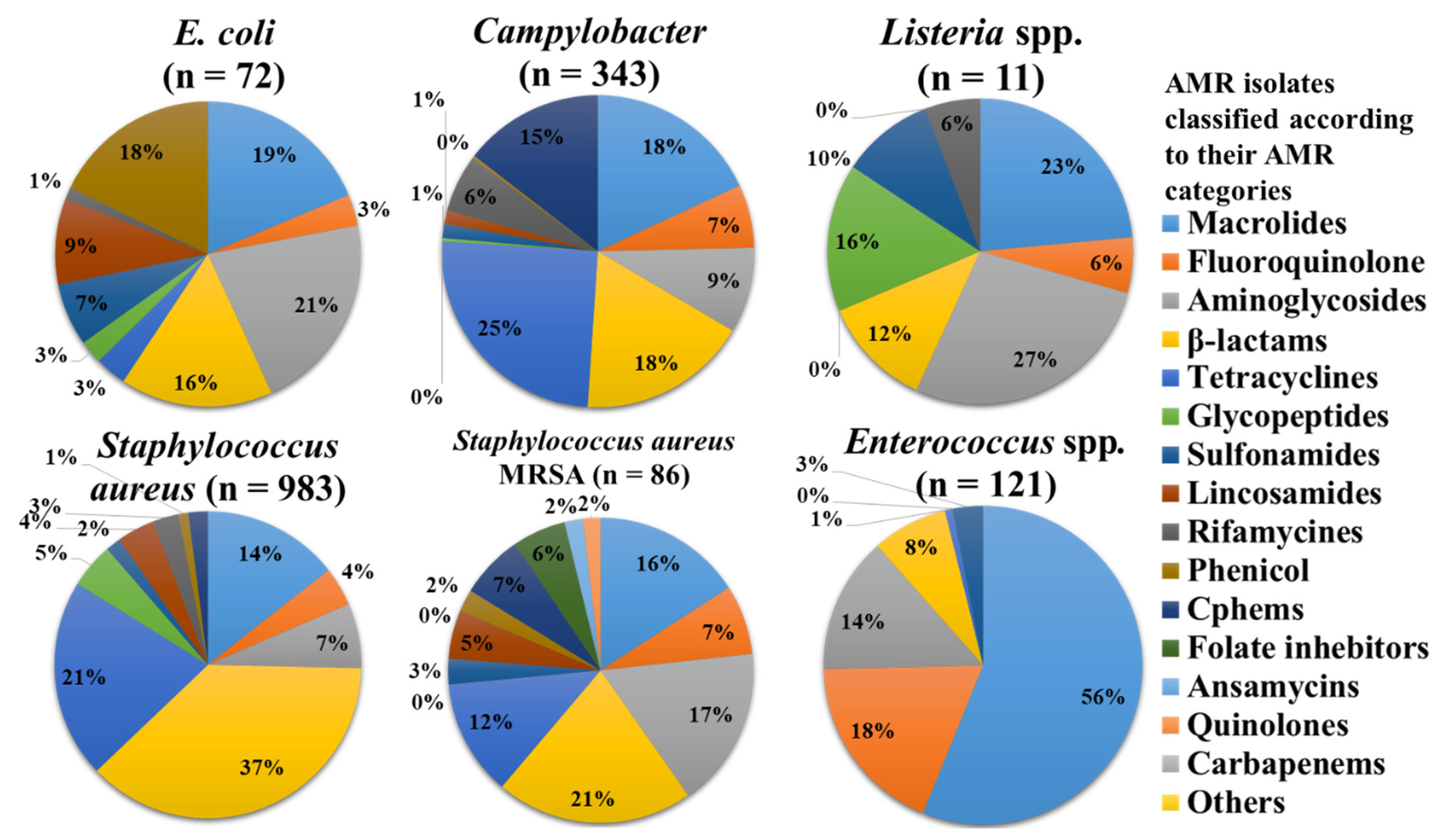
3.3. Gram-Positive Bacteria
3.3.1. Enterococcus
3.3.2. Staphylococcus
3.3.3. Listeria spp.
3.4. Gram-Negative Bacteria
3.4.1. Campylobacter
3.4.2. Escherichia coli
3.4.3. Salmonella
3.4.4. Cronobacter
3.4.5. Vibrio spp.
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef]
- Von Wintersdorff, C.J.H.; Penders, J.; Van Niekerk, J.M.; Mills, N.D.; Majumder, S.; Van Alphen, L.B.; Savelkoul, P.H.M.; Wolffs, P.F.G. Dissemination of antimicrobial resistance in microbial ecosystems through horizontal gene transfer. Front. Microbiol. 2016, 7, 173. [Google Scholar] [CrossRef]
- Varela, M.F.; Stephen, J.; Lekshmi, M.; Ojha, M.; Wenzel, N.; Sanford, L.M.; Hernandez, A.J.; Parvathi, A.; Kumar, S.H. Bacterial Resistance to Antimicrobial Agents. Antibiotics 2021, 10, 593. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Strategy for Containment of Antimicrobial Resistance; National Academies Press: Washington, DC, USA, 2001.
- EU Action on Antimicrobial Resistance. Available online: https://ec.europa.eu/health/antimicrobial-resistance/eu-action-antimicrobial-resistance_en (accessed on 25 May 2022).
- Brogan, D.M.; Mossialos, E. A critical analysis of the review on antimicrobial resistance report and the infectious disease financing facility. Glob. Health 2016, 12, 8. [Google Scholar] [CrossRef]
- Tackling Drug-Resistant Infections Globally: Final Report and Recommendations—The Review on Antimicrobial Resistance Chaired By Jim O’neill. 2016. Available online: https://wellcomecollection.org/works/thvwsuba (accessed on 25 May 2022).
- European Union. Prohibits the Use of Antibiotics as Growth Promoters. Available online: https://www.cabi.org/animalscience/news/15063 (accessed on 25 May 2022).
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, J.; Zheng, B.; Zhao, L.; Li, S.; Li, L. Changes in Chinese Policies to Promote the Rational Use of Antibiotics. PLoS Med. 2013, 10, e1001556. [Google Scholar] [CrossRef]
- Maron, D.F.; Smith, T.J.S.; Nachman, K.E. Restrictions on antimicrobial use in food animal production: An international regulatory and economic survey. Glob. Health 2013, 9, 48. [Google Scholar] [CrossRef]
- Bulletin of Ministry of Agriculture and Rural Affairs. Available online: http://www.moa.gov.cn/nybgb/2021/202112/ (accessed on 25 May 2022).
- The U.S. Government and Antimicrobial Resistance | Center for Strategic and International Studies. Available online: https://www.csis.org/analysis/us-government-and-antimicrobial-resistance (accessed on 25 May 2022).
- FARAD. Compounding Guide for the Food Animal Veterinarian 1 Compounding Guide for the Food Animal Veterinarian. Available online: http://www.farad.org/publications/miscellaneous/faradcompoundingguide.pdf (accessed on 26 May 2022).
- Zhang, L.; Fu, Y.; Xiong, Z.; Ma, Y.; Wei, Y.; Qu, X.; Zhang, H.; Zhang, J.; Liao, M. Highly prevalent multidrug-resistant Salmonella from chicken and pork meat at retail markets in Guangdong, China. Front. Microbiol. 2018, 9, 2104. [Google Scholar] [CrossRef]
- Li, Y.; Xie, T.; Pang, R.; Wu, Q.; Zhang, J.; Lei, T.; Xue, L.; Wu, H.; Wang, J.; Ding, Y.; et al. Food-Borne Vibrio parahaemolyticus in China: Prevalence, Antibiotic Susceptibility, and Genetic Characterization. Front. Microbiol. 2020, 11, 1670. [Google Scholar] [CrossRef]
- Li, W.; Li, H.; Zheng, S.; Wang, Z.; Sheng, H.; Shi, C.; Shi, X.; Niu, Q.; Yang, B. Prevalence, serotype, antibiotic susceptibility, and genotype of Salmonella in eggs from poultry farms and marketplaces in Yangling, Shaanxi province, China. Front. Microbiol. 2020, 11, 1482. [Google Scholar] [CrossRef]
- Ma, L.; Wang, Y.; Shen, J.; Zhang, Q.; Wu, C. Tracking Campylobacter contamination along a broiler chicken production chain from the farm level to retail in China. Int. J. Food Microbiol. 2014, 181, 77–84. [Google Scholar] [CrossRef]
- Xie, T.; Wu, Q.; Xu, X.; Zhang, J.; Guo, W. Prevalence and population analysis of Vibrio parahaemolyticus in aquatic products from South China markets. FEMS Microbiol. Lett. 2015, 362, fnv178. [Google Scholar] [CrossRef]
- Sun, C.; Wang, Y.; Ma, S.; Zhang, S.; Liu, D.; Wang, Y.; Wu, C. Surveillance of antimicrobial resistance in Escherichia coli and enterococci from food products at retail in Beijing, China. Food Control 2021, 119, 107483. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, M.; Luo, J.; Chen, J.; Wang, Q.; Lu, S.; Ji, H. Antimicrobial resistance of Escherichia coli isolated from retail foods in northern Xinjiang, China. Food Sci. Nutr. 2020, 8, 2035–2051. [Google Scholar] [CrossRef]
- Yang, X.; Wu, Q.; Huang, J.; Wu, S.; Zhang, J.; Chen, L.; Wei, X.; Ye, Y.; Li, Y.; Wang, J.; et al. Prevalence and characterization of Salmonella isolated from raw vegetables in China. Food Control 2020, 109, 106915. [Google Scholar] [CrossRef]
- Ling, N.; Li, C.; Zhang, J.; Wu, Q.; Zeng, H.; He, W.; Ye, Y.; Wang, J.; Ding, Y.; Chen, M.; et al. Prevalence and molecular and antimicrobial characteristics of Cronobacter spp. Isolated from raw vegetables in China. Front. Microbiol. 2018, 9, 1149. [Google Scholar] [CrossRef]
- Wang, W.; Lin, X.; Jiang, T.; Peng, Z.; Xu, J.; Yi, L.; Li, F.; Fanning, S.; Baloch, Z. Prevalence and characterization of Staphylococcus aureus cultured from raw milk taken from dairy cows with mastitis in Beijing, China. Front. Microbiol. 2018, 9, 1123. [Google Scholar] [CrossRef]
- Xie, T.; Wu, G.; He, X.; Lai, Z.; Zhang, H.; Zhao, J. Prevalence and genetic diversity of Vibrio parahaemolyticus strains from salmon in Chinese markets. FEMS Microbiol. Lett. 2019, 366, fnz103. [Google Scholar] [CrossRef]
- Xu, X.; Cheng, J.; Wu, Q.; Zhang, J.; Xie, T. Prevalence, characterization, and antibiotic susceptibility of Vibrio parahaemolyticus isolated from retail aquatic products in North China. BMC Microbiol. 2016, 16, 32. [Google Scholar] [CrossRef]
- Lei, T.; Jiang, F.; He, M.; Zhang, J.; Zeng, H.; Chen, M.; Pang, R.; Wu, S.; Wei, L.; Wang, J.; et al. Prevalence, virulence, antimicrobial resistance, and molecular characterization of fluoroquinolone resistance of Vibrio parahaemolyticus from different types of food samples in China. Int. J. Food Microbiol. 2020, 317, 108461. [Google Scholar] [CrossRef]
- Wang, W.; Chen, J.; Shao, X.; Huang, P.; Zha, J.; Ye, Y. Occurrence and antimicrobial resistance of Salmonella isolated from retail meats in Anhui, China. Food Sci. Nutr. 2021, 9, 4701–4710. [Google Scholar] [CrossRef]
- Hu, Y.; Li, F.; Zheng, Y.; Jiao, X.; Guo, L. Isolation, molecular characterization and antibiotic susceptibility pattern of Vibrio parahaemolyticus from aquatic products in the Southern Fujian Coast, China. J. Microbiol. Biotechnol. 2020, 30, 856–867. [Google Scholar] [CrossRef]
- Tate, H.; Li, C.; Nyirabahizi, E.; Tyson, G.H.; Zhao, S.; Rice-Trujillo, C.; Jones, S.B.; Ayers, S.; M’Ikanatha, N.M.; Hanna, S.; et al. A National Antimicrobial Resistance Monitoring System survey of antimicrobial-resistant foodborne bacteria isolated from retail veal in the United States. Acta Med. Port. 2021, 84, 1749–1759. [Google Scholar] [CrossRef]
- Noormohamed, A.; Fakhr, M.K. Incidence and antimicrobial resistance profiling of campylobacter in retail chicken livers and gizzards. Foodborne Pathog. Dis. 2012, 9, 617–624. [Google Scholar] [CrossRef]
- Buyukcangaz, E.; Velasco, V.; Sherwood, J.S.; Stepan, R.M.; Koslofsky, R.J.; Logue, C.M. Molecular typing of Staphylococcus aureus and Methicillin-resistant S. aureus (MRSA) isolated from animals and retail meat in North Dakota, United States. Foodborne Pathog. Dis. 2013, 10, 608–617. [Google Scholar] [CrossRef]
- Abdalrahman, L.S.; Fakhr, M.K. Incidence, antimicrobial susceptibility, and toxin genes possession screening of Staphylococcus aureus in retail chicken livers and gizzards. Foods 2015, 4, 115–129. [Google Scholar] [CrossRef]
- Jackson, C.R.; Davis, J.A.; Barrett, J.B. Prevalence and characterization of methicillin-resistant Staphylococcus aureus isolates from retail meat and humans in Georgia. J. Clin. Microbiol. 2013, 51, 1199–1207. [Google Scholar] [CrossRef]
- Abdalrahman, L.S.; Wells, H.; Fakhr, M.K. Staphylococcus aureus is more prevalent in retail beef livers than in pork and other beef cuts. Pathogens 2015, 4, 182–198. [Google Scholar] [CrossRef]
- Abdalrahman, L.S.; Stanley, A.; Wells, H.; Fakhr, M.K. Isolation, virulence, and antimicrobial resistance of methicillin-resistant Staphylococcus aureus (MRSA) and methicillin sensitive Staphylococcus aureus (MSSA) strains from Oklahoma retail poultry meats. Int. J. Environ. Res. Public Health 2015, 12, 6148–6161. [Google Scholar] [CrossRef]
- Kim, C.; Almuqati, R.; Fatani, A.; Rahemi, A.; Kaseloo, P.; Wynn, C.; Nartea, T.; Ndegwa, E.; Rutto, L. Prevalence and antimicrobial resistance of foodborne pathogens in select fresh produce procured from farmers’ markets in Central Virginia. J. Food Saf. 2021, 41, e12895. [Google Scholar] [CrossRef]
- Thapaliya, D.; Forshey, B.M.; Kadariya, J.; Quick, M.K.; Farina, S.; O’ Brien, A.; Nair, R.; Nworie, A.; Hanson, B.; Kates, A.; et al. Prevalence and molecular characterization of Staphylococcus aureus in commercially available meat over a one-year period in Iowa, USA. Food Microbiol. 2017, 65, 122–129. [Google Scholar] [CrossRef]
- Safarpoor Dehkordi, F.; Gandomi, H.; Basti, A.A.; Misaghi, A.; Rahimi, E. Phenotypic and genotypic characterization of antibiotic resistance of methicillin-resistant Staphylococcus aureus isolated from hospital food. Antimicrob. Resist. Infect. Control 2017, 6, 104. [Google Scholar] [CrossRef]
- Del Collo, L.P.; Karns, J.S.; Biswas, D.; Lombard, J.E.; Haley, B.J.; Kristensen, R.C.; Kopral, C.A.; Fossler, C.P.; Van Kessel, J.A.S. Prevalence, antimicrobial resistance, and molecular characterization of Campylobacter spp. in bulk tank milk and milk filters from US dairies. J. Dairy Sci. 2017, 100, 3470–3479. [Google Scholar] [CrossRef]
- WHO—World Health Organization. Ranking of medically important antimicrobials for risk management of antimicrobial resistance due to non-human use. In Critically Important Antimicrobials for Human Medicine, 6th ed.; World Health Organization: Geneva, Switzerland, 2018; ISBN 978-92-4-151552-8. Available online: https://Apps.Who.Int/Iris/Bitstream/Handle/10665/312266/9789241515528-Eng.Pdf?Ua=1 (accessed on 27 May 2022).
- Transatlantic Taskforce on Antimicrobial Resistance (TATFAR) Report on Recommendation 18. 2016. Available online: https://www.cdc.gov/drugresistance/pdf/tatfar-report---recommendation-18.pdf (accessed on 27 May 2022).
- Centre for Genomic Pathogen Surveillance. A Systematic Review to Assess the Significance of the Food Chain in the Context of Antimicrobial Resistance (AMR) with Particular Reference to Pork and Poultry Meat, Dairy Products, Seafood and Fresh Produce on Retail Sale in the UK; Royal Veterinary College: London, UK, 2020; p. 5. Available online: https://www.food.gov.uk/sites/default/files/media/document/amr-systematic-review-final-report-2016_0.pdf (accessed on 27 May 2022).
- Evans, E.W.; Redmond, E.C. Domestic Kitchen Microbiological Contamination and Self-Reported Food Hygiene Practices of Older Adult Consumers. J. Food Prot. 2019, 82, 1326–1335. [Google Scholar] [CrossRef]
- Borrusso, P.A.; Quinlan, J.J. Prevalence of Pathogens and Indicator Organisms in Home Kitchens and Correlation with Unsafe Food Handling Practices and Conditions. J. Food Prot. 2017, 80, 590–597. [Google Scholar] [CrossRef]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne Illness Acquired in the United States—Major Pathogens. Emerg. Infect. Dis. 2011, 17, 7. [Google Scholar] [CrossRef]
- Wang, W.; Baloch, Z.; Jiang, T.; Zhang, C.; Peng, Z.; Li, F.; Fanning, S.; Ma, A.; Xu, J. Enterotoxigenicity and antimicrobial resistance of Staphylococcus aureus isolated from retail food in China. Front. Microbiol. 2017, 8, 2256. [Google Scholar] [CrossRef]
- Salmonella Homepage | CDC. Available online: https://www.cdc.gov/salmonella/index.html (accessed on 27 May 2022).
- Xiang, Y.; Li, F.; Dong, N.; Tian, S.; Zhang, H.; Du, X.; Zhou, X.; Xu, X.; Yang, H.; Xie, J.; et al. Investigation of a Salmonellosis Outbreak Caused by Multidrug Resistant Salmonella typhimurium in China. Front. Microbiol. 2020, 11, 801. [Google Scholar] [CrossRef]
- Microbiological Guidelines for Food. Available online: https://www.cfs.gov.hk/english/food_leg/files/food_leg_Microbiological_Guidelines_for_Food_e.pdf (accessed on 27 May 2022).


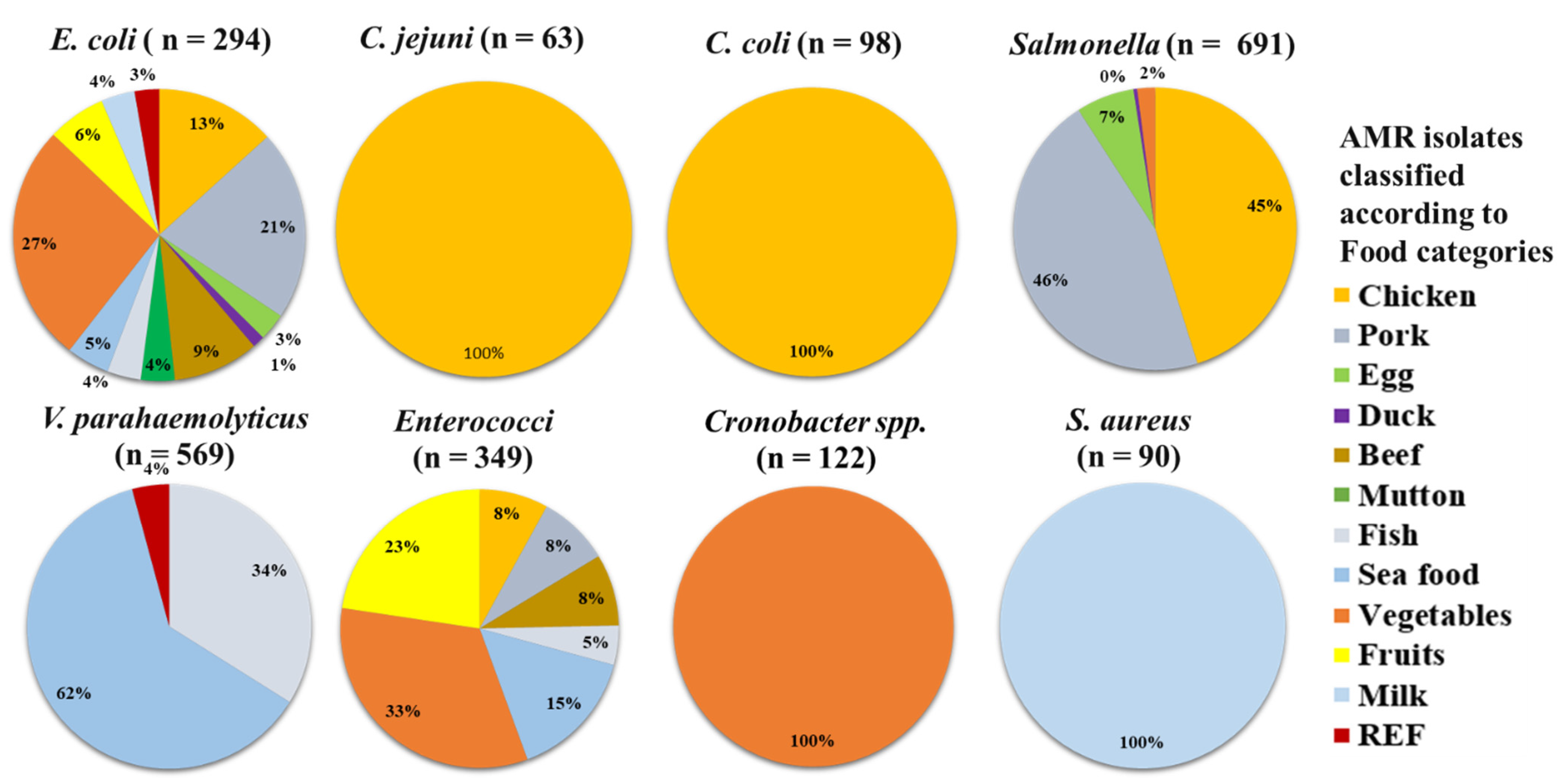


| Microbes | Chicken n = 1006 | Pork n = 558 | Egg n = 847 | Duck n = 41 | Beef n = 67 | Mutton n = 19 | Fish n = 1108 | Sea Food n = 1254 | Vegetables n = 1094 | Fruits n = 132 | Milk n = 216 | REF n = 622 | Total n = 6965 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n and (%) | n and (%) | n and (%) | n and (%) | n and (%) | n and (%) | n and (%) | n and (%) | n and (%) | n and (%) | n and (%) | n and (%) | n and (%) | |
| E. coli | 39 (3.9) | 62 (11.1) | 9 (1.1) | 4 (9.8) | 28 (41.8) | 11 (57.9) | 11 (1.0) | 14 (1.1) | 78 (7.1) | 19 (14.4) | 11 (5.1) | 8 (1.3) | 294 (4.2) |
| C. jejuni | 63 (6.3) | - | - | - | - | - | - | - | - | - | - | - | 63 (0.9) |
| C. coli | 98 (9.7) | - | - | - | - | - | - | - | - | - | - | - | 98 (1.4) |
| Salmonella | 312 (31) | 316 (56.6) | 46 (5.4) | 3 (7.3) | - | - | - | - | 14 (1.3) | - | - | - | 691 (9.9) |
| V. parahaemolyticus | - | - | - | - | - | - | 193 (17.4) | 352 (28.1) | - | - | - | 24 (3.9) | 569 (8.2) |
| Enterococci | 28 (2.8) | 29 (5.2) | - | - | 29 (43.3) | - | 16 (1.4) | 53 (4.2) | 115 (10.5) | 79 (59.8) | - | - | 349 (5.0) |
| Cronobacter spp. | - | - | - | - | - | - | - | - | 122 (11.2) | - | - | - | 122 (1.8) |
| S. aureus | - | - | - | - | - | - | - | - | - | - | 90 (41.7) | - | 90 (1.3) |
| Total infected | 540 (53.7) | 407 (72.9) | 55 (6.5) | 7 (17.1) | 57 (85.1) | 11 (57.9) | 220 (19.9) | 419 (33.4) | 329 (30.1) | 98 (74.2) | 101 (46.8) | 32 (5.1) | 2276 (32.7) |
| Microbes | Chicken n = 1564 | Beef n= 1253 | Pork n = 1461 | Meat n = 396 | Turkey n = 299 | Fish n = 55 | Milk n = 465 | Vegetables n = 194 | Others n = 366 | Total n = 6053 |
|---|---|---|---|---|---|---|---|---|---|---|
| n and (%) | n and (%) | n and (%) | n and (%) | n and (%) | n and (%) | n and (%) | n and (%) | n and (%) | n and (%) | |
| E. coli | - | - | - | 49 (12.4) | - | - | - | 23 (11.9) | - | 72 (1.2) |
| Salmonella | - | - | - | 2 (0.5) | - | - | - | - | - | 2 (0.0) |
| Campylobacter spp. | - | - | - | - | - | - | 143 (30.8) | 12 (6.2) | - | 155 (2.6) |
| C. jejuni | 69 (4.4) | - | - | - | - | - | - | - | - | 69 (1.1) |
| C. coli | 66 (4.2) | - | - | - | - | - | - | - | - | 66 (1.1) |
| Listeria spp. | - | - | - | - | - | - | - | 11 (5.7) | - | 11 (0.2) |
| L. monocytogenes | - | - | - | - | - | - | - | 3 (1.5) | - | 3 (0.0) |
| Staphylococcus aureus | 442 (28.3) | 1030 (82.2) | 510 (34.9) | 10 (2.5) | 86 (28.8) | 2 (3.6) | - | 4 (2.1) | 27 (7.4) | 2111 (34.9) |
| Staphylococcus aureus MRSA | 16 (1.0) | 6 (0.5) | 21 (1.4) | 6 (1.5) | 42 (14.0) | - | - | 4 (2.1) | 19 (5.2) | 114 (1.9) |
| Enterococcus | - | - | - | 121 (30.5) | - | - | - | - | - | 121 (2.0) |
| Total infected | 593 (37.9) | 1036 (82.7) | 531 (36.3) | 188 (47.5) | 128 (42.8) | 2 (3.6) | 143 (30.8) | 57 (29.4) | 46 (12.6) | 2724 (45.0) |
| S. No. | Country | Organizations | Role |
|---|---|---|---|
| 1. | China | Bureau of Animal and Plant Health Inspection and Quarantine (BAPHIQ) https://www.baphiq.gov.tw/ (accessed on 24 April 2022) | Global Action Plan on Antimicrobial Resistance, and the OIE Strategy on Antimicrobial Resistance and the Prudent Use of Antimicrobials. |
| 2. | National Action Plan (NAP) http://www.gov.cn/xinwen/2016-08/25/content_5102348.htm (accessed on 24 April 2022) | Regulate antimicrobial agents and antimicrobial resistance control. | |
| 3. | China Antimicrobial Resistance Surveillance System (CARSS) http://www.carss.cn/ (accessed on 24 April 2022) | AMR surveillance. | |
| 4. | China Antimicrobial Surveillance Network (CHINET) https://www.chinets.com/ (accessed on 24 April 2022) | Help clinicians to better understand the current status and trends of AMR and to correct inappropriate antibiotic prescribing. | |
| 5. | USA | National Antimicrobial Resistance Monitoring System (NARMS) https://www.cdc.gov/narms/index.html (accessed on 24 April 2022) | Track changes in the antimicrobial susceptibility of enteric (intestinal) bacteria found in ill people. |
| 6. | Centers for Disease Control and Prevention https://www.cdc.gov/ (accessed on 24 April 2022) | Carry out scientific research on new and ongoing pathogen threats. | |
| 7. | Food and Drug Administration (FDA) https://www.fda.gov/ (accessed on 24 April 2022) | Protecting public health by assuring that foods are wholesome, sanitary, and properly labeled. | |
| 8. | US Department of Agriculture (USDA) https://www.usda.gov/ (accessed on 24 April 2022) | Safeguard food, agriculture, natural resources, rural development, nutrition, and related issues based on public policy. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Himanshu; R. Prudencio, C.; da Costa, A.C.; Leal, E.; Chang, C.-M.; Pandey, R.P. Systematic Surveillance and Meta-Analysis of Antimicrobial Resistance and Food Sources from China and the USA. Antibiotics 2022, 11, 1471. https://doi.org/10.3390/antibiotics11111471
Himanshu, R. Prudencio C, da Costa AC, Leal E, Chang C-M, Pandey RP. Systematic Surveillance and Meta-Analysis of Antimicrobial Resistance and Food Sources from China and the USA. Antibiotics. 2022; 11(11):1471. https://doi.org/10.3390/antibiotics11111471
Chicago/Turabian StyleHimanshu, Carlos R. Prudencio, Antonio Charlys da Costa, Elcio Leal, Chung-Ming Chang, and Ramendra Pati Pandey. 2022. "Systematic Surveillance and Meta-Analysis of Antimicrobial Resistance and Food Sources from China and the USA" Antibiotics 11, no. 11: 1471. https://doi.org/10.3390/antibiotics11111471
APA StyleHimanshu, R. Prudencio, C., da Costa, A. C., Leal, E., Chang, C.-M., & Pandey, R. P. (2022). Systematic Surveillance and Meta-Analysis of Antimicrobial Resistance and Food Sources from China and the USA. Antibiotics, 11(11), 1471. https://doi.org/10.3390/antibiotics11111471









