Antibiotic Resistance to Mycobacterium tuberculosis and Potential Use of Natural and Biological Products as Alternative Anti-Mycobacterial Agents
Abstract
1. Introduction
2. Genetic Factors That Predispose to TB Risk
3. The Impact of Mtb on the Immune System
4. The Gut–Lung Axis
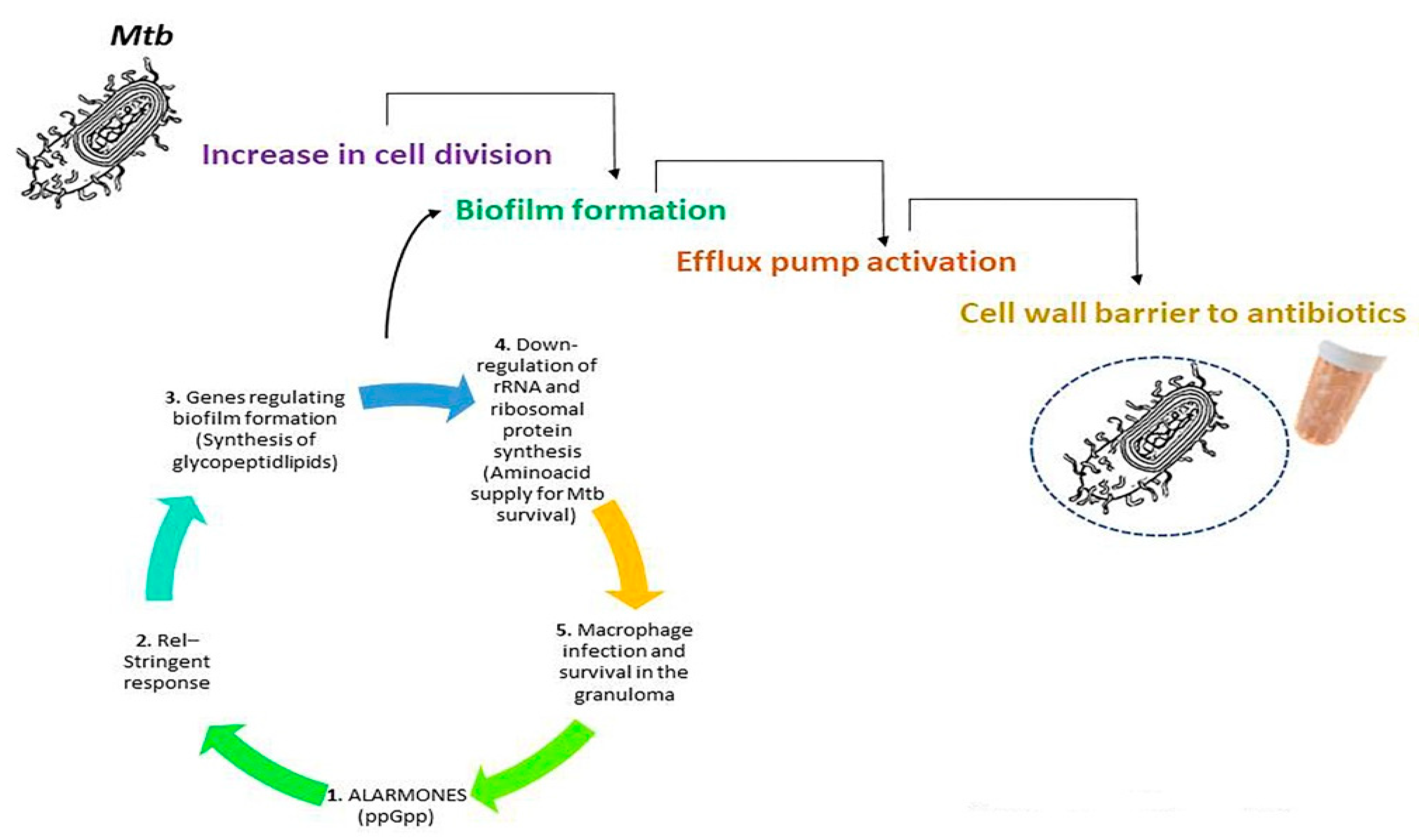
5. Mtb-Mediated Resistance against Antibiotics
6. Novel Treatment to Overcome Antibiotic Resistance against Mtb
6.1. Probiotics
6.2. Polyphenols
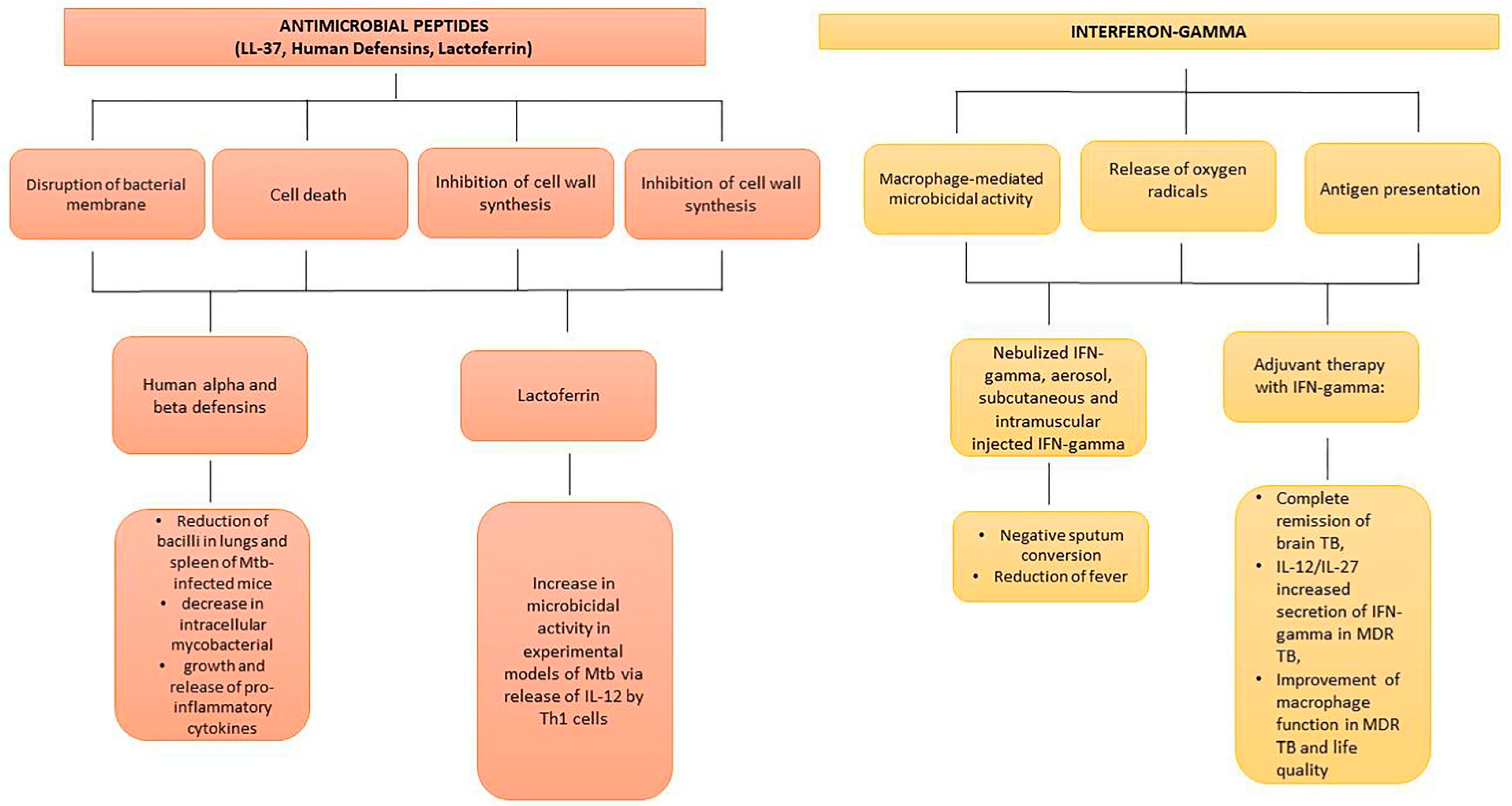
6.3. Antimicrobial Peptides
6.4. Interferon-Gamma and Mesenchymal Stem Cells
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Furin, J.; Cox, H.; Pai, M. Tuberculosis. Lancet 2019, 393, 1642–1656. [Google Scholar] [CrossRef]
- World Health Organization. Global Tuberculosis Report; World Health Organization: Geneva, Switzerland, 2020; ISBN 978-92-4-001313-1. [Google Scholar]
- Bruchfeld, J.; Correia-Neves, M.; Källenius, G. Tuberculosis and HIV Coinfection. Cold Spring Harb. Perspect. Med. 2015, 5, a017871. [Google Scholar] [CrossRef] [PubMed]
- Onyebujoh, P.; Zumla, A.; Ribeiro, I.; Rustomjee, R.; Mwaba, P.; Gomes, M.; Grange, J.M. Treatment of Tuberculosis: Present Status and Future Prospects. Bull. World Health Organ. 2005, 83, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Harichander, S.; Wiafe, E.; Mensah, K.B.; Bangalee, V.; Oosthuizen, F. The Incidence of TB and MDR-TB in Pediatrics and Therapeutic Options: A Systematic Review. Syst. Rev. 2022, 11, 157. [Google Scholar] [CrossRef]
- Vilchèze, C. Mycobacterial Cell Wall: A Source of Successful Targets for Old and New Drugs. Appl. Sci. 2020, 10, 2278. [Google Scholar] [CrossRef]
- Wehrli, W. Rifampin: Mechanisms of Action and Resistance. Clin. Infect. Dis. 1983, 5, S407–S411. [Google Scholar] [CrossRef]
- Zhang, Y. Mode of Action of Pyrazinamide: Disruption of Mycobacterium tuberculosis Membrane Transport and Energetics by Pyrazinoic Acid. J. Antimicrob. Chemother. 2003, 52, 790–795. [Google Scholar] [CrossRef]
- Sosnik, A.; Carcaboso, Á.M.; Glisoni, R.J.; Moretton, M.A.; Chiappetta, D.A. New Old Challenges in Tuberculosis: Potentially Effective Nanotechnologies in Drug Delivery. Adv. Drug Deliv. Rev. 2010, 62, 547–559. [Google Scholar] [CrossRef]
- Gupta, K.R.; Arora, G.; Mattoo, A.; Sajid, A. Stringent Response in Mycobacteria: From Biology to Therapeutic Potential. Pathogens 2021, 10, 1417. [Google Scholar] [CrossRef]
- Srivatsan, A.; Wang, J.D. Control of Bacterial Transcription, Translation and Replication by (p)PpGpp. Curr. Opin. Microbiol. 2008, 11, 100–105. [Google Scholar] [CrossRef]
- Mestre, A.A.; Zhou, P.; Chi, J.-T. Metazoan Stringent-like Response Mediated by MESH1 Phenotypic Conservation via Distinct Mechanisms. Comput. Struct. Biotechnol. J. 2022, 20, 2680–2684. [Google Scholar] [CrossRef] [PubMed]
- Traxler, M.F.; Summers, S.M.; Nguyen, H.-T.; Zacharia, V.M.; Hightower, G.A.; Smith, J.T.; Conway, T. The Global, PpGpp-Mediated Stringent Response to Amino Acid Starvation in Escherichia Coli. Mol. Microbiol. 2008, 68, 1128–1148. [Google Scholar] [CrossRef] [PubMed]
- Eymann, C.; Homuth, G.; Scharf, C.; Hecker, M. Bacillus Subtilis Functional Genomics: Global Characterization of the Stringent Response by Proteome and Transcriptome Analysis. J. Bacteriol. 2002, 184, 2500–2520. [Google Scholar] [CrossRef] [PubMed]
- Travis, B.A.; Schumacher, M.A. Diverse Molecular Mechanisms of Transcription Regulation by the Bacterial Alarmone PpGpp. Mol. Microbiol. 2022, 117, 252–260. [Google Scholar] [CrossRef]
- Buriánková, K.; Doucet-Populaire, F.; Dorson, O.; Gondran, A.; Ghnassia, J.-C.; Weiser, J.; Pernodet, J.-L. Molecular Basis of Intrinsic Macrolide Resistance in the Mycobacterium tuberculosis Complex. Antimicrob. Agents Chemother. 2004, 48, 143–150. [Google Scholar] [CrossRef]
- te Brake, L.H.M.; de Knegt, G.J.; de Steenwinkel, J.E.; van Dam, T.J.P.; Burger, D.M.; Russel, F.G.M.; van Crevel, R.; Koenderink, J.B.; Aarnoutse, R.E. The Role of Efflux Pumps in Tuberculosis Treatment and Their Promise as a Target in Drug Development: Unraveling the Black Box. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 271–291. [Google Scholar] [CrossRef]
- Fux, C.A.; Costerton, J.W.; Stewart, P.S.; Stoodley, P. Survival Strategies of Infectious Biofilms. Trends Microbiol. 2005, 13, 34–40. [Google Scholar] [CrossRef]
- Pai, M.; Behr, M.A.; Dowdy, D.; Dheda, K.; Divangahi, M.; Boehme, C.C.; Ginsberg, A.; Swaminathan, S.; Spigelman, M.; Getahun, H.; et al. Tuberculosis. Nat. Rev. Dis. Prim. 2016, 2, 16076. [Google Scholar] [CrossRef]
- Russell, D.G. Mycobacterium tuberculosis and the Intimate Discourse of a Chronic Infection: Mycobacterium’s Subversion of Host Function. Immunol. Rev. 2011, 240, 252–268. [Google Scholar] [CrossRef]
- Silva Miranda, M.; Breiman, A.; Allain, S.; Deknuydt, F.; Altare, F. The Tuberculous Granuloma: An Unsuccessful Host Defence Mechanism Providing a Safety Shelter for the Bacteria? Clin. Dev. Immunol. 2012, 2012, 1–14. [Google Scholar] [CrossRef]
- Bermudez, L.E.; Young, L.S. Tumor Necrosis Factor, Alone or in Combination with IL-2, but Not IFN-Gamma, Is Associated with Macrophage Killing of Mycobacterium Avium Complex. J. Immunol. 1988, 140, 3006–3013. [Google Scholar] [PubMed]
- Lutzky, V.P.; Ratnatunga, C.N.; Smith, D.J.; Kupz, A.; Doolan, D.L.; Reid, D.W.; Thomson, R.M.; Bell, S.C.; Miles, J.J. Anomalies in T Cell Function Are Associated with Individuals at Risk of Mycobacterium Abscessus Complex Infection. Front. Immunol. 2018, 9, 1319. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.S.; Kim, E.J.; Lee, S.-H.; Suh, G.Y.; Chung, M.P.; Kim, H.; Kwon, O.J.; Koh, W.-J. Decreased Cytokine Production in Patients with Nontuberculous Mycobacterial Lung Disease. Lung 2007, 185, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Charitos, I.A.; Bottalico, L. A successful history: Probiotics and their potential as antimicrobials. Expert Rev. Anti-Infect. Ther. 2019, 17, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Gavrilova, N.N.; Ratnikova, I.A.; Sadanov, A.K.; Bayakisheva, K.; Tourlibaeva, Z.J.; Belikova, O.A. Application of probiotics in complex treatment of tuberculosis. Int. J. Eng. Res. Appl. 2014, 4, 13–18. [Google Scholar]
- Magrone, T.; Magrone, M.; Russo, M.A.; Jirillo, E. Recent Advances on the Anti-Inflammatory and Antioxidant Properties of Red Grape Polyphenols: In Vitro and In Vivo Studies. Antioxidants 2019, 9, 35. [Google Scholar] [CrossRef]
- Watson, R.R. Polyphenols: Prevention and Treatment of Human Disease, 2nd ed.; Elsevier: San Diego, CA, USA, 2018. [Google Scholar]
- Magrone, T.; Russo, M.A.; Jirillo, E. Antimicrobial Peptides in Human Disease: Therapeutic Approaches. Second of Two Parts. Curr. Pharm. Des. 2018, 24, 1148–1156. [Google Scholar] [CrossRef]
- Haney, E.F.; Straus, S.K.; Hancock, R.E.W. Reassessing the Host Defense Peptide Landscape. Front. Chem. 2019, 7, 43. [Google Scholar] [CrossRef]
- Magrone, T.; Jirillo, E. Immunity to Tuberculosis and Novel Therapeutic Strategies. MAGRONE, Thea; JIRILLO, Emilio. Immunity to tuberculosis and novel therapeutic strategies. Clin. Immunol. Endocr. Metab. Drugs 2014, 1, 46–60. [Google Scholar] [CrossRef]
- Thye, T.; Vannberg, F.O.; Wong, S.H.; Owusu-Dabo, E.; Osei, I.; Gyapong, J.; Sirugo, G.; Sisay-Joof, F.; Enimil, A.; Chinbuah, M.A.; et al. Genome-wide association analyses identifies a susceptibility locus for tuberculosis on chromosome 18q11.2. Nat. Genet. 2010, 42, 739–741. [Google Scholar] [CrossRef]
- Zheng, R.; Li, Z.; He, F.; Liu, H.; Chen, J.; Chen, J.; Xie, X.; Zhou, J.; Chen, H.; Wu, X.; et al. Genome-Wide Association Study Identifies Two Risk Loci for Tuberculosis in Han Chinese. Nat. Commun. 2018, 9, 4072. [Google Scholar] [CrossRef] [PubMed]
- Thye, T.; Owusu-Dabo, E.; Vannberg, F.O.; van Crevel, R.; Curtis, J.; Sahiratmadja, E.; Balabanova, Y.; Ehmen, C.; Muntau, B.; Ruge, G.; et al. Common Variants at 11p13 Are Associated with Susceptibility to Tuberculosis. Nat. Genet. 2012, 44, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Chimusa, E.R.; Zaitlen, N.; Daya, M.; Möller, M.; van Helden, P.D.; Mulder, N.J.; Price, A.L.; Hoal, E.G. Genome-Wide Association Study of Ancestry-Specific TB Risk in the South African Coloured Population. Hum. Mol. Genet. 2014, 23, 796–809. [Google Scholar] [CrossRef] [PubMed]
- Quistrebert, J.; Orlova, M.; Kerner, G.; Ton, L.T.; Luong, N.T.; Danh, N.T.; Vincent, Q.B.; Jabot-Hanin, F.; Seeleuthner, Y.; Bustamante, J.; et al. Genome-Wide Association Study of Resistance to Mycobacterium tuberculosis Infection Identifies a Locus at 10q26.2 in Three Distinct Populations. PLoS Genet. 2021, 17, e1009392. [Google Scholar] [CrossRef]
- Sveinbjornsson, G.; Gudbjartsson, D.F.; Halldorsson, B.V.; Kristinsson, K.G.; Gottfredsson, M.; Barrett, J.C.; Gudmundsson, L.J.; Blondal, K.; Gylfason, A.; Gudjonsson, S.A.; et al. HLA Class II Sequence Variants Influence Tuberculosis Risk in Populations of European Ancestry. Nat. Genet. 2016, 48, 318–322. [Google Scholar] [CrossRef]
- Huang, L.; Holtzinger, A.; Jagan, I.; BeGora, M.; Lohse, I.; Ngai, N.; Nostro, C.; Wang, R.; Muthuswamy, L.B.; Crawford, H.C.; et al. Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids. Nat. Med. 2015, 21, 1364–1371. [Google Scholar] [CrossRef]
- Rosain, J.; Kong, X.-F.; Martinez-Barricarte, R.; Oleaga-Quintas, C.; Ramirez-Alejo, N.; Markle, J.; Okada, S.; Boisson-Dupuis, S.; Casanova, J.-L.; Bustamante, J. Mendelian Susceptibility to Mycobacterial Disease: 2014-2018 Update. Immunol. Cell Biol. 2019, 97, 360–367. [Google Scholar] [CrossRef]
- Alahari, A.; Trivelli, X.; Guérardel, Y.; Dover, L.G.; Besra, G.S.; Sacchettini, J.C.; Reynolds, R.C.; Coxon, G.D.; Kremer, L. Thiacetazone, an Antitubercular Drug That Inhibits Cyclopropanation of Cell Wall Mycolic Acids in Mycobacteria. PLoS ONE 2007, 2, e1343. [Google Scholar] [CrossRef]
- Mi, J.; Gong, W.; Wu, X. Advances in Key Drug Target Identification and New Drug Development for Tuberculosis. BioMed Res. Int. 2022, 2022, 5099312. [Google Scholar] [CrossRef]
- Roy, S.; Schmeier, S.; Kaczkowski, B.; Arner, E.; Alam, T.; Ozturk, M.; Tamgue, O.; Parihar, S.P.; Kawaji, H.; Itoh, M.; et al. Transcriptional landscape of Mycobacterium tuberculosis infection in macrophages. Sci. Rep. 2018, 8, 6758. [Google Scholar] [CrossRef]
- Wei, M.; Wang, L.; Wu, T.; Xi, J.; Han, Y.; Yang, X.; Zhang, D.; Fang, Q.; Tang, B. NLRP3 Activation Was Regulated by DNA Methylation Modification during Mycobacterium tuberculosis Infection. BioMed Res. Int. 2016, 2016, 4323281. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Leung, E.T.; Wong, H.K.; Lui, G.; Lee, N.; To, K.F.; Choy, K.W.; Chan, R.C.; Ip, M. Unraveling methylation changes of host macrophages in Mycobacterium tuberculosis infection. Tuberculosis 2016, 98, 139–148. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, P.; Kumar, R.; Bhardwaj, A. dPABBs: A Novel in silico Approach for Predicting and Designing Anti-biofilm Peptides. Sci. Rep. 2016, 6, 21839. [Google Scholar] [CrossRef] [PubMed]
- Behrouzi, A.; Hadifar, S.; Amanzadeh, A.; Riazi Rad, F.; Vaziri, F.; Siadat, S.D. Aberrant Methylation of Host Macrophages Induced by Tuberculosis Infection. World J. Microbiol. Biotechnol. 2019, 35, 168. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Prakhar, P.; Rajmani, R.S.; Mahadik, K.; Borbora, S.M.; Balaji, K.N. Histone Methyltransferase SET8 Epigenetically Reprograms Host Immune Responses to Assist Mycobacterial Survival. J. Infect. Dis. 2017, 216, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Marín Franco, J.L.; Genoula, M.; Corral, D.; Duette, G.; Ferreyra, M.; Maio, M.; Dolotowicz, M.B.; Aparicio-Trejo, O.E.; Patiño-Martínez, E.; Charton, A.; et al. Host-Derived Lipids from Tuberculous Pleurisy Impair Macrophage Microbicidal-Associated Metabolic Activity. Cell Rep. 2020, 33, 108547. [Google Scholar] [CrossRef]
- Gleeson, L.E.; Sheedy, F.J.; Palsson-McDermott, E.M.; Triglia, D.; O’Leary, S.M.; O’Sullivan, M.P.; O’Neill, L.A.J.; Keane, J. Cutting Edge: Mycobacterium tuberculosis Induces Aerobic Glycolysis in Human Alveolar Macrophages That Is Required for Control of Intracellular Bacillary Replication. J. Immunol. 2016, 196, 2444–2449. [Google Scholar] [CrossRef]
- Wu, K.-T.; Chou, W.-Y.; Wang, C.-J.; Chen, C.-Y.; Ko, J.-Y.; Chen, P.-C.; Cheng, J.-H.; Yang, Y.-J. Efficacy of Extracorporeal Shockwave Therapy on Calcified and Noncalcified Shoulder Tendinosis: A Propensity Score Matched Analysis. Biomed Res. Int. 2019, 2019, 2958251. [Google Scholar] [CrossRef]
- Cooper, A.M.; Roberts, A.D.; Rhoades, E.R.; Callahan, J.E.; Getzy, D.M.; Orme, I.M. The Role of Interleukin-12 in Acquired Immunity to Mycobacterium tuberculosis Infection. Immunology 1995, 84, 423–432. [Google Scholar]
- Verreck, F.A.W.; de Boer, T.; Langenberg, D.M.L.; Hoeve, M.A.; Kramer, M.; Vaisberg, E.; Kastelein, R.; Kolk, A.; de Waal-Malefyt, R.; Ottenhoff, T.H.M. Human IL-23-Producing Type 1 Macrophages Promote but IL-10-Producing Type 2 Macrophages Subvert Immunity to (Myco)Bacteria. Proc. Natl. Acad. Sci. USA 2004, 101, 4560–4565. [Google Scholar] [CrossRef]
- Chandra, P.; Grigsby, S.J.; Philips, J.A. Immune Evasion and Provocation by Mycobacterium tuberculosis. Nat. Rev. Microbiol. 2022, 25, 1–17. [Google Scholar] [CrossRef]
- Robinson, C.M.; Nau, G.J. Interleukin-12 and Interleukin-27 Regulate Macrophage Control of Mycobacterium tuberculosis. J. Infect. Dis. 2008, 198, 359–366. [Google Scholar] [CrossRef]
- Zorn, E.; Nelson, E.A.; Mohseni, M.; Porcheray, F.; Kim, H.; Litsa, D.; Bellucci, R.; Raderschall, E.; Canning, C.; Soiffer, R.J.; et al. IL-2 Regulates FOXP3 Expression in Human CD4+CD25+ Regulatory T Cells through a STAT-Dependent Mechanism and Induces the Expansion of These Cells in Vivo. Blood 2006, 108, 1571–1579. [Google Scholar] [CrossRef]
- Ahmed, A.; Vyakarnam, A. Emerging Patterns of Regulatory T Cell Function in Tuberculosis. Clin. Exp. Immunol. 2020, 202, 273–287. [Google Scholar] [CrossRef]
- Orme, I.M.; Robinson, R.T.; Cooper, A.M. The Balance between Protective and Pathogenic Immune Responses in the TB-Infected Lung. Nat. Immunol. 2015, 16, 57–63. [Google Scholar] [CrossRef]
- Shah, T.; Shah, Z.; Baloch, Z.; Cui, X. The Role of Microbiota in Respiratory Health and Diseases, Particularly in Tuberculosis. Biomed Pharm. 2021, 143, 112108. [Google Scholar] [CrossRef]
- Varela-Trinidad, G.U.; Domínguez-Díaz, C.; Solórzano-Castanedo, K.; Íñiguez-Gutiérrez, L.; Hernández-Flores, T.J.; Fafutis-Morris, M. Probiotics: Protecting Our Health from the Gut. Microorganisms 2022, 10, 1428. [Google Scholar] [CrossRef]
- Budden, K.F.; Gellatly, S.L.; Wood, D.L.A.; Cooper, M.A.; Morrison, M.; Hugenholtz, P.; Hansbro, P.M. Emerging Pathogenic Links between Microbiota and the Gut-Lung Axis. Nat. Rev. Microbiol. 2017, 15, 55–63. [Google Scholar] [CrossRef]
- Wang, J.; Li, F.; Wei, H.; Lian, Z.-X.; Sun, R.; Tian, Z. Respiratory Influenza Virus Infection Induces Intestinal Immune Injury via Microbiota-Mediated Th17 Cell-Dependent Inflammation. J. Exp. Med. 2014, 211, 2397–2410. [Google Scholar] [CrossRef]
- Schuijt, T.J.; Lankelma, J.M.; Scicluna, B.P.; de Sousa e Melo, F.; Roelofs, J.J.T.H.; de Boer, J.D.; Hoogendijk, A.J.; de Beer, R.; de Vos, A.; Belzer, C.; et al. The Gut Microbiota Plays a Protective Role in the Host Defence against Pneumococcal Pneumonia. Gut 2016, 65, 575–583. [Google Scholar] [CrossRef]
- Arnold, I.C.; Hutchings, C.; Kondova, I.; Hey, A.; Powrie, F.; Beverley, P.; Tchilian, E. Helicobacter Hepaticus Infection in BALB/c Mice Abolishes Subunit-Vaccine-Induced Protection against M. Tuberculosis. Vaccine 2015, 33, 1808–1814. [Google Scholar] [CrossRef] [PubMed]
- Majlessi, L.; Sayes, F.; Bureau, J.-F.; Pawlik, A.; Michel, V.; Jouvion, G.; Huerre, M.; Severgnini, M.; Consolandi, C.; Peano, C.; et al. Colonization with Helicobacter Is Concomitant with Modified Gut Microbiota and Drastic Failure of the Immune Control of Mycobacterium tuberculosis. Mucosal Immunol. 2017, 10, 1178–1189. [Google Scholar] [CrossRef]
- Negatu, D.A.; Liu, J.J.J.; Zimmerman, M.; Kaya, F.; Dartois, V.; Aldrich, C.C.; Gengenbacher, M.; Dick, T. Whole-Cell Screen of Fragment Library Identifies Gut Microbiota Metabolite Indole Propionic Acid as Antitubercular. Antimicrob. Agents Chemother. 2018, 62, e01571-17. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, S.H.E. Indole Propionic Acid: A Small Molecule Links between Gut Microbiota and Tuberculosis. Antimicrob. Agents Chemother. 2018, 62, e00389-18. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Mendonca, L.; Dhariwal, A.; Fontes, G.; Menzies, D.; Xia, J.; Divangahi, M.; King, I.L. Intestinal Dysbiosis Compromises Alveolar Macrophage Immunity to Mycobacterium tuberculosis. Mucosal Immunol. 2019, 12, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Winglee, K.; Eloe-Fadrosh, E.; Gupta, S.; Guo, H.; Fraser, C.; Bishai, W. Aerosol Mycobacterium tuberculosis Infection Causes Rapid Loss of Diversity in Gut Microbiota. PLoS ONE 2014, 9, e97048. [Google Scholar] [CrossRef]
- Luo, M.; Liu, Y.; Wu, P.; Luo, D.-X.; Sun, Q.; Zheng, H.; Hu, R.; Pandol, S.J.; Li, Q.-F.; Han, Y.-P.; et al. Alternation of Gut Microbiota in Patients with Pulmonary Tuberculosis. Front. Physiol. 2017, 8, 822. [Google Scholar] [CrossRef]
- Maji, A.; Misra, R.; Dhakan, D.B.; Gupta, V.; Mahato, N.K.; Saxena, R.; Mittal, P.; Thukral, N.; Sharma, E.; Singh, A.; et al. Gut Microbiome Contributes to Impairment of Immunity in Pulmonary Tuberculosis Patients by Alteration of Butyrate and Propionate Producers. Environ. Microbiol. 2018, 20, 402–419. [Google Scholar] [CrossRef]
- Lachmandas, E.; van den Heuvel, C.N.A.M.; Damen, M.S.M.A.; Cleophas, M.C.P.; Netea, M.G.; van Crevel, R. Diabetes Mellitus and Increased Tuberculosis Susceptibility: The Role of Short-Chain Fatty Acids. J. Diabetes Res. 2016, 2016, 6014631. [Google Scholar] [CrossRef]
- Pai, M.; Furin, J. Tuberculosis Innovations Mean Little If They Cannot Save Lives. Elife 2017, 6, e25956. [Google Scholar] [CrossRef]
- Prasad, S.; Shilpa, V.P.; Abbas, H.S.; Kotakonda, M. Mechanisms of Antimicrobial Resistance: Highlights on Current Advance Methods for Detection of Drug Resistance and Current Pipeline Antitubercular Agents. Curr. Pharm. Biotechnol. 2022, 23, 1824–1836. [Google Scholar] [CrossRef] [PubMed]
- Ramón-García, S.; Martín, C.; Thompson, C.J.; Aínsa, J.A. Role of the Mycobacterium tuberculosis P55 Efflux Pump in Intrinsic Drug Resistance, Oxidative Stress Responses, and Growth. Antimicrob. Agents Chemother. 2009, 53, 3675–3682. [Google Scholar] [CrossRef]
- Bottalico, L.; Charitos, I.A.; Potenza, M.A.; Montagnani, M.; Santacroce, L. The war against bacteria, from the past to present and beyond. Expert Rev. Anti-Infect. Ther. 2022, 20, 681–706. [Google Scholar] [CrossRef] [PubMed]
- Ehrt, S.; Schnappinger, D.; Rhee, K.Y. Metabolic Principles of Persistence and Pathogenicity in Mycobacterium tuberculosis. Nat. Rev. Microbiol. 2018, 16, 496–507. [Google Scholar] [CrossRef]
- Hauryliuk, V.; Atkinson, G.C.; Murakami, K.S.; Tenson, T.; Gerdes, K. Recent Functional Insights into the Role of (p)PpGpp in Bacterial Physiology. Nat. Rev. Microbiol. 2015, 13, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, J. Significance of the Differential Peptidome in Multidrug-Resistant Tuberculosis. BioMed Res. Int. 2019, 2019, 5653424. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, G.C.; Tenson, T.; Hauryliuk, V. The RelA/SpoT Homolog (RSH) Superfamily: Distribution and Functional Evolution of PpGpp Synthetases and Hydrolases across the Tree of Life. PLoS ONE 2011, 6, e23479. [Google Scholar] [CrossRef] [PubMed]
- Mishra, J.; Rajput, R.; Singh, K.; Bansal, A.; Misra, K. Antioxidant-Rich Peptide Fractions Derived from High-Altitude Chinese Caterpillar Medicinal Mushroom Ophiocordyceps Sinensis (Ascomycetes) Inhibit Bacterial Pathogens. Int. J. Med. Mushrooms 2019, 21, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Barry, C.E.; Boshoff, H.I.; Dartois, V.; Dick, T.; Ehrt, S.; Flynn, J.; Schnappinger, D.; Wilkinson, R.J.; Young, D. The Spectrum of Latent Tuberculosis: Rethinking the Biology and Intervention Strategies. Nat. Rev. Microbiol. 2009, 7, 845–855. [Google Scholar] [CrossRef]
- Banu, S.; Honoré, N.; Saint-Joanis, B.; Philpott, D.; Prévost, M.-C.; Cole, S.T. Are the PE-PGRS Proteins of Mycobacterium tuberculosis Variable Surface Antigens? Mol. Microbiol. 2002, 44, 9–19. [Google Scholar] [CrossRef]
- Ramakrishnan, L.; Federspiel, N.A.; Falkow, S. Granuloma-Specific Expression of Mycobacterium Virulence Proteins from the Glycine-Rich PE-PGRS Family. Science 2000, 288, 1436–1439. [Google Scholar] [CrossRef] [PubMed]
- De Maio, F.; Battah, B.; Palmieri, V.; Petrone, L.; Corrente, F.; Salustri, A.; Palucci, I.; Bellesi, S.; Papi, M.; Rubino, S.; et al. PE_PGRS3 of Mycobacterium tuberculosis Is Specifically Expressed at Low Phosphate Concentration, and Its Arginine-Rich C-Terminal Domain Mediates Adhesion and Persistence in Host Tissues When Expressed in Mycobacterium Smegmatis. Cell Microbiol. 2018, 20, e12952. [Google Scholar] [CrossRef] [PubMed]
- Dutta, N.K.; Klinkenberg, L.G.; Vazquez, M.J.; Segura-Carro, D.; Colmenarejo, G.; Ramon, F.; Rodriguez-Miquel, B.; Mata-Cantero, L.; Porras-De Francisco, E.; Chuang, Y.M.; et al. Inhibiting the stringent response blocks Mycobacterium tuberculosis entry into quiescence and reduces persistence. Sci. Adv. 2019, 5, eaav2104. [Google Scholar] [CrossRef] [PubMed]
- Dahl, J.L.; Kraus, C.N.; Boshoff, H.I.M.; Doan, B.; Foley, K.; Avarbock, D.; Kaplan, G.; Mizrahi, V.; Rubin, H.; Barry, C.E. The Role of RelMtb-Mediated Adaptation to Stationary Phase in Long-Term Persistence of Mycobacterium tuberculosis in Mice. Proc. Natl. Acad. Sci. USA 2003, 100, 10026–10031. [Google Scholar] [CrossRef]
- Dutta, N.K.; Karakousis, P.C. Latent tuberculosis infection: Myths, models, and molecular mechanisms. Microbiol. Mol. Biol. Rev. 2014, 78, 343–371. [Google Scholar] [CrossRef]
- Chakraborty, P.; Kumar, A. The Extracellular Matrix of Mycobacterial Biofilms: Could We Shorten the Treatment of Mycobacterial Infections? Microb. Cell 2019, 6, 105–122. [Google Scholar] [CrossRef]
- Basaraba, R.J.; Ojha, A.K. Mycobacterial Biofilms: Revisiting Tuberculosis Bacilli in Extracellular Necrotizing Lesions. Microbiol. Spectr. 2017, 5, 10.1128. [Google Scholar] [CrossRef]
- Petchiappan, A.; Naik, S.Y.; Chatterji, D. RelZ-Mediated Stress Response in Mycobacterium Smegmatis: PGpp Synthesis and Its Regulation. J. Bacteriol. 2020, 202, e00444-19. [Google Scholar] [CrossRef]
- Santacroce, L.; Man, A.; Charitos, I.A.; Haxhirexha, K.; Topi, S. Current knowledge about the connection between health status and gut microbiota from birth to elderly. A narrative review. Front. Biosci. (Landmark Ed.) 2021, 26, 135–148. [Google Scholar] [CrossRef]
- Genestet, C.; Bernard-Barret, F.; Hodille, E.; Ginevra, C.; Ader, F.; Goutelle, S.; Lina, G.; Dumitrescu, O.; Lyon, T.B.; Lyon TB study group. Antituberculous Drugs Modulate Bacterial Phagolysosome Avoidance and Autophagy in Mycobacterium tuberculosis-Infected Macrophages. Tuberculosis 2018, 111, 67–70. [Google Scholar] [CrossRef]
- Liu, Y.; Tran, D.Q.; Rhoads, J.M. Probiotics in Disease Prevention and Treatment. J. Clin. Pharmacol. 2018, 58, S164–S179. [Google Scholar] [CrossRef] [PubMed]
- Pamer, E.G. Resurrecting the intestinal microbiota to combat antibiotic-resistant pathogens. Science 2016, 352, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Hörmannsperger, G.; Haller, D. Molecular crosstalk of probiotic bacteria with the intestinal immune system: Clinical relevance in the context of inflammatory bowel disease. Int. J. Med. Microbiol. 2010, 300, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Ghadimi, D.; Fölster-Holst, R.; de Vrese, M.; Winkler, P.; Heller, K.J.; Schrezenmeir, J. Effects of Probiotic Bacteria and Their Genomic DNA on TH1/TH2-Cytokine Production by Peripheral Blood Mononuclear Cells (PBMCs) of Healthy and Allergic Subjects. Immunobiology 2008, 213, 677–692. [Google Scholar] [CrossRef]
- Cardona, P.J. The Progress of Therapeutic Vaccination with Regard to Tuberculosis. Front. Microbiol. 2016, 7, 1536. [Google Scholar] [CrossRef]
- Montané, E.; Barriocanal, A.M.; Arellano, A.L.; Valderrama, A.; Sanz, Y.; Perez-Alvarez, N.; Cardona, P.; Vilaplana, C.; Cardona, P.-J. Pilot, Double-Blind, Randomized, Placebo-Controlled Clinical Trial of the Supplement Food Nyaditum Resae® in Adults with or without Latent TB Infection: Safety and Immunogenicity. PLoS ONE 2017, 12, e0171294. [Google Scholar] [CrossRef]
- Rahim, M.A.; Seo, H.; Kim, S.; Tajdozian, H.; Barman, I.; Lee, Y.; Lee, S.; Song, H.-Y. In Vitro Anti-Tuberculosis Effect of Probiotic Lacticaseibacillus rhamnosus PMC203 Isolated from Vaginal Microbiota. Sci. Rep. 2022, 12, 8290. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, J.; Xu, L.; Cai, J.; Zhao, S.; Ma, A. Lactobacillus Casei Modulates Inflammatory Cytokines and Metabolites during Tuberculosis Treatment: A Post Hoc Randomized Controlled Trial. Asia. Pac. J. Clin. Nutr. 2022, 31, 66–77. [Google Scholar] [CrossRef]
- Lee, Y.; Seo, H.; Kim, S.; Rahim, M.D.A.; Yoon, Y.; Jung, J.; Lee, S.; Beom Ryu, C.; Song, H.-Y. Activity of Lactobacillus Crispatus Isolated from Vaginal Microbiota against Mycobacterium tuberculosis. J. Microbiol. 2021, 59, 1019–1030. [Google Scholar] [CrossRef]
- Cao, R.; Teskey, G.; Islamoglu, H.; Gutierrez, M.; Salaiz, O.; Munjal, S.; Fraix, M.P.; Sathananthan, A.; Nieman, D.C.; Venketaraman, V. Flavonoid Mixture Inhibits Mycobacterium tuberculosis Survival and Infectivity. Molecules 2019, 24, 851. [Google Scholar] [CrossRef]
- Raju, A.; Degani, M.S.; Khambete, M.P.; Ray, M.K.; Rajan, M.G. Antifolate Activity of Plant Polyphenols against Mycobacterium tuberculosis. Phytother. Res. 2015, 29, 1646–1651. [Google Scholar] [CrossRef] [PubMed]
- Salih, E.Y.A.; Julkunen-Tiitto, R.; Luukkanen, O.; Sipi, M.; Fahmi, M.K.M.; Fyhrquist, P.J. Potential Anti-Tuberculosis Activity of the Extracts and Their Active Components of Anogeissus leiocarpa (DC.) Guill. and Perr. with Special Emphasis on Polyphenols. Antibiotics 2020, 9, 364. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.K.; Saha, S.; Poria, K.; Samanta, T.; Gautam, S.; Mukhopadhyay, J. A Saponin-Polybromophenol Antibiotic (CU1) from Cassia Fistula Bark Against Multi-Drug Resistant Bacteria Targeting RNA Polymerase. Curr. Res. Pharm. Drug Discov. 2022, 3, 100090. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, S.; Chang, K.; Sakai, M.; Shimizu, N.; Yamada, M.; Tanaka, T.; Nakazawa, H.; Ichinose, F.; Yamada, Y.; Ishigami, A.; et al. Inflammatory Stimuli Induce Inhibitory S-Nitrosylation of the Deacetylase SIRT1 to Increase Acetylation and Activation of P53 and P65. Sci. Signal 2014, 7, ra106. [Google Scholar] [CrossRef]
- Yang, H.; Chen, J.; Chen, Y.; Jiang, Y.; Ge, B.; Hong, L. Sirtuin Inhibits, M. Tuberculosis -Induced Apoptosis in Macrophage through Glycogen Synthase Kinase-3β. Arch. Biochem. Biophys. 2020, 694, 108612. [Google Scholar] [CrossRef]
- Magana, M.; Pushpanathan, M.; Santos, A.L.; Leanse, L.; Fernandez, M.; Ioannidis, A.; Giulianotti, M.A.; Apidianakis, Y.; Bradfute, S.; Ferguson, A.L.; et al. The Value of Antimicrobial Peptides in the Age of Resistance. Lancet Infect. Dis. 2020, 20, e216–e230. [Google Scholar] [CrossRef]
- Gaglione, R.; Pizzo, E.; Notomista, E.; de la Fuente-Nunez, C.; Arciello, A. Host Defence Cryptides from Human Apolipoproteins: Applications in Medicinal Chemistry. Curr. Top. Med. Chem. 2020, 20, 1324–1337. [Google Scholar] [CrossRef]
- Mehta, K.; Sharma, P.; Mujawar, S.; Vyas, A. Role of Antimicrobial Peptides in Treatment and Prevention of Mycobacterium tuberculosis: A Review. Int. J. Pept. Res. Ther. 2022, 28, 132. [Google Scholar] [CrossRef]
- Jadhav, K.; Singh, R.; Ray, E.; Singh, A.K.; Verma, R.K. Taming the devil: Antimicrobial peptides for safer TB therapeutics. Curr. Protein Pept. Sci. 2022. [Google Scholar] [CrossRef]
- Travis, S.M.; Anderson, N.N.; Forsyth, W.R.; Espiritu, C.; Conway, B.D.; Greenberg, E.P.; McCray, P.B., Jr.; Lehrer, R.I.; Welsh, M.J.; Tack, B.F. Bactericidal activity of mammalian cathelicidin-derived peptides. Infect. Immun. 2000, 68, 2748–2755. [Google Scholar] [CrossRef]
- Hancock, R.E.; Sahl, H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial Peptides: Diversity, Mechanism of Action and Strategies to Improve the Activity and Biocompatibility In Vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.; Gomes, M.S. Immuno-Stimulatory Peptides as a Potential Adjunct Therapy against Intra-Macrophagic Pathogens. Molecules 2017, 22, 1297. [Google Scholar] [CrossRef]
- Dürr, U.H.; Sudheendra, U.S.; Ramamoorthy, A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim. Biophys. Acta 2006, 1758, 1408–1425. [Google Scholar] [CrossRef]
- Yang, B.; Good, D.; Mosaiab, T.; Liu, W.; Ni, G.; Kaur, J.; Liu, X.; Jessop, C.; Yang, L.; Fadhil, R.; et al. Significance of LL-37 on Immunomodulation and Disease Outcome. Biomed Res. Int. 2020, 2020, 8349712. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-like Receptor Triggering of a Vitamin D-Mediated Human Antimicrobial Response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef] [PubMed]
- Mily, A.; Rekha, R.S.; Kamal, S.M.M.; Arifuzzaman, A.S.M.; Rahim, Z.; Khan, L.; Haq, M.A.; Zaman, K.; Bergman, P.; Brighenti, S.; et al. Significant Effects of Oral Phenylbutyrate and Vitamin D3 Adjunctive Therapy in Pulmonary Tuberculosis: A Randomized Controlled Trial. PLoS ONE 2015, 10, e0138340. [Google Scholar] [CrossRef] [PubMed]
- Torres-Juarez, F.; Cardenas-Vargas, A.; Montoya-Rosales, A.; González-Curiel, I.; Garcia-Hernandez, M.H.; Enciso-Moreno, J.A.; Hancock, R.E.W.; Rivas-Santiago, B. LL-37 Immunomodulatory Activity during Mycobacterium tuberculosis Infection in Macrophages. Infect. Immun. 2015, 83, 4495–4503. [Google Scholar] [CrossRef]
- Cobongela, S.; Makatini, M.M.; Mdluli, P.S.; Sibuyi, N. Acyldepsipeptide Analogues: A Future Generation Antibiotics for Tuberculosis Treatment. Pharmaceutics 2022, 14, 1956. [Google Scholar] [CrossRef]
- Desvignes, L.; Wolf, A.J.; Ernst, J.D. Dynamic Roles of Type I and Type II IFNs in Early Infection with Mycobacterium tuberculosis. J. Immunol. 2012, 188, 6205–6215. [Google Scholar] [CrossRef]
- Petruccioli, E.; Scriba, T.J.; Petrone, L.; Hatherill, M.; Cirillo, D.M.; Joosten, S.A.; Ottenhoff, T.H.; Denkinger, C.M.; Goletti, D. Correlates of Tuberculosis Risk: Predictive Biomarkers for Progression to Active Tuberculosis. Eur. Respir. J. 2016, 48, 1751–1763. [Google Scholar] [CrossRef] [PubMed]
- Berns, S.A.; Isakova, J.A.; Pekhtereva, P.I. Therapeutic Potential of Interferon-Gamma in Tuberculosis. Admet Dmpk 2022, 10, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Ernst, J.D. The Immunological Life Cycle of Tuberculosis. Nat. Rev. Immunol. 2012, 12, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Hickman-Davis, J.M.; Fang, F.C.; Nathan, C.; Shepherd, V.L.; Voelker, D.R.; Wright, J.R. Lung surfactant and reactive oxygen-nitrogen species: Antimicrobial activity and host-pathogen interactions. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001, 281, L517–L523. [Google Scholar] [CrossRef]
- Fortes, A.; Pereira, K.; Antas, P.R.Z.; Franken, C.L.M.C.; Dalcolmo, M.; Ribeiro-Carvalho, M.M.; Cunha, K.S.; Geluk, A.; Kritski, A.; Kolk, A.; et al. Detection of in Vitro Interferon-Gamma and Serum Tumour Necrosis Factor-Alpha in Multidrug-Resistant Tuberculosis Patients. Clin. Exp. Immunol. 2005, 141, 541–548. [Google Scholar] [CrossRef]
- Brooks, B.M.; Hart, C.A.; Coleman, J.W. Differential Effects of Beta-Lactams on Human IFN-Gamma Activity. J. Antimicrob. Chemother. 2005, 56, 1122–1125. [Google Scholar] [CrossRef]
- Gao, X.-F.; Yang, Z.-W.; Li, J. Adjunctive Therapy with Interferon-Gamma for the Treatment of Pulmonary Tuberculosis: A Systematic Review. Int. J. Infect. Dis. 2011, 15, 594–600. [Google Scholar] [CrossRef]
- Condos, R.; Rom, W.N.; Schluger, N.W. Treatment of Multidrug-Resistant Pulmonary Tuberculosis with Interferon-Gamma via Aerosol. Lancet 1997, 349, 1513–1515. [Google Scholar] [CrossRef]
- Park, S.-K.; Cho, S.; Lee, I.-H.; Jeon, D.-S.; Hong, S.-H.; Smego, R.A.; Cho, S.-N. Subcutaneously Administered Interferon-Gamma for the Treatment of Multidrug-Resistant Pulmonary Tuberculosis. Int. J. Infect. Dis. 2007, 11, 434–440. [Google Scholar] [CrossRef]
- Suárez-Méndez, R.; García-García, I.; Fernández-Olivera, N.; Valdés-Quintana, M.; Milanés-Virelles, M.T.; Carbonell, D.; Machado-Molina, D.; Valenzuela-Silva, C.M.; López-Saura, P.A. Adjuvant interferon gamma in patients with drug-resistant pulmonary tuberculosis: A pilot study. BMC Infect. Dis. 2004, 4, 44. [Google Scholar] [CrossRef]
- Raad, I.; Hachem, R.; Leeds, N.; Sawaya, R.; Salem, Z.; Atweh, S. Use of Adjunctive Treatment with Interferon-Gamma in an Immunocompromised Patient Who Had Refractory Multidrug-Resistant Tuberculosis of the Brain. Clin. Infect. Dis. 1996, 22, 572–574. [Google Scholar] [CrossRef] [PubMed]
- Pisarenko, M.S. Peculiarities of IFN-g secretion in drug-resistant pulmonary tuberculosis. Fundam. Res. 2013, 9, 444–447. [Google Scholar]
- Khan, T.A.; Mazhar, H.; Saleha, S.; Tipu, H.N.; Muhammad, N.; Abbas, M.N. Interferon-Gamma Improves Macrophages Function against M. Tuberculosis in Multidrug-Resistant Tuberculosis Patients. Chemother. Res. Pr. 2016, 2016, 7295390. [Google Scholar] [CrossRef] [PubMed]
- Ballini, A.; De Frenza, G.; Cantore, S.; Papa, F.; Grano, M.; Mastrangelo, F.; Tetè, S.; Grassi, F.R. In vitro stem cell cultures from human dental pulp and periodontal ligament: New prospects in dentistry. Int. J. Immunopathol. Pharmacol. 2007, 20, 9–16. [Google Scholar] [CrossRef]
- Charitos, I.A.; Ballini, A.; Cantore, S.; Boccellino, M.; Di Domenico, M.; Borsani, E.; Nocini, R.; Di Cosola, M.; Santacroce, L.; Bottalico, L. Stem Cells: A Historical Review about Biological, Religious, and Ethical Issues. Stem. Cells Int. 2021, 2021, 9978837. [Google Scholar] [CrossRef]
- Raghuvanshi, S.; Sharma, P.; Singh, S.; Van Kaer, L.; Das, G. Mycobacterium tuberculosis evades host immunity by recruiting mesenchymal stem cells. Proc. Natl. Acad. Sci. USA 2010, 107, 21653–21658. [Google Scholar] [CrossRef]
- Das, B.; Kashino, S.S.; Pulu, I.; Kalita, D.; Swami, V.; Yeger, H.; Felsher, D.W.; Campos-Neto, A. CD271(+) bone marrow mesenchymal stem cells may provide a niche for dormant Mycobacterium tuberculosis. Sci. Transl. Med. 2013, 5, 170ra13. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, F.; Li, W.; Dang, J.L.; Yuan, J.; Wang, J.; Zeng, D.L.; Sun, C.X.; Liu, Y.Y.; Ao, Q.; et al. Human Gingiva-Derived Mesenchymal Stem Cells Modulate Monocytes/Macrophages and Alleviate Atherosclerosis. Front. Immunol. 2018, 9, 878. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, Q.; Ye, Z.; Li, Y.; Che, Z.; Huang, M.; Zeng, J. Mesenchymal Stem Cells and Tuberculosis: Clinical Challenges and Opportunities. Front. Immunol. 2021, 12, 695278. [Google Scholar] [CrossRef]
- Maslennikov, A.A.; Obolonkova, N.I.; Belgorod State National Research University. Efficiency of ingaron in the treatment of patients with destructive pulmonary bacteriologicaly proven tuberculosis. Res. Result 2016, 2, 10–16. [Google Scholar] [CrossRef][Green Version]
- Diallo, D.; Somboro, A.M.; Diabate, S.; Baya, B.; Kone, A.; Sarro, Y.S.; Kone, B.; Diarra, B.; Diallo, S.; Diakite, M.; et al. Antituberculosis Therapy and Gut Microbiota: Review of Potential Host Microbiota Directed-Therapies. Front. Cell. Infect. Microbiol. 2021, 11, 673100. [Google Scholar] [CrossRef] [PubMed]
- Marzulli, G.; Magrone, T.; Kawaguchi, K.; Kumazawa, Y.; Jirillo, E. Fermented Grape Marc (FGM): Immunomodulating Properties and Its Potential Exploitation in the Treatment of Neurodegenerative Diseases. Curr. Pharm. Des. 2012, 18, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.S.; Costa, R.P.; Gomes, P.; Gomes, M.S.; Silva, T.; Teixeira, C. Antimicrobial Peptides as Potential Anti-Tubercular Leads: A Concise Review. Pharmaceuticals 2021, 14, 323. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wu, R.; Zhao, B.; Zeiner, R.; Chien, P.; You, M. Live-Cell Imaging of (p)ppGpp with RNA-based Fluorescent Sensors. bioRxiv 2021, 60, 24070–24074. [Google Scholar] [CrossRef]
- El Omari, K.; Hamze, M.; Alwan, S.; Osman, M.; Jama, C.; Chihib, N.E. In-vitro evaluation of the antibacterial activity of the essential oils of Micromeria barbata, Eucalyptus globulus and Juniperus excelsa against strains of Mycobacterium tuberculosis (including MDR), Mycobacterium kansasii and Mycobacterium gordonae. J. Infect. Public Health 2019, 12, 615–618. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arrigoni, R.; Ballini, A.; Topi, S.; Bottalico, L.; Jirillo, E.; Santacroce, L. Antibiotic Resistance to Mycobacterium tuberculosis and Potential Use of Natural and Biological Products as Alternative Anti-Mycobacterial Agents. Antibiotics 2022, 11, 1431. https://doi.org/10.3390/antibiotics11101431
Arrigoni R, Ballini A, Topi S, Bottalico L, Jirillo E, Santacroce L. Antibiotic Resistance to Mycobacterium tuberculosis and Potential Use of Natural and Biological Products as Alternative Anti-Mycobacterial Agents. Antibiotics. 2022; 11(10):1431. https://doi.org/10.3390/antibiotics11101431
Chicago/Turabian StyleArrigoni, Roberto, Andrea Ballini, Skender Topi, Lucrezia Bottalico, Emilio Jirillo, and Luigi Santacroce. 2022. "Antibiotic Resistance to Mycobacterium tuberculosis and Potential Use of Natural and Biological Products as Alternative Anti-Mycobacterial Agents" Antibiotics 11, no. 10: 1431. https://doi.org/10.3390/antibiotics11101431
APA StyleArrigoni, R., Ballini, A., Topi, S., Bottalico, L., Jirillo, E., & Santacroce, L. (2022). Antibiotic Resistance to Mycobacterium tuberculosis and Potential Use of Natural and Biological Products as Alternative Anti-Mycobacterial Agents. Antibiotics, 11(10), 1431. https://doi.org/10.3390/antibiotics11101431







