Recent Progress in Phage Therapy to Modulate Multidrug-Resistant Acinetobacter baumannii, including in Human and Poultry
Abstract
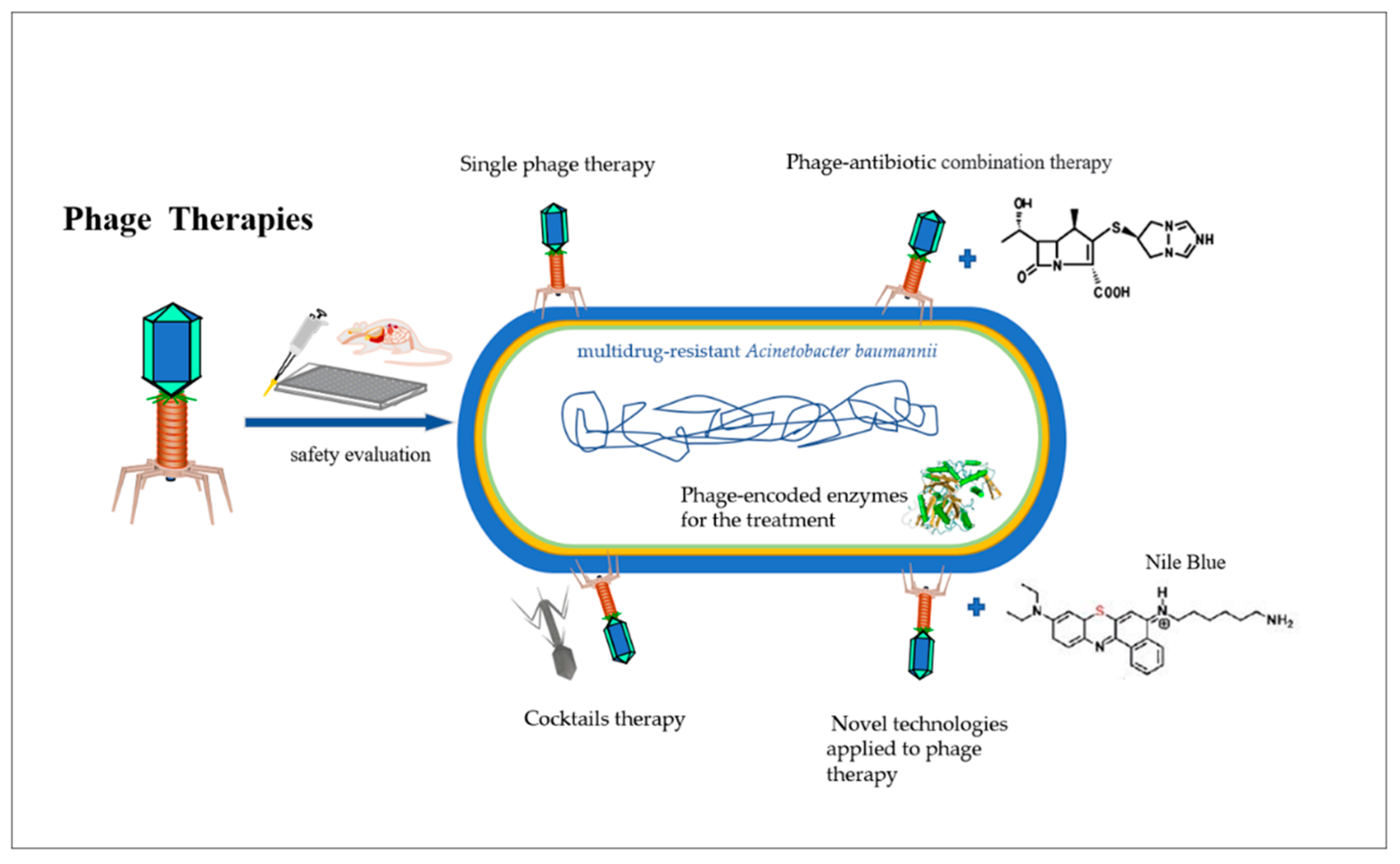
1. Introduction
2. Phage Therapy on Human Infection
2.1. Single Phage Therapy
2.2. Cocktail Therapy
2.3. Phage–Antibiotic Synergy
2.4. Phage-Encoded Enzymes for the Treatment of A. baumannii
2.4.1. Endolysins
2.4.2. Depolymerases
2.5. Novel Technologies Applied to Phage Therapy
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Howard, A.; O’Donoghue, M.; Feeney, A.; Sleator, R.D. Acinetobacter baumannii: An emerging opportunistic pathogen. Virulence 2012, 3, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, R.-Q.; Wang, L.; Yang, W.-T.; Zou, Q.-H.; Xiao, D. Proteomic Analyses of Acinetobacter baumannii Clinical Isolates to Identify Drug Resistant Mechanism. Front. Cell. Infect. Microbiol. 2021, 11, 625430. [Google Scholar] [CrossRef] [PubMed]
- Ayoub Moubareck, C.; Hammoudi Halat, D. Insights into Acinetobacter baumannii: A Review of Microbiological, Virulence, and Resistance Traits in a Threatening Nosocomial Pathogen. Antibiotics 2020, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Grygorcewicz, B.; Wojciuk, B.; Roszak, M.; Łubowska, N.; Błażejczak, P.; Jursa-Kulesza, J.; Rakoczy, R.; Masiuk, H.; Dołęgowska, B. Environmental Phage-Based Cocktail and Antibiotic Combination Effects on Acinetobacter baumannii Biofilm in a Human Urine Model. Microb. Drug Resist. 2021, 27, 25–35. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Antimicrobial Resistance: 2014 Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014.
- Papp-Wallace, K.M.; Endimiani, A.; Taracila, M.A.; Bonomo, R.A. Carbapenems: Past, Present, and Future. Antimicrob. Agents Chemother. 2011, 55, 4943–4960. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.R.; Lee, J.H.; Park, M.; Park, K.S.; Bae, I.K.; Kim, Y.B.; Cha, C.J.; Jeong, B.C.; Lee, S.H. Biology of Acinetobacter baumannii: Pathogenesis, Antibiotic Resistance Mechanisms, and Prospective Treatment Options. Front. Cell. Infect. Microbiol. 2017, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.; Al-Saryi, N.; Al-Kadmy, I.M.S.; Aziz, S.N. Multidrug-resistant Acinetobacter baumannii as an emerging concern in hospitals. Mol. Biol. Rep. 2021, 48, 6987–6998. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.; Alvi, I.A.; Rehman, S.U. Insight into Acinetobacter baumannii: Pathogenesis, global resistance, mechanisms of resistance, treatment options, and alternative modalities. Infect. Drug Resist. 2018, 11, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Nang, S.C.; Azad, M.A.K.; Velkov, T.; Zhou, Q.; Li, J. Rescuing the Last-Line Polymyxins: Achievements and Challenges. Pharmacol. Rev. 2021, 73, 679–728. [Google Scholar] [CrossRef] [PubMed]
- As, S.G.; Priyadharsini, J.V. CLSI based antibiogram profile and the detection of MDR and XDR strains of Acinetobacter baumannii isolated from urine samples. Med. J. Islamic Repub. Iran 2019, 33, 3. [Google Scholar]
- Assimakopoulos, S.F.; Karamouzos, V.; Lefkaditi, A.; Sklavou, C.; Kolonitsiou, F.; Christofidou, M.; Fligou, F.; Gogos, C.; Marangos, M. Triple combination therapy with high-dose ampicillin/sulbactam, high-dose tigecycline and colistin in the treatment of ventilator-associated pneumonia caused by pan-drug resistant Acinetobacter baumannii: A case series study. Le Infez. Med. 2019, 27, 11–16. [Google Scholar]
- Goic-Barisic, I.; Seruga Music, M.; Kovacic, A.; Tonkic, M.; Hrenovic, J. Pan Drug-Resistant Environmental Isolate of Acinetobacter baumannii from Croatia. Microb. Drug Resist. 2017, 23, 494–496. [Google Scholar] [CrossRef] [PubMed]
- Mottet, A.; Tempio, G. Global poultry production: Current state and future outlook and challenges. World’s Poult. Sci. J. 2017, 73, 245–256. [Google Scholar] [CrossRef]
- Chapot, L.; Whatford, L.; Compston, P.; Tak, M.; Cuevas, S.; Garza, M.; Bennani, H.; Bin Aslam, H.; Hennessey, M.; Limon, G.; et al. A Global Media Analysis of the Impact of the COVID-19 Pandemic on Chicken Meat Food Systems: Key Vulnerabilities and Opportunities for Building Resilience. Sustainability 2021, 13, 9435. [Google Scholar] [CrossRef]
- More, S.J. European perspectives on efforts to reduce antimicrobial usage in food animal production. Ir. Veter. J. 2020, 73, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nocera, F.; Attili, A.-R.; De Martino, L. Acinetobacter baumannii: Its Clinical Significance in Human and Veterinary Medicine. Pathogens 2021, 10, 127. [Google Scholar] [CrossRef]
- Kumar, S.; Anwer, R.; Yadav, M.; Sehrawat, N.; Kumar, V.; Sharma, A.K. Isolation and Characterization of Acinetobacter baumannii from Chicken Meat Samples in North India. Asian, J. Biol. Life Sci. 2021, 10, 462–468. [Google Scholar] [CrossRef]
- Release, N. WHO publishes list of bacteria for which new antibiotics are urgently needed. Neurosciences 2017, 38, 444–445. [Google Scholar]
- Sharma, S.; Chatterjee, S.; Datta, S.; Prasad, R.; Dubey, D.; Prasad, R.K.; Vairale, M.G. Bacteriophages and its applications: An overview. Folia Microbiol. 2016, 62, 17–55. [Google Scholar] [CrossRef]
- Mäntynen, S.; Laanto, E.; Oksanen, H.M.; Poranen, M.M.; Díaz-Muñoz, S.L. Black box of phage–bacterium interactions: Exploring alternative phage infection strategies. Open Biol. 2021, 11, 210188. [Google Scholar] [CrossRef]
- Kortright, K.E.; Chan, B.K.; Koff, J.L.; Turner, P.E. Phage Therapy: A Renewed Approach to Combat Antibiotic-Resistant Bacteria. Cell Host Microbe 2019, 25, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.; Pires, D.P.; Costa, A.R.; Azeredo, J. Phage Therapy: Going Temperate? Trends Microbiol. 2019, 27, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Jeannot, K.; Xiao, Y.; Knapp, C.W. Editorial: Horizontal Gene Transfer Mediated Bacterial Antibiotic Resistance. Front. Microbiol. 2019, 10, 1933. [Google Scholar] [CrossRef] [PubMed]
- Principi, N.; Silvestri, E.; Esposito, S. Advantages and limitations of bacteriophages for the treatment of bacterial infections. Front. Pharmacol. 2019, 10, 513. [Google Scholar] [CrossRef] [PubMed]
- Łobocka, M.; Dąbrowska, K.; Górski, A. Engineered Bacteriophage Therapeutics: Rationale, Challenges and Future. BioDrugs Clin. Immunother. Biopharm. Gene Ther. 2021, 35, 255–280. [Google Scholar] [CrossRef]
- Isler, B.; Doi, Y.; Bonomo, R.A.; Paterson, D.L. New Treatment Options against Carbapenem-Resistant Acinetobacter baumannii Infections. Antimicrob. Agents Chemother. 2019, 63, e01110-18. [Google Scholar] [CrossRef]
- LaVergne, S.; Hamilton, T.; Biswas, B.; Kumaraswamy, M.; Schooley, R.T.; Wooten, D. Phage Therapy for a Multidrug-Resistant Acinetobacter baumannii Craniectomy Site Infection. Open Forum Infect. Dis. 2018, 5, ofy064. [Google Scholar]
- Khan, F.M.; Gondil, V.S.; Li, C.; Jiang, M.; Li, J.; Yu, J.; Wei, H.; Yang, H. A Novel Acinetobacter baumannii Bacteriophage Endolysin LysAB54 With High Antibacterial Activity Against Multiple Gram-Negative Microbes. Front. Cell. Infect. Microbiol. 2021, 11, 637313. [Google Scholar] [CrossRef]
- Jiang, L.; Tan, J.; Hao, Y.; Wang, Q.; Yan, X.; Wang, D.; Tuo, L.; Wei, Z.; Huang, G. Isolation and Characterization of a Novel Myophage Abp9 Against Pandrug Resistant Acinetobacater baumannii. Front. Microbiol. 2020, 11, 506068. [Google Scholar] [CrossRef]
- Schooley, R.T.; Biswas, B.; Gill, J.J.; Hernandez-Morales, A.; Lancaster, J.; Lessor, L.; Barr, J.J.; Reed, S.L.; Rohwer, F.; Benler, S.; et al. Development and Use of Personalized Bacteriophage-Based Therapeutic Cocktails To Treat a Patient with a Disseminated Resistant Acinetobacter baumannii Infection. Antimicrob. Agents Chemother. 2017, 61, e00954-17. [Google Scholar]
- Loc-Carrillo, C.; Abedon, S.T. Pros and cons of phage therapy. Bacteriophage 2011, 1, 111–114. [Google Scholar] [CrossRef] [PubMed]
- SBochkareva, S.; Aleshkin, A.V.; Ershova, O.N.; Novikova, L.I.; Zeigarnik, M.V. Anti-phage antibody response in phage therapy against healthcare-associated infections (HAIs). Infect. Dis. 2017, 15, 1535–1540. [Google Scholar]
- Díaz-Muñoz, S.L.; Koskella, B. Bacteria–Phage Interactions in Natural Environments. Adv. Appl. Microbiol. 2014, 89, 135–183. [Google Scholar] [PubMed]
- Sari, M.; Pilvi, R.; Matti, J. On-Demand Isolation of Bacteriophages Against Drug-Resistant Bacteria for Personalized Phage Therapy. Front. Microbiol. 2015, 6, 1271. [Google Scholar]
- Matsuzaki, S.; Rashel, M.; Uchiyama, J.; Sakurai, S.; Ujihara, T.; Kuroda, M.; Ikeuchi, M.; Tani, T.; Fujieda, M.; Wakiguchi, H.; et al. Bacteriophage therapy: A revitalized therapy against bacterial infectious diseases. J. Infect. Chemother. Off. J. Jpn. Soc. Chemother. 2005, 11, 211–219. [Google Scholar] [CrossRef]
- Krylov, V.; Shaburova, O.; Krylov, S.; Pleteneva, E. A Genetic Approach to the Development of New Therapeutic Phages to Fight Pseudomonas Aeruginosa in Wound Infections. Viruses 2012, 5, 15–53. [Google Scholar] [CrossRef]
- Weld, R.; Butts, C.; Heinemann, J.A. Models of phage growth and their applicability to phage therapy. J. Theor. Biol. 2004, 227, 1–11. [Google Scholar] [CrossRef]
- Gupta, R.; Prasad, Y. Efficacy of polyvalent bacteriophage P-27/HP to control multidrug resistant Staphylococcus aureus associated with human infections. Curr. Microbiol. 2011, 62, 255–260. [Google Scholar] [CrossRef]
- Górski, A.; Jończyk-Matysiak, E.; Łusiak-Szelachowska, M.; Międzybrodzki, R.; Weber-Dąbrowska, B.; Borysowski, J. The Potential of Phage Therapy in Sepsis. Front. Immunol. 2017, 8, 1783. [Google Scholar] [CrossRef]
- Skurnik, M.; Pajunen, M.; Kiljunen, S. Biotechnological challenges of phage therapy. Biotechnol. Lett. 2007, 29, 995–1003. [Google Scholar] [CrossRef]
- Wienhold, S.-M.; Lienau, J.; Witzenrath, M. Towards Inhaled Phage Therapy in Western Europe. Viruses 2019, 11, 295. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, M.T.; Khoshbayan, A.; Chegini, Z.; Farahani, I.; Shariati, A. Bacteriophages, a New Therapeutic Solution for Inhibiting Multidrug-Resistant Bacteria Causing Wound Infection: Lesson from Animal Models and Clinical Trials. Drug Des. Dev. Ther. 2020, 14, 1867–1883. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.K.; Abedon, S.T.; Loc-Carrillo, C. Phage cocktails and the future of phage therapy. Future Microbiol. 2013, 8, 769–783. [Google Scholar] [CrossRef] [PubMed]
- McCallin, S.; Sarker, S.A.; Sultana, S.; Oechslin, F.; Brüssow, H. Metagenome analysis of Russian and Georgian Pyophage cocktails and a placebo-controlled safety trial of single phage versus phage cocktail in healthy Staphylococcus aureus carriers. Environ. Microbiol. 2018, 20, 3278–3293. [Google Scholar] [CrossRef] [PubMed]
- Molina, F.; Simancas, A.; Ramírez, M.; Tabla, R.; Roa, I.; Rebollo, J.E. A New Pipeline for Designing Phage Cocktails Based on Phage-Bacteria Infection Networks. Front. Microbiol. 2021, 12, 564532. [Google Scholar] [CrossRef]
- Luong, T.; Salabarria, A.C.; Roach, D.R. Phage Therapy in the Resistance Era: Where Do We Stand and Where Are We Going? Clin. Ther. 2020, 42, 1659–1680. [Google Scholar] [CrossRef]
- Oliveira, H.; São-José, C.; Azeredo, J. Phage-Derived Peptidoglycan Degrading Enzymes: Challenges and Future Prospects for In Vivo Therapy. Viruses 2018, 10, 292. [Google Scholar] [CrossRef]
- Ran, B.; Yuan, Y.; Xia, W.; Li, M.; Yao, Q.; Wang, Z.; Wang, L.; Li, X.; Xu, Y.; Peng, X. A photo-sensitizable phage for multidrug-resistant Acinetobacter baumannii therapy and biofilm ablation. Chem. Sci. 2021, 12, 1054–1061. [Google Scholar] [CrossRef]
- Jeon, J.; Kim, J.-W.; Yong, D.; Lee, K.; Chong, Y. Complete Genome Sequence of the Podoviral Bacteriophage YMC/09/02/B1251 ABA BP, Which Causes the Lysis of an OXA-23-Producing Carbapenem-Resistant Acinetobacter baumannii Isolate from a Septic Patient. J. Virol. 2012, 86, 12437–12438. [Google Scholar] [CrossRef]
- Hua, Y.; Luo, T.; Yang, Y.; Dong, D.; Wang, R.; Wang, Y.; Xu, M.; Guo, X.; Hu, F.; He, P. Phage Therapy as a Promising New Treatment for Lung Infection Caused by Carbapenem-Resistant Acinetobacter baumannii in Mice. Front. Microbiol. 2018, 8, 2659. [Google Scholar] [CrossRef]
- Wu, M.; Hu, K.; Xie, Y.; Liu, Y.; Mu, D.; Guo, H.; Zhang, Z.; Zhang, Y.; Chang, D.; Shi, Y. A Novel Phage PD-6A3, and Its Endolysin Ply6A3, With Extended Lytic Activity Against Acinetobacter baumannii. Front. Microbiol. 2018, 9, 3302. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-L.; Kuo, C.-F.; Yeh, C.-M.; Chen, J.-R.; Cheng, M.-F.; Hung, C.-H. Efficacy of φkm18p phage therapy in a murine model of extensively drug-resistant Acinetobacter baumannii infection. Infect. Drug Resist. 2018, 11, 2301–2310. [Google Scholar] [CrossRef] [PubMed]
- Grygorcewicz, B.; Roszak, M.; Golec, P.; Śleboda-Taront, D.; Łubowska, N.; Górska, M.; Jursa-Kulesza, J.; Rakoczy, R.; Wojciuk, B.; Dołęgowska, B. Antibiotics Act with vB_AbaP_AGC01 Phage against Acinetobacter baumannii in Human Heat-Inactivated Plasma Blood and Galleria mellonella Models. Int. J. Mol. Sci. 2020, 21, 4390. [Google Scholar] [CrossRef] [PubMed]
- Styles, K.M.; Thummeepak, R.; Leungtongkam, U.; Smith, S.E.; Christie, G.; Millard, A.; Moat, J.; Dowson, C.G.; Wellington, E.M.H.; Sitthisak, S.; et al. Investigating Bacteriophages Targeting the Opportunistic Pathogen Acinetobacter baumannii. Antibiotics 2020, 9, 200. [Google Scholar] [CrossRef]
- Tawakol, M.M.; Nabil, N.M.; Samy, A. Evaluation of bacteriophage efficacy in reducing the impact of single and mixed infections with Escherichia coli and infectious bronchitis in chickens. Infect. Ecol. Epidemiol. 2019, 9, 1686822. [Google Scholar] [CrossRef]
- Hesse, S.; Adhya, S. Phage Therapy in the Twenty-First Century: Facing the Decline of the Antibiotic Era; Is It Finally Time for the Age of the Phage? Annu. Rev. Microbiol. 2019, 73, 155–174. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, L.; Li, X.; Tan, D.; Cong, C.; Xu, Y. Efficacy of a phage cocktail in controlling phage resistance development in multidrug resistant Acinetobacter baumannii. Virus Res. 2019, 272, 197734. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Dai, J.; Guo, M.; Li, J.; Zhou, X.; Li, F.; Gao, Y.; Qu, H.; Lu, H.; Jin, J.; et al. Pre-optimized phage therapy on secondary Acinetobacter baumannii infection in four critical COVID-19 patients. Emerg. Microbes Infect. 2021, 10, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Upadhaya, S.D.; Ahn, J.M.; Cho, J.H.; Kim, J.Y.; Kang, D.K.; Kim, S.W.; Kim, H.B.; Kim, I.H. Bacteriophage cocktail supplementation improves growth performance, gut microbiome and production traits in broiler chickens. J. Anim. Sci. Biotechnol. 2021, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.-H.; Wang, J.-L.; Wen, F.-S.; Chang, K.-M.; Kuo, C.-F.; Lin, C.-H.; Luo, H.-R.; Hung, C.-H. Isolation and Characterization of φkm18p, a Novel Lytic Phage with Therapeutic Potential against Extensively Drug Resistant Acinetobacter baumannii. PLoS ONE 2012, 7, e46537. [Google Scholar] [CrossRef]
- Jasim, H.N.; Hafidh, R.R.; Abdulamir, A.S. Formation of therapeutic phage cocktail and endolysin to highly multi-drug resistant Acinetobacter baumannii: In vitro and in vivo study. Iran, J. Basic Med. Sci. 2018, 21, 1100–1108. [Google Scholar]
- Nilsson, A.S. Phage therapy—Constraints and possibilities. Upsala J. Med. Sci. 2014, 119, 192–198. [Google Scholar] [CrossRef]
- Al Amin, A.; Hoque, M.N.; Siddiki, A.Z.; Saha, S.; Kamal, M. Antimicrobial resistance situation in animal health of Bangladesh. Vet. World 2020, 13, 2713–2727. [Google Scholar] [CrossRef] [PubMed]
- Richards, P.J.; Connerton, P.L.; Connerton, I.F. Phage Biocontrol of Campylobacter jejuni in Chickens Does Not Produce Collateral Effects on the Gut Microbiota. Front. Microbiol. 2019, 10, 476. [Google Scholar] [CrossRef]
- Xu, S.; Campisi, E.; Li, J.; Fischetti, V.A. Decontamination of Escherichia coli O157:H7 on fresh Romaine lettuce using a novel bacteriophage lysin. Int. J. Food Microbiol. 2021, 341, 109068. [Google Scholar] [CrossRef]
- Tagliaferri, T.L.; Jansen, M.; Horz, H.-P. Fighting Pathogenic Bacteria on Two Fronts: Phages and Antibiotics as Combined Strategy. Front. Cell. Infect. Microbiol. 2019, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Oechslin, F. Resistance Development to Bacteriophages Occurring during Bacteriophage Therapy. Viruses 2018, 10, 351. [Google Scholar] [CrossRef]
- Ma, C.; Wang, N.; Xu, Y. Bacteriophage combined with antibiotics in the control of multidrug-resistant Acinetobacter baumannii. Chin. J. Antibiot. 2018, 43, 9. [Google Scholar]
- Tan, X.; Chen, H.; Zhang, M.; Zhao, Y.; Jiang, Y.; Liu, X.; Huang, W.; Ma, Y. Clinical Experience of Personalized Phage Therapy Against Carbapenem-Resistant Acinetobacter baumannii Lung Infection in a Patient with Chronic Obstructive Pulmonary Disease. Front. Cell. Infect. Microbiol. 2021, 11, 631585. [Google Scholar] [CrossRef]
- Jansen, M.; Wahida, A.; Latz, S.; Krüttgen, A.; Häfner, H.; Buhl, E.M.; Ritter, K.; Horz, H.-P. Enhanced antibacterial effect of the novel T4-like bacteriophage KARL-1 in combination with antibiotics against multi-drug resistant Acinetobacter baumannii. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Grygorcewicz, B.; Roszak, M.; Rakoczy, R.; Augustyniak, A.; Konopacki, M.; Jablonska, J.; Serwin, N.; Cecerska-Heryc, E.; Kordas, M.; Galant, K.; et al. PhageScore-based analysis of Acinetobacter baumannii infecting phages antibiotic interaction in liquid medium. Arch. Microbiol. 2022, 204, 421. [Google Scholar] [CrossRef]
- Liu, C.G.; Green, S.I.; Min, L.; Clark, J.R.; Salazar, K.C.; Terwilliger, A.L.; Kaplan, H.B.; Trautner, B.W.; Ramig, R.F.; Maresso, A.W. Phage-Antibiotic Synergy Is Driven by a Unique Combination of Antibacterial Mechanism of Action and Stoichiometry. mBio 2020, 11, e01462-20. [Google Scholar]
- Young, R. Bacteriophage lysis: Mechanism and regulation. Microbiol. Rev. 1992, 56, 430–481. [Google Scholar] [CrossRef] [PubMed]
- Schmelcher, M.; Loessner, M.J. Bacteriophage endolysins-extending their application to tissues and the bloodstream. Curr. Opin. Biotechnol. 2021, 68, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Blasco, L.; Ambroa, A.; Trastoy, R.; Bleriot, I.; Moscoso, M.; Fernández-Garcia, L.; Perez-Nadales, E.; Fernández-Cuenca, F.; Torre-Cisneros, J.; Oteo-Iglesias, J.; et al. In vitro and in vivo efficacy of combinations of colistin and different endolysins against clinical strains of multi-drug resistant pathogens. Sci. Rep. 2020, 10, 7163. [Google Scholar] [CrossRef]
- Abdelrahman, F.; Easwaran, M.; Daramola, O.I.; Ragab, S.; Lynch, S.; Oduselu, T.J.; Khan, F.M.; Ayobami, A.; Adnan, F.; Torrents, E.; et al. Phage-Encoded Endolysins. Antibiotics 2021, 10, 124. [Google Scholar] [CrossRef]
- Rahman, M.U.; Wang, W.; Sun, Q.; Shah, J.A.; Li, C.; Sun, Y.; Li, Y.; Zhang, B.; Chen, W.; Wang, S. Endolysin, a Promising Solution against Antimicrobial Resistance. Antibiotics 2021, 10, 1277. [Google Scholar] [CrossRef] [PubMed]
- Plotka, M.; Kapusta, M.; Dorawa, S.; Kaczorowska, A.-K.; Kaczorowski, T. Ts2631 Endolysin from the Extremophilic Thermus scotoductus Bacteriophage vB_Tsc2631 as an Antimicrobial Agent against Gram-Negative Multidrug-Resistant Bacteria. Viruses 2019, 11, 657. [Google Scholar] [CrossRef] [PubMed]
- Antonova, N.P.; Vasina, D.V.; Lendel, A.M.; Usachev, E.V.; Makarov, V.V.; Gintsburg, A.L.; Tkachuk, A.P.; Gushchin, V.A. Broad Bactericidal Activity of the Myoviridae Bacteriophage Lysins LysAm24, LysECD7, and LysSi3 against Gram-Negative ESKAPE Pathogens. Viruses 2019, 11, 284. [Google Scholar] [CrossRef] [PubMed]
- Vasina, D.V.; Antonova, N.P.; Grigoriev, I.V.; Yakimakha, V.S.; Lendel, A.M.; Nikiforova, M.A.; Pochtovyi, A.A.; Remizov, T.A.; Usachev, E.V.; Shevlyagina, N.V.; et al. Discovering the Potentials of Four Phage Endolysins to Combat Gram-Negative Infections. Front. Microbiol. 2021, 12, 748718. [Google Scholar] [CrossRef] [PubMed]
- Lichtenberg, J.; Calvo, E.P.; Madsen, K.; Lund, T.; Birkved, F.K.; van Cauwenberghe, S.; Mourier, M.; Wulf-Andersen, L.; Jansman, A.; Lopez-Ulibarri, R. Safety evaluation of a novel muramidase for feed application. Regul. Toxicol. Pharmacol. 2017, 89, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Boroojeni, F.G.; Männer, K.; Rieger, J.; Calvo, E.P.; Zentek, J. Evaluation of a microbial muramidase supplementation on growth performance, apparent ileal digestibility, and intestinal histology of broiler chickens. Poult. Sci. 2019, 98, 2080–2086. [Google Scholar] [CrossRef] [PubMed]
- Sais, M.; Barroeta, A.C.; López-Colom, P.; Nofrarías, M.; Majó, N.; Lopez-Ulibarri, R.; Calvo, E.P.; Martín-Orúe, S.M. Evaluation of dietary supplementation of a novel microbial muramidase on gastrointestinal functionality and growth performance in broiler chickens. Poult. Sci. 2020, 99, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Salmond, G.P.C.; Fineran, P.C. A century of the phage: Past, present and future. Nat. Rev. Microbiol. 2015, 13, 777–786. [Google Scholar] [CrossRef]
- Pires, D.P.; Oliveira, H.; Melo, L.D.; Sillankorva, S.; Azeredo, J. Bacteriophage-encoded depolymerases: Their diversity and biotechnological applications. Appl. Microbiol. Biotechnol. 2016, 100, 2141–2151. [Google Scholar] [CrossRef]
- Latka, A.; Maciejewska, B.; Majkowska-Skrobek, G.; Briers, Y.; Drulis-Kawa, Z. Bacteriophage-encoded virion-associated enzymes to overcome the carbohydrate barriers during the infection process. Appl. Microbiol. Biotechnol. 2017, 101, 3103–3119. [Google Scholar] [CrossRef]
- Wang, C.; Li, P.; Zhu, Y.; Huang, Y.; Gao, M.; Yuan, X.; Niu, W.; Liu, H.; Fan, H.; Qin, Y.; et al. Identification of a Novel Acinetobacter baumannii Phage-Derived Depolymerase and Its Therapeutic Application in Mice. Front. Microbiol. 2020, 11, 1407. [Google Scholar] [CrossRef]
- Al Mahmud, S.; Roy, R.; Sugiokto, F.; Islam, N.; Lin, M.-D.; Lin, L.-C.; Lin, N.-T. Phage φAB6-Borne Depolymerase Combats Acinetobacter baumannii Biofilm Formation and Infection. Antibiotics 2021, 10, 279. [Google Scholar] [CrossRef]
- Maes, S.; Vackier, T.; Huu, S.N.; Heyndrickx, M.; Steenackers, H.; Sampers, I.; Raes, K.; Verplaetse, A.; De Reu, K. Occurrence and characterisation of biofilms in drinking water systems of broiler houses. BMC Microbiol. 2019, 19, 1–15. [Google Scholar] [CrossRef]
- Hirakawa, K.; Ota, K.; Hirayama, J.; Oikawa, S.; Kawanishi, S. Nile Blue Can Photosensitize DNA Damage through Electron Transfer. Chem. Res. Toxicol. 2014, 27, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Sommer, J.; Trautner, C.; Witte, A.K.; Fister, S.; Schoder, D.; Rossmanith, P.; Mester, P.-J. Don’t Shut the Stable Door after the Phage Has Bolted—The Importance of Bacteriophage Inactivation in Food Environments. Viruses 2019, 11, 468. [Google Scholar] [CrossRef] [PubMed]
- El-Gohary, F.A.; Huff, W.E.; Huff, G.R.; Rath, N.C.; Zhou, Z.Y.; Donoghue, A.M. Environmental augmentation with bacteriophage prevents colibacillosis in broiler chickens. Poult. Sci. 2014, 93, 2788–2792. [Google Scholar] [CrossRef] [PubMed]
- Penziner, S.; Schooley, R.T.; Pride, D.T. Animal Models of Phage Therapy. Front. Microbiol. 2021, 12, 631794. [Google Scholar] [CrossRef] [PubMed]
| Advantage | Limitations |
|---|---|
| Narrow antimicrobial spectrum [32] | There is no definite optimal dosage and/or administration plan. Adaptive anti-phage immunity may develop through multiple dosing [33] |
| Abundant in water, soil and other ecological environment [34] | Technical challenges accompany the preparation of phagocytic mixture in advance [35] |
| Fewer side-effects [36] | Can promote horizontal gene transfer through transduction, which may lead to the spread of drug resistance [37] |
| Low environmental impact [32] | Lack of reproducibility amongst results from different in vivo and in vitro studies [38] |
| Low impact on broader microbial communities [39] | The immune response of the body may affect phage activity [40] |
| Low phage characterization and isolation cost [41] | Stability and shelf life [42] |
| Effective against bacterial biofilms [43] | Convoluted rational design (pharmacodynamics/pharmacokinetics) [44] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Lin, Y.; Galgano, S.; Houdijk, J.; Xie, W.; Jin, Y.; Lin, J.; Song, W.; Fu, Y.; Li, X.; et al. Recent Progress in Phage Therapy to Modulate Multidrug-Resistant Acinetobacter baumannii, including in Human and Poultry. Antibiotics 2022, 11, 1406. https://doi.org/10.3390/antibiotics11101406
Zhang Y, Lin Y, Galgano S, Houdijk J, Xie W, Jin Y, Lin J, Song W, Fu Y, Li X, et al. Recent Progress in Phage Therapy to Modulate Multidrug-Resistant Acinetobacter baumannii, including in Human and Poultry. Antibiotics. 2022; 11(10):1406. https://doi.org/10.3390/antibiotics11101406
Chicago/Turabian StyleZhang, Yan, Yuanqing Lin, Salvatore Galgano, Jos Houdijk, Weiquan Xie, Yajie Jin, Jiameng Lin, Wuqiang Song, Yijuan Fu, Xiuying Li, and et al. 2022. "Recent Progress in Phage Therapy to Modulate Multidrug-Resistant Acinetobacter baumannii, including in Human and Poultry" Antibiotics 11, no. 10: 1406. https://doi.org/10.3390/antibiotics11101406
APA StyleZhang, Y., Lin, Y., Galgano, S., Houdijk, J., Xie, W., Jin, Y., Lin, J., Song, W., Fu, Y., Li, X., Chui, W., Kan, W., Jia, C., Hu, G., & Li, T. (2022). Recent Progress in Phage Therapy to Modulate Multidrug-Resistant Acinetobacter baumannii, including in Human and Poultry. Antibiotics, 11(10), 1406. https://doi.org/10.3390/antibiotics11101406






