Abstract
We aimed to describe the prevalence, risk factors, morbidity and mortality associated with the occurrence of bacteraemia during the postoperative ICU stay after lung transplantation (LT). We conducted a retrospective single-centre study that included all consecutive patients who underwent LT between January 2015 and October 2021. We analysed all the blood cultures drawn during the postoperative ICU stay, as well as samples from suspected infectious sources in case of bacteraemia. Forty-six bacteria were isolated from 45 bacteraemic patients in 33/303 (10.9%) patients during the postoperative ICU stay. Staphylococcus aureus (17.8%) was the most frequent bacteria, followed by Pseudomonas aeruginosa (15.6%) and Enterococcus faecium (15.6%). Multidrug-resistant bacteria accounted for 8/46 (17.8%) of the isolates. The most common source of bacteraemia was pneumonia (38.3%). No pre- or intraoperative risk factor for bacteraemia was identified. Recipients who experienced bacteraemia required more renal replacement therapy, invasive mechanical ventilation, norepinephrine support, tracheotomy and more days of hospitalization during the ICU stay. After adjustment for age, sex, type of LT procedure and the need for intraoperative ECMO, the occurrence of bacteraemia was associated with a higher mortality rate in the ICU (aOR = 3.55, 95% CI [1.56–8.08], p = 0.003). Bacteraemia is a major source of concern for lung transplant recipients.
1. Introduction
Lung transplantation (LT) is a life-saving therapy for patients with end-stage lung disease. Posttransplant infections account for 17% and 33% of the causes of death between 0 and 30 days and between 31 days and 1 year, respectively [1]. Bacteraemia, defined as the presence and detection of bacteria in the blood, is one of the top ten causes of death in the United States and Europe [2]. Although bacteraemia has been shown to increase morbidity and mortality among solid organ transplant recipients [3], it remains insufficiently described in LT [4,5,6].
During the postoperative stay in the intensive care unit (ICU), lung transplant patients receive high doses of immunosuppressive drugs, putting them at high risk for infectious complications [7], and are exposed to many other complications [8,9]. Therefore, we aim to describe the prevalence, risk factors, morbidity and mortality associated with the occurrence of bacteraemia during the posttransplant ICU stay, which have not yet been reported.
2. Results
Between January 2015 and October 2021, 303 patients underwent LT. Demographic data, underlying diseases and indications for LT are presented in Table 1.

Table 1.
Comparison of demographics and preoperative characteristics between lung transplant recipients with and without bacterial bloodstream infection.
2.1. Prevalence of Bacteraemia and Characteristics
Forty-six bacteria were isolated from forty-five cases of bacteraemia occurring in 33/303 (10.9%) patients during the postoperative ICU stay. The median (IQR) time from LT to the onset of bacteraemia was 8 (1–23) days. The distribution of first episodes of bacteraemia according to Gram stain and time from LT is presented in Figure 1.
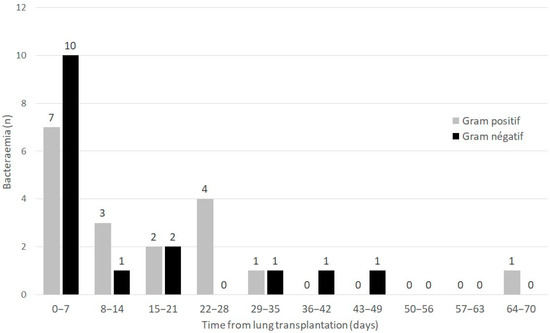
Figure 1.
Distribution of first episodes of bacteraemia according to Gram stain and time from lung transplantation.
Monomicrobial bacteraemia (n = 44) was observed in 32/33 (97.8%) patients (one patient had a mixed Staphylococcus aureus and Proteus mirabilis bacteraemia). Staphylococcus aureus (17.8%) was the most frequent bacteria, followed by Pseudomonas aeruginosa (15.6%) and Enterococcus faecium (15.6%). MDR bacteria accounted for 8/46 (17.8%) of the isolates, exclusively involving Gram-negative bacilli (GNB), with the exception of coagulase-negative staphylococci. MDR GNB included multidrug-resistant P. aeruginosa (resistance to ceftazidime, cefepime, ciprofloxacin, meropenem) (n = 1), extended-spectrum β-lactamase (ESBL)-producing Enterobacter cloacae (n = 1), ESBL-producing Klebsiella pneumonia (n = 3), ESBL-producing Escherichia coli (n = 2) and AmpC β-lactamase-overproducing Klebsiella aerogenes (n = 1). No methicillin-resistant S. aureus strains were isolated.
The most common source of bacteraemia was pneumonia (38.3%). However, the sources remained unknown in 31.2% of cases. Some cases of bacteraemia had multiple sources. One case due to K. pneumonia and one due to E. faecium both had two sources (pneumonia and pleural infection and chest wall infection and pleural infection, respectively). The microbiological characteristics are displayed in Table 2. One patient experienced a recurrence of bacteraemia during the posttransplant ICU stay. The first incidence of bacteraemia was identified as P. aeruginosa and was due to a chest wall infection 33 days after LT. A recurrence due to pleural infection occurred at day 68. Only one Candida-albicans-positive blood culture of unknown origin was observed during the postoperative ICU stay, drawn on ICU admission.

Table 2.
Bacteria identified in positive blood cultures stratified by the source.
2.2. Pre-Existing Risk Factors at ICU Admission Associated with the Occurrence of Bacteraemia during the Postoperative ICU Stay
No pre-existing risk factors at ICU admission were associated with the occurrence of bacteraemia during the postoperative ICU stay (Table 1).
2.3. ICU Morbidity Associated with the Occurrence of Bacteraemia
Recipients who experienced bacteraemia had higher SAPS II scores on postoperative ICU admission. In addition, during their ICU stays, they had higher acute kidney injury KDIGO scores, required more renal replacement therapy, invasive mechanical ventilation, norepinephrine support, tracheotomy and had longer ICU stays (Table 3).

Table 3.
Comparison of morbidity and mortality during the posttransplant ICU stay between patients with and without bacteraemia.
2.4. ICU and One-Year Mortality Rates Associated with the Occurrence of Bacteraemia
A total of 36.4% of recipients with postoperative bacteraemia died in the ICU compared to 12.6% for patients without bacteraemia (p < 0.001). After adjustment for age, sex, type of LT procedure and the need for intraoperative ECMO, the occurrence of bacteraemia was associated with a higher mortality rate in the ICU (aOR = 3.55, 95% CI (1.56–8.08), p = 0.003).
The 1-year survival was inferior for recipients who had bacteraemia occurring during the postoperative ICU stay (57.6%) compared to that of recipients without bacteraemia (77.4%). The Kaplan–Meier survival curve and log-rank test (p = 0.03) are shown in Figure 2. After adjustment for age, sex, type of LT and the need for ECMO, the difference in the incidence of bacteraemia did not reach statistical significance to independently decrease 1-year survival (HR = 1.70, 95% CI (0.93–3.09), p = 0.09).
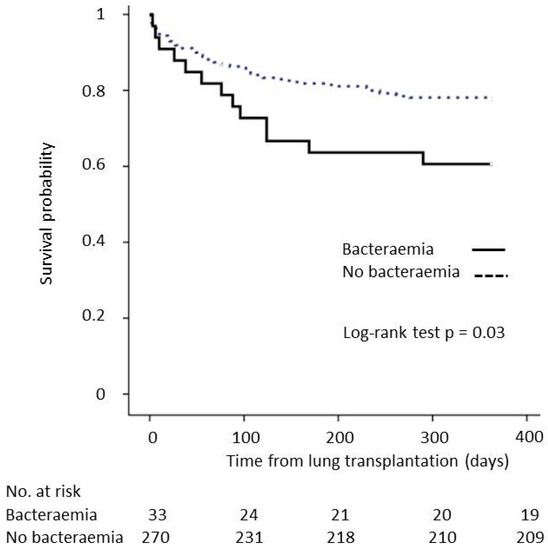
Figure 2.
Impact of bacteraemia occurring during the posttransplant ICU stay on 1-year survival.
2.5. Impact of the Adequacy of 48 h Perioperative ABX to Bacteraemia Occurring during the First Week after LT on ICU Morbidity and Mortality
Sixteen (16/33 = 48.5%) patients had bacteraemia within the first week of LT. Forty-eight-hour perioperative ABX had effective in vitro activity against the bacterial strains of bacteraemia occurring during the first week in 11 patients (amoxicillin/clavulanic acid (n = 6), cefazolin (n = 2), piperacillin/tazobactam (n = 2), cefepime (n = 1)) and was ineffective in 5 patients (amoxicillin/clavulanic acid (n = 2), cefazolin (n = 2), piperacillin/tazobactam + ciprofloxacin (n = 1)). Patients with and without effective 48 h perioperative ABX had similar rates of ICU mortality (60% vs. 27.3%, p = 0.30), length of ICU stay (26 (10–51) vs. 16 (7–69) days, p = 1), duration of invasive mechanical ventilation (25 (10–27) vs. 3 (1–28) days, p = 0.42) and duration of norepinephrine support (9 (7–10) vs. 2 (1–9) days, p = 0.30).
2.6. Comparison of ICU Morbidity and Mortality between Patients with Postoperative Bacteraemia Occurrence before 7 Days vs. after 7 Days
The rate of ICU mortality was similar between patients with postoperative bacteraemia occurrence before days vs. after 7 days (37.5% vs. 35.3%, p = 0.50). However, patients who developed bacteraemia early (before 7 days) had a longer duration of invasive mechanical ventilation (8 (1–31) vs. 38 (32–55) days, p = 0.01) and ICU length of stay (18 (7–60) vs. 55 (41–96) days, p = 0.01) than patients who developed bacteraemia after 7 days. The duration of norepinephrine support did not significantly differ between the groups (3 (1–10) vs. 4 (2–19) days, p = 0.4)
2.7. Comparison of ICU Morbidity and Mortality between Patients with Gram-Positive vs. Gram-Negative Postoperative Bacteraemia
Patients with Gram-positive bacteraemia had a similar rate of ICU mortality (27.8% vs. 43.8%, p = 0.15), duration of invasive mechanical ventilation (33 (13–49) vs. 32 (2–44) days, p = 0.65), norepinephrine support (6 (2–16) vs. 3 (1–15) days, p = 1) and ICU length of stay (45 (22–96) vs. 44 (22–89) days, p = 0.91) as patients with Gram-negative bacteraemia.
2.8. Impact of Multidrug-Resistant Isolates on ICU Mortality of Recipients with Bacteraemia
Patients with MDR bacteraemia had a similar rate of ICU mortality (37.5% vs. 36%, p = 1), duration of invasive mechanical ventilation (49 (35–55) vs. 22 (3–40) days, p = 0.06), norepinephrine support (7 (3–12) vs. 3 (1–14) days, p = 0.61) and ICU length of stay (95 (48–121) vs. 37 (16–56) days, p = 0.08) as patients with non-MDR bacteraemia.
3. Discussion
This study examined the largest cohort of lung transplant recipients studied for bacteraemia, on par with that of Husain et al. [4]. However, significant differences exist. Our cohort did not include patients with cystic fibrosis and primarily consisted of patients with COPD and ILD, and we focused on describing bacteraemia and its impact on postoperative ICU stay. Only three studies published more than 15 years ago have examined the impact of bacteraemia in LT [4,5,6]. These studies reported rates of bacteraemia ranging from 11.5% to 25%, higher than our observed rate of 10.5%. This difference could be explained by the absence of cystic fibrosis cases in our cohort, a risk factor for bacteraemia that has already been identified [5]. In addition, we focused only on bacteraemia occurring during the postoperative ICU stay.
Bacteraemia has been consistently associated with decreased survival in lung transplant recipients. Here, we showed that bacteraemia drastically increased early morbidity and mortality during the postoperative ICU stay and could impact 1-year survival. As in critically ill nontransplant patients in whom bacteraemia is associated with organ failure and mortality [10,11], this complication could echo the severity of a patient’s condition. In the setting of LT, recipients are exposed to major risk factors for bacteraemia, namely ICU hospitalization, immunosuppression, nosocomial pneumonia and the need for mechanical ventilation. The rate of bacteraemia observed here was, consequently, two to five times more frequent than in nontransplant patients [10,11]. The high mortality associated with bacteraemia may be related to the fact that one-third of the bacteraemia incidents had an unknown source, which may make it more difficult to treat. In addition, bacteraemia has previously been shown to be an independent risk factor for mortality in nosocomial pneumonia [12], which was the most common source in our cohort.
In terms of public health, patients with bacteraemia required more invasive mechanical ventilation, renal replacement therapy and a longer length of ICU stay, which are very costly. Nosocomial bloodstream infections in critically ill patients were already shown to cause a significant economic burden [11].
Bacteraemia was most commonly due to S. aureus, P. aeruginosa, and E. faecium, the latter being found at a somewhat surprising level compared with those reported in previous studies [4,5,6]. However, in two-thirds of the cases, the source was not identified. MDR isolates were found only among GNB and predominantly involved ESBL-producing Enterobacterales. Neither MDR nor GNB were associated with higher mortality in recipients with bacteraemia. The rate of 17.8% MDR documented in the isolates was lower than the 48% rate reported by Husain et al. However, in their study, MDR involved all the Burkholderia cepacia isolates and 75% of the S. aureus strains, whereas in our cohort, B. cepacia was never found, as cystic fibrosis was not included. Furthermore, methicillin-resistant Staphylococcus aureus was not isolated, which is consistent with its higher prevalence in the US than in Europe [13,14,15].
Bacteraemia is likely a surrogate marker of severity, but unfortunately, we were unable to determine any preexisting factors at postoperative ICU admission that were associated with the occurrence of bacteraemia to identify a subpopulation of at-risk patients. Fifty percent of the bacteraemia cases occured early, within 8 postoperative days, despite the absence of induction therapy by T-cell depletion, which has been identified as a risk factor [4]. Cystic fibrosis and mechanical ventilation before LT have also been identified as risk factors for bacteraemia [5]. However, we could not evaluate these variables because we did not perform LT under these conditions. The lack of impact of adequate antibiotic prophylaxis on the occurrence of bacteraemia in the first week after LT was particularly disappointing. This lack of effect may be related to the fact that curative antibiotic therapy initiated upon the detection of bacteraemia was sufficient to treat it effectively, which was reinforced by the observation of a single recurrent bacteraemia related to pleural infection after chest wall infection. The emergence of new rapid molecular and phenotypic tests for the identification and susceptibility profile of positive blood cultures could further reduce the time to effective treatment and the misuse of antibiotics [16,17,18]. Additional biomarkers could also be of interest, such as procalcitonin, for the early detection of a quick ascent of an inflammation surrogate that should decrease after surgery [19]. In the future, point-of-care diagnostics could also be expected to accelerate the therapeutic process.
This study had some limitations related to its retrospective and monocentric nature with a limited number of cases of bacteraemia, which calls for great caution in interpreting the data and generalizing the results.
4. Materials and Methods
4.1. Study Design
We conducted a retrospective single-centre study that included all consecutive patients who underwent LT between January 2015 and October 2021. Retransplantations and ex vivo lung perfusion procedures were not included.
The study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of CEERB Paris Nord, which waived the need for signed informed consent (Institutional Review Board -IRB 00006477- Université Paris Cité, AP-HP.Nord).
We analysed all blood cultures drawn during the postoperative ICU stay, as well as samples from potential infectious sources in case of bacteraemia, defined as sites suspected of being infected by and responsible for bacteraemia.
The primary objectives were to describe (i) the prevalence of bacteraemia in the postoperative ICU stay, (ii) the bacterial species involved and their antibiotic susceptibility profiles, (iii) the potential pre- and perioperative risk factors for bacteraemia and (iv) the consequences of bacteraemia in terms of outcome (ICU morbidity and mortality).
As exploratory outcomes, we assessed (i) the impact of the adequacy of 48 h perioperative antibiotic prophylaxis (ABX) on bacteraemia occurring during the first week after LT morbidity and mortality in the ICU, (ii) the comparison of morbidity and mortality in the ICU between patients with postoperative bacteraemia occurrence before 7 days vs. after 7 days, (iii) the comparison of morbidity and mortality in the ICU between patients with Gram-positive vs. Gram-negative postoperative bacteraemia and (iv) the impact of multidrug-resistant (MDR) bacteria on morbidity and mortality in the ICU for recipients with bacteraemia.
4.2. Microbiological Features and Definitions
Each blood culture set included two bottles (aerobic and anaerobic). Ten millilitres of blood was taken under sterile conditions and injected into each bottle. All bottles were handled using a BD BACTEC™ blood culture system (Becton-Dickinson), which gave an automatic computerized alert when bacterial growth was detected and recorded the time elapsed between the introduction of the bottle in the apparatus and the alert signal. Bottles were incubated for 5 days at 35 °C (or extended to 8 days if infective endocarditis was suspected).
The diagnostic procedures conducted for all positive bottles included Gram staining, bacterial isolation using standard bacteriologic techniques and bacterial identification at the species level with a matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) Microflex LT Biotyper (Bruker Daltonics, Bremen, Germany). Bacterial susceptibility to antibiotics was determined using the disk-diffusion method according to EUCAST [20,21].
Bacteraemia was defined as the isolation of bacteria from a blood culture in the presence of clinical signs of infection, according to the Centers for Disease Control and Prevention criteria (central-line-associated bloodstream infection (CLABSI) and noncentral-line-associated bloodstream infection) [22].
For the usual bacteria considered to be skin contaminants (coagulase-negative staphylococci (CNS), Bacillus spp., Corynebacterium spp. or Cutibacterium acnes), bacteraemia was considered if more than two positive blood cultures were identified in a 3-day period [23].
The source of the bacteraemia (pneumonia, pleural infection, chest wall infection, intra-abdominal infection or other) was defined as a site sampled with a positive microbiological culture, according to the infection threshold where applicable, with the same bacterial species and the same antibiotic susceptibility as those isolated from the blood culture. If no source was identified, the bacteraemia was classified as being of an unknown source.
Recurrent bacteraemia was defined as the occurrence of a subsequent episode of the same microorganism with the same antibiotic susceptibility profile in a blood culture during the study period at least one week after the resolution of a previous episode (based on the resolution of symptoms and subsequent negative blood cultures with an appropriate duration of antibiotic therapy and appropriate source control, if indicated) [24].
Patients with bacteraemia were treated with appropriate antibiotic therapy, i.e., bacteria were susceptible according to the antibiotic susceptibility testing, for a duration depending on the infectious source, combined with source control if indicated: 7 days for pneumonia, 21 days for pleural infection, 7 days for catheter-related infection, 7 days for intra-abdominal infection, 7 days for chest wall infection and 6 weeks for infective endocarditis.
4.3. Data Collection
We collected:
- All positive blood cultures drawn during the posttransplant ICU stay; the time from LT to onset of bacteraemia, the type of bacterial species and the presence of MDR profiles were recorded [25].
- The demographic and pre-existing characteristics of patients before postoperative ICU admission, including the following: age, sex, body mass index (BMI), primary diagnosis of chronic pulmonary disease, cytomegalovirus mismatch (recipient/donor+), past medical history of diabetes and revascularized ischaemic heart disease, high-emergency LT, extracorporeal membrane oxygenation (ECMO) as a bridge to transplant and mean pulmonary arterial pressure (mPAP) measured by a right-heart catheterization at listing. High-emergency LT is a national prioritization system for the most severe patients with fibrosis, cystic fibrosis or pulmonary hypertension that was introduced in France in 2007 [26].
- Intraoperative characteristics: type of LT (i.e., single or bilateral), maximum graft cold ischaemic time, intraoperative blood transfusion of more than three packed red blood cells (PRBC) and intraoperative ECMO.
- Postoperative outcomes in ICU: simplified acute physiology score II (SAPS II) and sequential organ failure assessment (SOFA) score at ICU admission, acute kidney injury stage 3 of KDIGO (Kidney Disease: Improving Global Outcomes), renal replacement therapy, duration of mechanical ventilation, duration of norepinephrine support, ECMO in ICU, tracheotomy, ICU length of stay and mortality rates at day 30 and 1 year.
4.4. Perioperative Management
Surgical transplantation procedures and perioperative care, including postoperative management, were standardized for all patients according to our local protocol already published elsewhere [19]. The immunosuppressive regimen included mycophenolate mofetil, corticosteroids and tacrolimus. Induction therapy was not performed.
Perioperative antibiotic prophylaxis (ABX) was defined by the antibiotic regimen started intraoperatively. The standard perioperative ABX was cefazolin, as recommended in “Clinical practice guidelines for antimicrobial prophylaxis in surgery” [27]. However, ABX was modified for 1) antibiotic therapy given to the donor before lung harvesting and 2) adapted antibiotic therapy in cases of bronchoalveolar colonization ≤ 3 months documented with cefazolin-resistant bacteria. ABX was started intraoperatively and continued for 48 h after surgery, as recommended [28]. Antibiotic therapy was adapted to microbiological cultures obtained from bronchoalveolar lavage (BAL) performed on postoperative ICU admission. If a BAL culture was negative without evidence of infection, ABX was stopped after 48 h.
4.5. Statistical Analysis
The baseline characteristics within each group were described with numbers and percentages for qualitative variables and with medians and interquartile ranges for quantitative variables.
Univariate logistic regression and a calculation of unadjusted odds ratios (ORs) and 95% CIs were used to assess (1) risk factors pre-existing to ICU admission and associated with bacteraemia, (2) postoperative outcomes in the ICU associated with bacteraemia, (3) the impact of the adequacy of 48 h perioperative ABX to bacteraemia occurring during the first week after LT on morbidity and mortality in the ICU, (4) the comparison of morbidity and mortality in the ICU between patients with postoperative bacteraemia occurrence before 7 days vs. after 7 days, (5) the comparison of morbidity and mortality in the ICU between patients with Gram-positive vs. Gram-negative postoperative bacteraemia and (6) the impact of multidrug resistance on morbidity and mortality in the ICU for bacteraemic recipients.
The impact of bacteraemia on ICU mortality was assessed by multivariate logistic regression adjusted for age, sex, type of LT procedure and intraoperative ECMO to calculate adjusted OR (aOR) and 95% CI.
To analyse the impact of bacteraemia on 1-year survival, the probability of all-cause death was estimated using the Kaplan–Meier method and compared with the log-rank test. The risk of 1-year all-cause mortality was estimated before and after adjustment using the Cox proportional hazards model. We selected clinically relevant covariates analysed in a univariate regression to fit the multivariate method. The results were expressed as hazard ratios (HRs) and 95% confidence intervals (95% CIs).
All reported p-values were two-sided, and the level of statistical significance was specified a priori as less than 0.05. Statistical analysis and data management were performed using BM SPSS Statistics version 20 (IBM Corp., Armonk, NY, USA)
5. Conclusions
Bacteraemia is a major source of concern for lung transplant recipients that has been reported in more than 10% of these patients during the postoperative ICU stay and decreases their survival both in the ICU and at 1 year. As no risk factors have been identified, all recipients should be considered at risk. Future studies seem essential to evaluate the efficacy of the early detection of bacteraemia, including studies with rapid microbiologic diagnostic tests or biomarkers.
Author Contributions
Conceptualization, A.T.-D., B.L.-J., Y.C. and P.M. (Philippe Montravers); methodology, A.T.-D., B.L.-J., E.A., H.M., J.M. and P.M. (Philippe Montravers); validation, A.T.-D., E.A., N.Z., Y.C. and H.M.; formal analysis, A.T.-D., S.T., E.A., N.G. and P.M. (Philippe Montravers); investigation, A.T.-D., M.G., S.T., N.Z., P.M. (Pierre Mordant) and G.W.; data curation, A.T.-D. and N.G.; writing—original draft preparation, A.T.-D., M.G., S.T., N.G. and P.M. (Philippe Montravers); writing—review and editing, A.T.-D., P.M. (Pierre Mordant), Y.C., H.M., G.W., J.M. and P.M. (Philippe Montravers); project administration B.L.-J., N.Z. and P.M. (Philippe Montravers). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of CEERB Paris Nord, which waived the need for signed informed consent (Institutional Review Board -IRB 00006477- Université Paris Cité, AP-HP.Nord).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We warmly thank the list of investigators: Service d’Anesthésie-Réanimation: Dan Longrois, Alexandre Mignon, Aurélie Snauwaert, Parvine Tashk, Maksud Assadi, Jules Stern, Sacha Rozencwajg, Adnan El Kalai, Christian de Tymowski, Ali Jendoubi, Aurélie Gouel, Fabien Lion, Laura Soldan, Adela Harpan, Marie-Pierre Dilly, Yassine Rkik, Atanas Sabahov, Claire Depont, Elie Kantor, Laetitia Desplanque, Nils Carrara, Sonia Yung, Morgan Roue, Sophie Provenchère, Julia Voulgaropoulos, Alexandra Younes, Charles Moulin, Bozena Wachoswka, Corentin Gouezel, Elie Succar, Mohamed Foufa, Laila Guezouli, Lea Copelovici, Iulia Balcan, Jose Luis Carrasco, Julien Do vale, Lucie Mariani, Hadrien Portefaix, VincentMellano, Emmanuelle Busch. Service de Pneumologie B et Transplantation Pulmonaire: Cendrine Godet, Gaelle Weisenburger, Tiphaine Goletto, Chahine Medraoui, Gilles Jebrak, Armelle Marceau, Domitille Mouren, Mathilde Salpin, Charlotte Thibaut de Menonville, Alice Savary, Malika Hammouda, Lucie Genet, Gwenn Frère, Laurie Torus, Agnès Abadie, Diego Ferreira, Sandrine Tissot, Linda Hajouji-Idrissi, Zohra Brouk. Service de Chirurgie Vasculaire, Thoracique et Transplantation pulmonaire: Arnaud Roussel, Quentin Pellenc, Jean Senemaud, Pierre Cerceau, Regis Renard, Paul Labed, Iannis Ben Abdallah.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chambers, D.C.; Cherikh, W.S.; Harhay, M.O.; Hayes, D.; Hsich, E.; Khush, K.K.; Meiser, B.; Potena, L.; Rossano, J.W.; Toll, A.E.; et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-Sixth Adult Lung and Heart-Lung Transplantation Report-2019; Focus Theme: Donor and Recipient Size Match. J. Heart Lung Transplant. 2019, 38, 1042–1055. [Google Scholar] [CrossRef]
- Goto, M.; Al-Hasan, M.N. Overall Burden of Bloodstream Infection and Nosocomial Bloodstream Infection in North America and Europe. Clin. Microbiol. Infect. 2013, 19, 501–509. [Google Scholar] [CrossRef]
- Kritikos, A.; Manuel, O. Bloodstream Infections after Solid-Organ Transplantation. Virulence 2016, 7, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Husain, S.; Chan, K.M.; Palmer, S.M.; Hadjiliadis, D.; Humar, A.; McCurry, K.R.; Wagener, M.M.; Singh, N. Bacteremia in Lung Transplant Recipients in the Current Era. Am. J. Transplant. 2006, 6, 3000–3007. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.M.; Alexander, B.D.; Sanders, L.L.; Edwards, L.J.; Reller, L.B.; Davis, R.D.; Tapson, V.F. Significance of Blood Stream Infection after Lung Transplantation: Analysis in 176 Consecutive Patients. Transplantation 2000, 69, 2360–2366. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.; Cervera, C.; Gavaldá, J.; Rovira, M.; de la Cámara, R.; Jarque, I.; Montejo, M.; de la Torre-Cisneros, J.; Miguel Cisneros, J.; Fortún, J.; et al. Bloodstream Infections among Transplant Recipients: Results of a Nationwide Surveillance in Spain. Am. J. Transplant. 2007, 7, 2579–2586. [Google Scholar] [CrossRef]
- Nosotti, M.; Tarsia, P.; Morlacchi, L.C. Infections after Lung Transplantation. J. Thorac. Dis. 2018, 10, 3849–3868. [Google Scholar] [CrossRef]
- Snell, G.I.; Yusen, R.D.; Weill, D.; Strueber, M.; Garrity, E.; Reed, A.; Pelaez, A.; Whelan, T.P.; Perch, M.; Bag, R.; et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction, Part I: Definition and Grading—A 2016 Consensus Group Statement of the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2017, 36, 1097–1103. [Google Scholar] [CrossRef]
- Crespo, M.M.; McCarthy, D.P.; Hopkins, P.M.; Clark, S.C.; Budev, M.; Bermudez, C.A.; Benden, C.; Eghtesady, P.; Lease, E.D.; Leard, L.; et al. ISHLT Consensus Statement on Adult and Pediatric Airway Complications after Lung Transplantation: Definitions, Grading System, and Therapeutics. J. Heart Lung Transplant. 2018, 37, 548–563. [Google Scholar] [CrossRef]
- Prowle, J.R.; Echeverri, J.E.; Ligabo, E.V.; Sherry, N.; Taori, G.C.; Crozier, T.M.; Hart, G.K.; Korman, T.M.; Mayall, B.C.; Johnson, P.D.R.; et al. Acquired Bloodstream Infection in the Intensive Care Unit: Incidence and Attributable Mortality. Crit. Care 2011, 15, R100. [Google Scholar] [CrossRef]
- Pittet, D.; Tarara, D.; Wenzel, R.P. Nosocomial Bloodstream Infection in Critically Ill Patients. Excess Length of Stay, Extra Costs, and Attributable Mortality. JAMA 1994, 271, 1598–1601. [Google Scholar] [CrossRef] [PubMed]
- Magret, M.; Lisboa, T.; Martin-Loeches, I.; Máñez, R.; Nauwynck, M.; Wrigge, H.; Cardellino, S.; Díaz, E.; Koulenti, D.; Rello, J.; et al. Bacteremia Is an Independent Risk Factor for Mortality in Nosocomial Pneumonia: A Prospective and Observational Multicenter Study. Crit. Care 2011, 15, R62. [Google Scholar] [CrossRef] [PubMed]
- de Kraker, M.E.A.; Jarlier, V.; Monen, J.C.M.; Heuer, O.E.; van de Sande, N.; Grundmann, H. The Changing Epidemiology of Bacteraemias in Europe: Trends from the European Antimicrobial Resistance Surveillance System. Clin. Microbiol. Infect. 2013, 19, 860–868. [Google Scholar] [CrossRef]
- Dantes, R.; Mu, Y.; Belflower, R.; Aragon, D.; Dumyati, G.; Harrison, L.H.; Lessa, F.C.; Lynfield, R.; Nadle, J.; Petit, S.; et al. National Burden of Invasive Methicillin-Resistant Staphylococcus Aureus Infections, United States, 2011. JAMA Intern. Med. 2013, 173, 1970–1978. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.Y.; Mojica, N.; Jiang, W.; Cosgrove, S.E.; Septimus, E.; Morgan, D.J.; Laxminarayan, R. Trends in Methicillin-Resistant Staphylococcus Aureus Hospitalizations in the United States, 2010–2014. Clin. Infect. Dis. 2017, 65, 1921–1923. [Google Scholar] [CrossRef]
- Giacobbe, D.R.; Giani, T.; Bassetti, M.; Marchese, A.; Viscoli, C.; Rossolini, G.M. Rapid Microbiological Tests for Bloodstream Infections Due to Multidrug Resistant Gram-Negative Bacteria: Therapeutic Implications. Clin. Microbiol. Infect. 2020, 26, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Salimnia, H.; Fairfax, M.R.; Lephart, P.R.; Schreckenberger, P.; DesJarlais, S.M.; Johnson, J.K.; Robinson, G.; Carroll, K.C.; Greer, A.; Morgan, M.; et al. Evaluation of the FilmArray Blood Culture Identification Panel: Results of a Multicenter Controlled Trial. J. Clin. Microbiol. 2016, 54, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Komarow, L.; Virk, A.; Rajapakse, N.; Schuetz, A.N.; Dylla, B.; Earley, M.; Lok, J.; Kohner, P.; Ihde, S.; et al. Randomized Trial Evaluating Clinical Impact of RAPid IDentification and Susceptibility Testing for Gram-Negative Bacteremia: RAPIDS-GN. Clin. Infect. Dis. 2021, 73, e39–e46. [Google Scholar] [CrossRef]
- Desmard, M.; Benbara, A.; Boudinet, S.; Mal, H.; Dehoux, M.; Thabut, G.; Montravers, P. Post-Operative Kinetics of Procalcitonin after Lung Transplantation. J. Heart Lung Transplant. 2015, 34, 189–194. [Google Scholar] [CrossRef]
- EUCAST: Disk Diffusion Methodology. Available online: https://www.eucast.org/ast_of_bacteria/disk_diffusion_methodology (accessed on 12 September 2022).
- Gajic, I.; Kabic, J.; Kekic, D.; Jovicevic, M.; Milenkovic, M.; Mitic Culafic, D.; Trudic, A.; Ranin, L.; Opavski, N. Antimicrobial Susceptibility Testing: A Comprehensive Review of Currently Used Methods. Antibiotics 2022, 11, 427. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention Bloodstream Infection Event (Central Line-Associated Bloodstream Infection and Non-Central Line Associated Bloodstream Infection), National Healthcare Safety Network, January 2022. Available online: https://www.cdc.gov/nhsn/pdfs/pscmanual/4psc_clabscurrent.pdf (accessed on 12 September 2022).
- Weinstein, M.P.; Towns, M.L.; Quartey, S.M.; Mirrett, S.; Reimer, L.G.; Parmigiani, G.; Reller, L.B. The Clinical Significance of Positive Blood Cultures in the 1990s: A Prospective Comprehensive Evaluation of the Microbiology, Epidemiology, and Outcome of Bacteremia and Fungemia in Adults. Clin. Infect. Dis. 1997, 24, 584–602. [Google Scholar] [CrossRef] [PubMed]
- Al-Hasan, M.N.; Eckel-Passow, J.E.; Baddour, L.M. Recurrent Gram-Negative Bloodstream Infection: A 10-Year Population-Based Cohort Study. J. Infect. 2010, 61, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Boussaud, V.; Mal, H.; Trinquart, L.; Thabut, G.; Danner-Boucher, I.; Dromer, C.; Raymond, C.S.; Reynaud-Gaubert, M.; Kessler, R.; Philit, F.; et al. One-Year Experience With High-Emergency Lung Transplantation in France. Transplantation 2012, 93, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
- Bratzler, D.W.; Dellinger, E.P.; Olsen, K.M.; Perl, T.M.; Auwaerter, P.G.; Bolon, M.K.; Fish, D.N.; Napolitano, L.M.; Sawyer, R.G.; Slain, D.; et al. Clinical Practice Guidelines for Antimicrobial Prophylaxis in Surgery. Am. J. Health-Syst. Pharm. 2013, 70, 195–283. [Google Scholar] [CrossRef]
- Abbo, L.M.; Grossi, P.A.; The AST ID Community of Practice. Surgical Site Infections: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13589. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).