Abstract
Before the emergence of plasmid-mediated colistin resistance, colistin was once considered the last drug of choice for infections caused by carbapenem-resistant bacteria. Currently, researchers are relentlessly exploring possible alternative therapies that could efficiently curb the spread of drug resistance. In this study, we aim to investigate the synergistic antibacterial activity of tetrandrine in combination with colistin against mcr-1-harboring Escherichia coli. We examined the antibacterial activity of tetrandrine in combination with colistin in vivo and in vitro and examined the bacterial cells by fluorescence, scanning, and transmission electron microscopy (TEM) to explore their underlying mechanism of action. We further performed a computational analysis of MCR-1 protein and tetrandrine to determine the interaction interface of these two molecules. We confirmed that neither colistin nor tetrandrine could, on their own, inhibit the growth of mcr-1-positive E. coli. However, in combination, tetrandrine synergistically enhanced colistin activity to inhibit the growth of E. coli both in vivo and in vitro. Similarly, molecular docking showed that tetrandrine interacted with the three crucial amino acids of the MCR-1 protein in the active site, which might inhibit MCR-1 from binding to its substrates, cause MCR-1 to lose its ability to confer resistance. This study confirmed that tetrandrine and colistin have the ability to synergistically overcome the issue of colistin resistance in mcr-1-harboring E. coli.
1. Introduction
Escherichia coli is a gram-negative bacterium that causes infections in humans, poultry, and animals. In typical cases, antibiotics can kill or inhibit the growth of E. coli. However, after acquiring antibiotic-resistance genes, their treatment with routinely used antibiotics becomes difficult. Different resistance mechanisms have been investigated in E. coli [1,2,3,4], among which extended-spectrum β-lactamase (ESBL) production due to various genes, such as blaCTX-M, blaTEM, and blaSHV, has been most studied [5,6]. In such cases, the carbapenems were considered the best therapeutic options prior to the development of resistance, due to improper use, by acquiring blaNDM, blaKPC, blaOXA, and many other resistance genes [7,8,9]. As a result, colistin became one of the last-resort antibiotics against these superbugs. Initially, only chromosomal-based resistance was developed against colistin. Nonetheless, it was not much of a threat, because it was only transmitted vertically [10]. However, in late 2015, the first plasmid-mediated colistin resistance gene, mobilized colistin-resistance (mcr-1), was detected in China in pigs and humans; this initiated the post-antibiotic era, because all available antibiotic options were countered by bacterial resistance mechanisms [11]. Nowadays, carbapenem-resistant Enterobacterales harboring mcr have been reported worldwide [12,13,14]; therefore, colistin is alternatively used to cure those infections that lead to increases of the colistin resistance [15,16].
Researchers from various regions around the world have been investigating many alternative ways to cure such superbugs, and bacteriophage therapy, nano-medicines, and herb extracts have shown good activity. Colistin was also considered to be one of the last-resort treatments used to treat infections caused by multidrug-resistant bacteria [17]. Due to the narrow therapeutic action and nephrotoxicity of polymyxin, several recent studies have used a combination of colistin with other existing antibiotics and herbal medicines to find alternative therapies to eradicate colistin resistance and slow down its dissemination [18,19,20,21]. Some recent studies found that drug-combination therapies could be successful by using drug-repurposing strategies [22,23]. Among these, approaches using extracts of natural herbs are easy and have fewer side effects [24]. The alkaloid extract of Stephania tetrandra plant named “tetrandrine”, with the chemical structure “6,6,7,12-tetra methoxy-2,2” [25], is one of the herbal extracts that have many beneficial aspects against tumors, Candida mycosis, heart arrhythmia, and blood pressure.
The synergistic antibacterial activity of tetrandrine with other drugs has already been reported against Staphylococcus aureus, Mycobacterium smegmatis, and Salmonella species [26]. It has been reported that tetrandrine has an inhibitory effect on the efflux pump of bacteria, which helps in the absorption and retention of drugs inside the bacterial cells and increases their antibacterial activity [27]. In the present study, we investigate its effect in combination with colistin against the plasmid-mediated mcr-1-harboring, colistin-resistant E. coli isolated from the feces of healthy livestock in Pakistan. Their synergism was determined by checkerboard assay, and further analyses of its activity were carried out through electron microscopy. The findings of this study could provide a baseline for further research work in order to establish an alternative therapeutic option for treating colistin-resistant E. coli infections.
2. Results
2.1. Antibacterial Activity
The antibacterial activity of colistin and tetrandrine, alone and in combination, was determined. All mcr-1-positive isolates had a MIC ≥ 2 µg/mL and showed resistance to colistin. Similarly, the MIC of tetrandrine was found in the range of 320 to 1280 µg/mL, indicating that tetrandrine alone is not a suitable inhibitor of the growth of mcr-1-positive isolates. Interestingly, when colistin and tetrandrine were combined in the checkerboard assay, the FICI values were recorded as <0.5 for all 20 tested mcr-1-positive isolates, indicating a synergistic activity between tetrandrine and colistin. Similarly,the MIC concentrations of tetrandrine and colistin were applied against the tested isolates. We found a ≥ 4- to ≥ 256-fold increase in the MIC for colistin (Table 1). Similarly, the time-kill assay showed that neither colistin nor tetrandrine alone reduced the growth of E. coli, but, with combination treatment, a dramatic reduction in bacterial growth was recorded (Figure 1). Similar results were observed with fluorescence microscopy (Figure 2). These results suggest that tetrandrine enhanced the activity of colistin and inhibited the growth of mcr-1-harboring, colistin-resistant E. coli.

Table 1.
Susceptibility values of colistin and tetrandrine alone and in combination. The table represents the MIC, FICI, and fold change of colistin MIC values in a total of 20 tested E. coli strains.
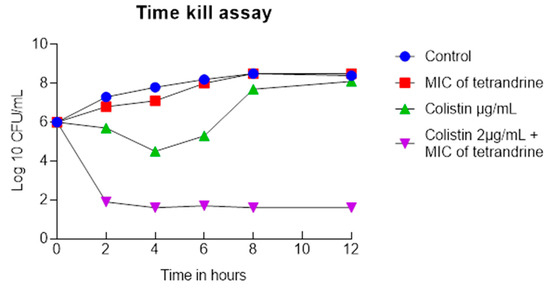
Figure 1.
Colistin and tetrandrine in combination inhibits the growth of colistin-resistant E. coli. Time kill assay of colistin and tetrandrine alone and in combination shows that neither colistin nor tetrandrine inhibits the growth of E. coli. In contrast, the growth of E. coli was significantly reduced when treated with a combination of colistin and tetrandrine.
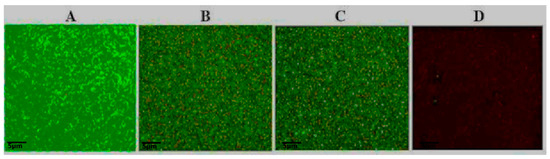
Figure 2.
Colistin and tetrandrine, in combination, kill colistin-resistant E. coli. Fluorescence microscopic observation of (A) control: cells growing in the absence of colistin and tetrandrine. (B) Colistin alone does not inhibit the growth of E. coli, (C) tetrandrine alone does not inhibit the growth of E. coli, and (D) cells treated with colistin plus tetrandrine in combination show numerous dead cells.
2.2. Scanning Electron Microscopic Observation
The SEM observation was performed to examine the morphological and membrane-structure modification of mcr-1-harboring E. coli to explore the synergistic antibacterial activity of colistin and tetrandrine. Bacteria were incubated for 4 h with colistin (2 µg/mL) and tetrandrine (MIC value), alone and in combination. Micrographs show untreated E. coli (Figure 3A), E. coli treated with colistin alone (Figure 3B), and E. coli treated with tetrandrine alone (Figure 3C). The untreated cells and cells treated with colistin or tetrandrine alone had an unvarying rod shape with an intact membrane, smooth surface, and no visible morphological modifications. On the other hand, multiple dents, holes, and deep hollows were seen in the colistin-plus-tetrandrine-treated bacteria (Figure 3D). Many protruding and distorted cells, and some permanently lysed cells, were observed in the SEM images.

Figure 3.
Colistin and tetrandrine in combination causes membrane damage and kills colistin-resistant E. coli. Scanning electron microscopic observation of (A) control untreated cells showing normal E. coli morphology, (B) cells treated with colistin alone, (C) cells treated with tetrandrine, and (D) cells treated with a combination of colistin and tetrandrine showing rupture of outer membrane and leakage of cytoplasmic contents.
2.3. Transmission Electron Microscopy Observations
E. coli cells were examined by TEM to characterize the morphological alternations resulting from colistin-plus-tetrandrine treatment. Figure 4A–C reveal a stable structure with a smooth appearance, well-defined intact membranes, and relatively uniform electron density in the cytosol. On the other hand, a destabilized cell wall with a rough surface was observed when bacterial cells were treated with a combination of colistin and tetrandrine (Figure 4D). Furthermore, substantial alterations in shape and integrity or increased permeability, resulting in leaking of cytoplasmic material, were detected. The periplasmic space was distended and occupied by electron-dense material from the cytosol. Several vacuole-like structures were noted where electron density was abridged. There was also a considerable electron-dense region near the transparent regions. Furthermore, some additional membranous structures in the region of cells were found, and the detachment of the inner membrane from the outer membrane was also observed. These morphological alterations reveal the synergistic antibacterial activity of a combination of tetrandrine and colistin, which inhibits the growth of mcr-1-positive isolates.
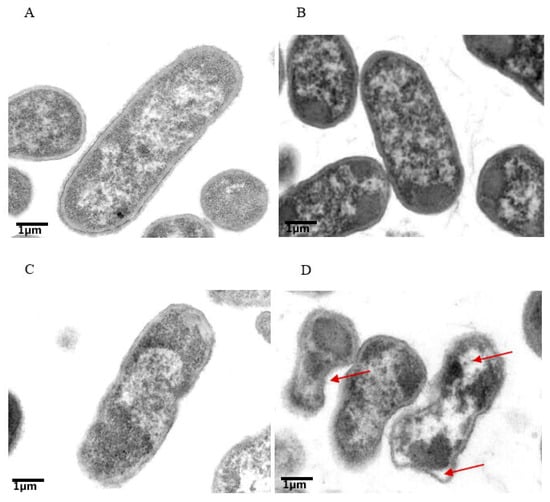
Figure 4.
Colistin and tetrandrine in combination causes membrane damage and kills colistin-resistant E. coli. Transmission electron microscopic observation of (A) control untreated cells with intake cell morphology, (B) cells treated with colistin alone, (C) cells treated with tetrandrine, and (D) Cells treated with a combination of colistin and tetrandrine showing discontinuation of membranes and vacuoles moving out of cells.
2.4. In Vivo Synergistic Activity
To confirm the in vitro results of the above experiments, we evaluated the synergistic potential in vivo in an animal infection model. Mid-log bacteria cultures were suspended to 107 CFU/mL, and 100 μL of suspension was inoculated into the right thigh muscle of neutropenic female mice. One hour later, a placebo (normal saline control), tetrandrine (18.75 mg/kg), and/or colistin (12.5 mg/kg) were administered. In the thighs of the control group, the bacterial loads were 6.5 log10 cfu/g after infection. The bacterial loads in the colistin- and tetrandrine-treated mice groups were 5.6 log10 cfu/g and 5.4 log10 cfu/g, respectively. In contrast, the bacterial load in the colistin-plus-tetrandrine combined-treated group reduced dramatically, which was noted as 2.76 log10 cfu/g (Figure 5). This indicates the synergistic antibacterial activity of a combination of colistin and tetrandrine on mcr-1-harboring E. coli (p < 0.05).
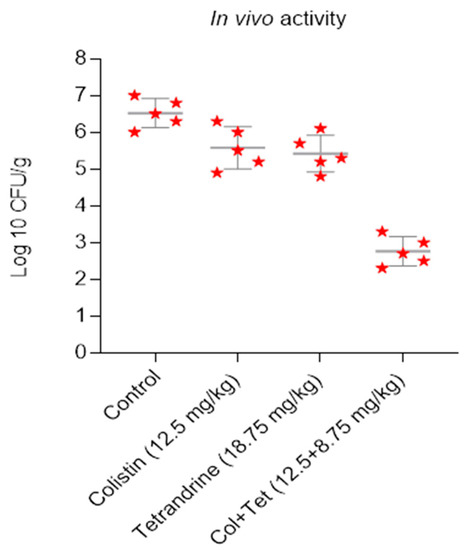
Figure 5.
Colistin and tetrandrine together inhibit infection by colistin-resistant E. coli in neutropenic mice. In vivo activity of colistin and tetrandrine alone and in combination in neutropenic mice, infected with colistin-resistant E. coli, show that neither colistin nor tetrandrine alone inhibits the growth of E. coli, but treatment with colistin and tetrandrine in combination reduces the bacterial load.
2.5. Molecular Docking
The molecular docking results show that tetrandrine can bond with Leu419, Ala420, and Tyr476 by hydrogen bonding, and that the free energy of binding was 8.34 kcal/mol, which was strong evidence of direct physical interaction between tetrandrine and MCR-1 protein (Figure 6).
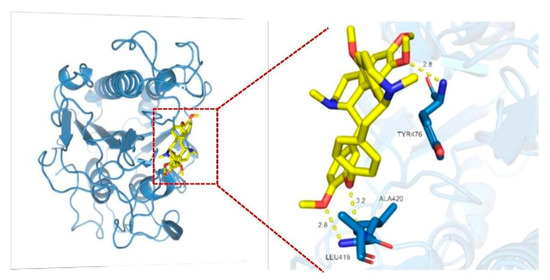
Figure 6.
Molecular docking of tetrandrine and MCR-1 protein show that tetrandrine forms hydrogen bonding with three amino acids of MCR-1 protein in its active sites, suggestive of inhibition of MCR-1 in terms of colistin resistance.
3. Discussion
Antimicrobial resistance is a worldwide health concern, due to which the mortality rate, hospital stay, and treatment costs increase [28]. Before 2015, the most hazardous bacterial-resistance mechanisms were carbapenemase and ESBL production. In the advent of drug resistance, colistin was considered the last treatment option for a cure; colistin has severe side effects in the form of nephro- and neurotoxicity [29,30,31]. The colistin option was in peril when its improper usage was high in human and livestock infection therapy; was also used as a food supplement for poultry and animal farming [32,33,34,35]. Due to its extensive use, there was a continuous worry regarding acquiring the plasmid-mediated resistance against this last drug of choice [36,37,38]. In 2015, the first plasmid-mediated colistin-resistant gene (mcr-1) was detected in pig and human origin samples [11]. The detection of this gene represents a step in the post-antibiotic, era because the researcher found that it has horizontal transferability and could transfer to both the same and different species via conjugation. The mcr-1 gene encodes phosphoethanolamine (pEtN) transferase enzymes that modify the lipid-A portion of the bacterial outer membrane and reduce the affinity of colistin to perform the antibacterial activity [39]. After the development of this resistance, researchers from all over the world started searching for alternative or novel therapeutic options such as bacteriophage therapy, synthetics drugs formulation, and medicinal plant extracts to cure the infections caused by superbugs [40].
In the alternative therapeutic options, the extract of medicinal plants is cheap, easy, and has fewer side effects [41]. Tetrandrine is one of the herbal extracts obtained from the roots of S. tetrandra plant. Tetrandrine is a plant-based efflux pump inhibitor used to cure cardiovascular disorders as a Ca+2 antagonist [42]. Based on its efflux inhibitor potential, tetrandrine is also considered a drug adjuvant against multiple drug-resistant pathogens to improve the retention of drugs inside bacterial cells to perform antibacterial activity. Previous studies have shown that the synergistic activity of tetrandrine with ethidium bromide, fluconazole, and colistin against methicillin-resistant S. aureus, Candida albicans, and Salmonella species [26,43,44]. In the current study, aiming for similar synergism, we analyzed the synergistic activity of tetrandrine with colistin against mcr-1-harboring E. coli. Based on the literature search and to the best of our knowledge, this is the first study to report the therapeutic activity of tetrandrine against E. coli. We performed checkerboard and time-kill assays to determine their antibacterial activity alone and in combination. Our results show that neither colistin nor tetrandrine have antibacterial against mcr-1 positive E. coli. However, when used in combination, the MIC values decreased dramatically.
Furthermore, FICI indicated that potential synergism existed against all our mcr-1-harboring pathogens. Similar trends were also observed from the time-kill assay. We also determined their efficacy in vivo using a neutropenic mouse model. Interestingly, we found that, for the mouse group for which the tetrandrine and colistin were used in combination, the bacterial load was reduced two-fold compared to treatment with either agent alone. A similar in vivo trend has also been observed against Salmonella, but, in that case, the bacterial load was measured in the spleen and liver tissue, whereas we measured bacterial content in the thighs of mice [26]. These results indicate that a combination of tetrandrine and colistin could potentially treat localized and systematic infections.
SEM and TEM visualization were carried to observe the effect of tetrandrine and colistin on the morphology of bacterial cells. SEM micrographs of treated cells illustrated remarkable damage to the cell wall, displaying irregularly wrinkled membrane stacks and deep craters. Similarly, TEM images confirmed the disruption of the polar region and leakage of the cytoplasmic content. It has already been reported that the cell-wall degradation is usually caused by the weakening of the peptidoglycan layer [45]. Moreover, we performed a computational analysis of the MCR-1 protein and tetrandrine to find possible direct interactions between the two molecules. It was determined that tetrandrine interacts with three essential amino acids, including Leu419, Ala420, and Tyr476, of MCR-1. Due to this interaction, the MCR-1 might lose its function and affinity toward its substrates, because all three amino acids are located in the active site [46]. These results are consistent with an early report of a combination of tetrandrine and colistin against Salmonella species [26]. It is further suggested that tetrandrine and colistin might have the same synergistic effect against all mcr-1-harboring Gram-negative pathogens and need to be further investigated. Further studies are required to determine the synergistic activity of colistin and tetrandrine on chromosomal-based resistance to colistin in E. coli.
The limitation of the current study is that our E. coli were clinically isolates, and no mcr-1 positive reference study was used in the current study. Moreover, further research work is required to determine the cytotoxicity and bioavailability of a combined tetrandrine and colistin therapy.
4. Materials and Methods
4.1. Strains, Drugs, and Media
In total, 20 isolates were randomly selected from 75 mcr-1-positive isolates detected in our previous study from the fecal samples of healthy livestock in Pakistan [47]. Tetrandrine and colistin sulfate were purchased from the Xi’an Herb Bio-Tech Co., Ltd. (Xi’an, China) and Sigma-Aldrich Trading Co., Ltd. (Shanghai, China). All bacterial media used in this study were purchased from Hope Biotechnology Co., LTD (Qingdao, China).
4.2. Antimicrobial Susceptibility Testing and Checkerboard Assay
The minimum inhibitory concentrations (MICs) of colistin and tetrandrine were determined using the broth microdilution method, according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines [48]. The checkerboard assay was performed for determining the synergistic activity and fractional inhibitory concentration (FIC) indices of both compounds. Briefly, the 0.5 McFarland turbidity of overnight cultured and tested E. coli was achieved and further diluted at 1:100 in a Mueller-Hinton broth (MHB) medium. Subsequently, 100 µL of MHB was added to each well of a 96-well plate. The standard concentrations of tetrandrine (1024 µg/mL) were diluted along the ordinate, while the colistin sulfate was diluted along the abscissa. The plates were incubated at 37 °C for 18–24 h. The MIC of both compounds alone and in combination was determined, and the fractional inhibitory concentration index (FICI) was calculated using the below equation.
‘Synergy’ was considered to be when FICI ≤ 0.5, 0.5 < FICI ≤ 1 indicates ‘partial synergy’, FICI = 1 indicates an ‘additive effect’, and a FIC index > 1 indicates an ‘antagonistic’ effect [26,49].
4.3. Time-Kill Assay
For the time-kill assay, an mcr-1-positive strain was randomly selected, and the assay was performed according to the CLSI guidelines. Briefly, the selected strain was cultured overnight, then diluted 1:10,000 in MHB medium and incubated for 4 h at 37 °C to obtain early exponential-phase bacteria. The E. coli cells were then treated with tetrandrine (MIC value) and colistin (MIC value), both alone and in combination. The cultures were incubated at 37 °C, and 100 µL of aliquot was removed at 0, 2, 4, 8, and 12 h time points. Then 20 µL of each dilution was inoculated on MHA and incubated at 37 °C for 18–24 h. The number of colonies on each plate was counted. Synergistic activity was interpreted as a 2-log10 decrease in the number of CFU/mL between the combination and its most active single component after 12 h of incubation.
4.4. Fluorescence Staining
For fluorescence staining microscopy, an overnight culture of mcr-1-harboring E. coli was diluted in 1: 100 in MHB and incubated at 37 °C for 4 h to attain an early exponential growth phase. Then, the culture was divided into three tubes: the colistin (MIC value) tube, the tetrandrine (MIC value) tube, and the combined tube of colistin and tetrandrine. After 18 h of incubation, a small amount of bacterial culture was fixed and stained with fluorescent dyes SYTO 9 and propidium iodide (PI) on the coverslip. Then, the number of live cells was examined under a fluorescence microscope.
4.5. Scanning Electron Microscopy
For SEM analysis, exponentially grown mcr-1-positive E. coli cells were treated with colistin (MIC value) and tetrandrine (MIC value) alone and in combination for 4 h at 37 °C in Luria broth (LB). The cells were centrifuged and washed twice with phosphate-buffered saline (PBS). The pelleted cells were fixed with 2.5% (v/v) glutaraldehyde in PBS, incubated overnight at 4 °C, and then serially dehydrated using 30–100% ethanol. After substituting the ethanol with tertiary-butanol, the samples were released onto silver paper for vacuum freeze-drying. Finally, the dried cells were gold-coated and observed under the scanning electron microscope (SEM, Zeiss EVO18, Jena, Germany) at 10.0 kV.
4.6. Transmission Electron Microscopy
For the TEM observations, the E. coli cells were prepared as described earlier. The prepared bacterial pellets were subjected to a series of treatments according to the guidelines in the literature in order to perform TEM analysis. Ultrathin sections were prepared on formvar-coated grids (Plano, Wetzlar, Germany) and stained with 3% uranyl acetate. Microscopy was performed with a Hitachi H-9500) (Oberkochen, Germany) microscope at 120-keV electron energy. Zero-loss energy filtering (30) was applied for optimizing the contrast.
4.7. In Vivo Synergistic Activity
A female mouse model for E. coli infection was used to determine the synergistic effect of tetrandrine in combination with colistin. A total of 20 germ-free mice weighing 25 ± 2 g were treated with 150 mg/kg cyclophosphamide (Shanghai Biolang Biotechnology Co., Ltd, Shanghai, China) for 4 days, followed by a 100 mg/kg dose on the fifth day to induce neutropenia before bacterial inoculation. The mice were divided into four groups, with 5 in each group. The mcr-1-harboring E. coli isolates were selected for in vivo assay. Mid-log bacteria cultures were diluted to 107 CFU/mL, and 100 μL of suspension was inoculated into the right thigh muscle of each female mouse. After one hour, a placebo (normal saline, Group A), tetrandrine (18.75 mg/kg), and colistin (12.5 mg/kg) were administered in the following manner: tetrandrine only (Group B), colistin only (Group C), and tetrandrine + colistin (Group D). The mice were monitor for 48 h after treatment.
4.8. Molecular Docking of Tetrandrine and MCR-1 Protein
Molecular docking between the crystal structure of the MCR-1 protein (PDB ID: 5GRR) and tetrandrine (PubChem CID: 73078) was performed by AutoDock 4.3 and the Lamarckian Genetic Algorithm method on the Cygwin platform with default parameters to identify any possible direct interactions. The weighting parameters for the recording function included the hydrogen bond energy, hydrophobic interaction, spatial interaction, and the number of rotary keys in the legend. Convergence was measured to evaluate pairing. The lower the parameter, the more likely the ligand will bind to the active site. The docking result was visualized using the PyMOL software for the analysis of binding site residues.
4.9. Statistical Analysis
All numerical results are reported as the mean ± SD. The significance of differences between all groups was determined by the Student’s t-test and GraphPad Prism v8.0.2. The differences were considered significant at p ˂ 0.05.
5. Conclusions
In the present study, we determined that tetrandrine has the potential to synergistically enhance the activity of colistin against mcr-1-harboring E. coli both in vivo and in vitro. Computational analysis showed a direct interaction of tetrandrine with MCR-1 protein at the MCR-1 active site, indicating the ability of tetrandrine to inhibit substrate-MCR-1 binding. This study concluded that tetrandrine is a promising small molecule that can halt E. coli superbugs that show resistance to colistin. Further studies are required to determine their activity against chromosomal-based resistance in other colistin-resistant bacterial pathogens.
Author Contributions
Conceptualization, M.S. and F.Y.; methodology, M.S. and S.U.R.; software, M.S. and M.Z.; validation, S.U.R. and X.J.; formal analysis, H.B.; investigation, M.S.; resources M.S.; data curation, I.A. and X.L.; writing—M.S.; writing—review and editing, Y.Y., Y.Z. and X.J.; visualization, M.S. and H.B.; supervision, S.U.R.; project administration, M.S.; funding acquisition, M.S. and X.J. All authors have read and agreed to the published version of the manuscript.
Funding
This study has been graciously supported by the National Natural Science Foundation of China for International Young Scientists (No. 42150410383) and the 2020 Li Ka Shing Foundation Cross-Disciplinary Research Grant (project number: 2020LKSFG03E).
Institutional Review Board Statement
All animal procedures were approved by the ethical committee of the College of Veterinary Sciences and Animal Husbandry, Abdul Wali Khan University, Mardan, Pakistan. All work prescribed here was carried out following the Departmental Regulations on Experimental Animals handling guidelines of Microbiology (AWKUM/CVSAH/2020/09).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This study has been graciously supported by the National Natural Science Foundation of China for International Young Scientists and Li Ka Shing Foundation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Paitan, Y. Current Trends in Antimicrobial Resistance of Escherichia coli. Curr. Top. Microbiol. Immunol. 2018, 416, 181–211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, S.; Liu, X.; Lin, W.; Zhu, K. Equisetin Restores Colistin Sensitivity against Multi-Drug Resistant Gram-Negative Bacteria. Antibiotics 2021, 10, 1263. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, H.; Younas, S.; Qamar, M.U.; Junaid, K.; Abdalla, A.E.; Abosalif, K.O.A.; Alameen, A.A.M.; Elamir, M.Y.M.; Ahmad, N.; Hamam, S.S.M. Molecular epidemiology of extensively drug-resistant mcr encoded colistin-resistant bacterial strains co-expressing multifarious β-lactamases. Antibiotics 2021, 10, 467. [Google Scholar] [CrossRef]
- Ejaz, H.; Younas, S.; Abosalif, K.O.; Junaid, K.; Alzahrani, B.; Alsrhani, A.; Abdalla, A.E.; Ullah, M.I.; Qamar, M.U.; Hamam, S.S. Molecular analysis of bla SHV, bla TEM, and bla CTX-M in extended-spectrum β-lactamase producing Enterobacteriaceae recovered from fecal specimens of animals. PLoS ONE 2021, 16, e0245126. [Google Scholar] [CrossRef]
- Castanheira, M.; Simner, P.J.; Bradford, P.A. Extended-spectrum β-lactamases: An update on their characteristics, epidemiology and detection. JAC-Antimicrob. Resist. 2021, 3, dlab092. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.; ur Rahman, S.; Zhang, L.; Shahid, M.; Zhang, S.; Liu, G.; Gao, J.; Han, B. ESBL-Producing Escherichia coli from Cows Suffering Mastitis in China Contain Clinical Class 1 Integrons with CTX-M Linked to IS CR1. Front. Microbiol. 2016, 7, 1931. [Google Scholar] [CrossRef] [PubMed]
- VinodhKumar, O.R.; Karikalan, M.; Ilayaraja, S.; Sha, A.A.; Singh, B.R.; Sinha, D.K.; Chandra Mohan, S.; Pruthvishree, B.S.; Pawde, A.M.; Sharma, A.K. Multi-drug resistant (MDR), extended spectrum beta-lactamase (ESBL) producing and carbapenem resistant Escherichia coli in rescued Sloth bears (Melursus ursinus), India. Vet. Res. Commun. 2021, 45, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Heinz, E.; Ejaz, H.; Scott, J.B.; Wang, N.; Gujaran, S.; Pickard, D.; Wilksch, J.; Cao, H.; Haq, I.-U.; Dougan, G. Resistance mechanisms and population structure of highly drug resistant Klebsiella in Pakistan during the introduction of the carbapenemase NDM-1. Sci. Rep. 2019, 9, 2392. [Google Scholar] [CrossRef] [PubMed]
- Bilal, H.; Zhang, G.; Rehman, T.; Han, J.; Khan, S.; Shafiq, M.; Yang, X.; Yan, Z.; Yang, X. First Report of bla NDM-1 Bearing IncX3 Plasmid in Clinically Isolated ST11 Klebsiella pneumoniae from Pakistan. Microorganisms 2021, 9, 951. [Google Scholar] [CrossRef]
- Bilal, H.; Rehman, T.U.; Khan, M.A.; Hameed, F.; Jian, Z.G.; Han, J.; Yang, X. Molecular Epidemiology of mcr-1, bla (KPC-2,) and bla (NDM-1) Harboring Clinically Isolated Escherichia coli from Pakistan. Infect. Drug Resist. 2021, 14, 1467–1479. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Wang, Y.; Walsh, T.R.; Yi, L.X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Huang, H.; Dong, N.; Shu, L.; Lu, J.; Sun, Q.; Chan, E.W.-C.; Chen, S.; Zhang, R. Colistin-resistance gene mcr in clinical carbapenem-resistant Enterobacteriaceae strains in China, 2014–2019. Emerg. Microbes Infect. 2020, 9, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Bilal, H.; Hameed, F.; Khan, M.A.; Khan, S.; Yang, X.; Rehman, T.U. Detection of mcr-1 gene in extended-spectrum β-lactamase-producing Klebsiella pneumoniae from human urine samples in Pakistan. Jundishapur J. Microbiol. 2020, 13, e96646. [Google Scholar] [CrossRef]
- Kim, J.S.; Yu, J.K.; Jeon, S.J.; Park, S.-H.; Han, S.; Park, S.H.; Kang, M.; Jang, J.I.; Shin, E.-K.; Kim, J. Distribution of mcr genes among carbapenem-resistant Enterobacterales clinical isolates: High prevalence of mcr-positive Enterobacter cloacae complex in Seoul, Republic of Korea. Int. J. Antimicrob. Agents 2021, 58, 106418. [Google Scholar] [CrossRef] [PubMed]
- Paveenkittiporn, W.; Kamjumphol, W.; Ungcharoen, R.; Kerdsin, A. Whole-genome sequencing of clinically isolated carbapenem-resistant enterobacterales harboring mcr genes in Thailand, 2016–2019. Front. Microbiol. 2021, 11, 586368. [Google Scholar] [CrossRef]
- Bastidas-Caldes, C.; de Waard, J.H.; Salgado, M.S.; Villacís, M.J.; Coral-Almeida, M.; Yamamoto, Y.; Calvopiña, M. Worldwide Prevalence of mcr-mediated Colistin-Resistance Escherichia coli in Isolates of Clinical Samples, Healthy Humans, and Livestock—A Systematic Review and Meta-Analysis. Pathogens 2022, 11, 659. [Google Scholar] [CrossRef]
- Gogry, F.A.; Siddiqui, M.T.; Sultan, I.; Husain, F.M.; Al-Kheraif, A.A.; Ali, A.; Haq, Q.M.R. Colistin interaction and surface changes associated with mcr-1 conferred plasmid mediated resistance in E. coli and A. veronii strains. Pharmaceutics 2022, 14, 295. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Li, X.; Dong, L.; Hu, X.; Nie, T.; Lu, Y.; Lu, X.; Pang, J.; Li, G. Synergistic effect of colistin combined with PFK-158 against colistin-resistant Enterobacteriaceae. Antimicrob. Agents Chemother. 2019, 63, e00271-19. [Google Scholar] [CrossRef]
- Wang, Y.-M.; Kong, L.-C.; Liu, J.; Ma, H.-X. Synergistic effect of eugenol with Colistin against clinical isolated Colistin-resistant Escherichia coli strains. Antimicrob. Resist. Infect. Control. 2018, 7, 17. [Google Scholar] [CrossRef]
- Cannatelli, A.; Principato, S.; Colavecchio, O.L.; Pallecchi, L.; Rossolini, G.M. Synergistic activity of colistin in combination with resveratrol against colistin-resistant gram-negative pathogens. Front. Microbiol. 2018, 9, 1808. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, T.; Guo, Y.; Liu, S.; Wang, J.; Shen, Y.; Tang, S.; Wang, Y.; Deng, X. In vitro/vivo activity of potential MCR-1 inhibitor in combination with colistin againsts mcr-1-positive Klebsiella pneumonia. Front. Microbiol. 2018, 9, 1615. [Google Scholar] [CrossRef] [PubMed]
- Gogry, F.A.; Siddiqui, M.T.; Sultan, I.; Haq, Q.M.R. Current update on intrinsic and acquired colistin resistance mechanisms in bacteria. Front. Med. 2021, 8, 677720. [Google Scholar] [CrossRef] [PubMed]
- Peyclit, L.; Baron, S.A.; Rolain, J.-M. Drug repurposing to fight colistin and carbapenem-resistant bacteria. Front. Cell. Infect. Microbiol. 2019, 9, 193. [Google Scholar] [CrossRef] [PubMed]
- Chandra, H.; Singh, C.; Kumari, P.; Yadav, S.; Mishra, A.P.; Laishevtcev, A.; Brisc, C.; Brisc, M.C.; Munteanu, M.A.; Bungau, S. Promising Roles of Alternative Medicine and Plant-Based Nanotechnology as Remedies for Urinary Tract Infections. Molecules 2020, 25, 5593. [Google Scholar] [CrossRef]
- Luan, F.; He, X.; Zeng, N. Tetrandrine: A review of its anticancer potentials, clinical settings, pharmacokinetics and drug delivery systems. J. Pharm. Pharmacol. 2020, 72, 1491–1512. [Google Scholar] [CrossRef] [PubMed]
- Yi, K.; Liu, S.; Liu, P.; Luo, X.; Zhao, J.; Yan, F.; Pan, Y.; Liu, J.; Zhai, Y.; Hu, G. Synergistic antibacterial activity of tetrandrine combined with colistin against MCR-mediated colistin-resistant Salmonella. Biomed. Pharmacother. 2022, 149, 112873. [Google Scholar] [CrossRef] [PubMed]
- Sutter, M.C.; Wang, Y.X. Recent cardiovascular drugs from Chinese medicinal plants. Cardiovasc. Res. 1993, 27, 1891–1901. [Google Scholar] [CrossRef]
- McEwen, S.A.; Collignon, P.J. Antimicrobial Resistance: A One Health Perspective. Microbiol. Spectr. 2018, 6, 2. [Google Scholar] [CrossRef]
- Gai, Z.; Samodelov, S.L.; Kullak-Ublick, G.A.; Visentin, M. Molecular Mechanisms of Colistin-Induced Nephrotoxicity. Molecules 2019, 24, 653. [Google Scholar] [CrossRef]
- Zhou, Y.-F.; Liu, P.; Zhang, C.-J.; Liao, X.-P.; Sun, J.; Liu, Y.-H. Colistin Combined with Tigecycline: A Promising Alternative Strategy to Combat Escherichia coli Harboring bla NDM–5 and mcr-1. Front. Microbiol. 2020, 10, 2957. [Google Scholar] [CrossRef]
- Javed, H.; Saleem, S.; Zafar, A.; Ghafoor, A.; Shahzad, A.B.; Ejaz, H.; Junaid, K.; Jahan, S. Emergence of plasmid-mediated mcr genes from Gram-negative bacteria at the human-animal interface. Gut Pathog. 2020, 12, 54. [Google Scholar] [CrossRef] [PubMed]
- Stefaniuk, E.M.; Tyski, S. Colistin Resistance in Enterobacterales Strains—A Current View. Pol. J. Microbiol. 2019, 68, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Larbi, R.O.; Adeapena, W.; Ayim-Akonor, M.; Ansa, E.D.; Tweya, H.; Terry, R.F.; Labi, A.-K.; Harries, A.D. Antimicrobial, Multi-Drug and Colistin Resistance in Enterobacteriaceae in Healthy Pigs in the Greater Accra Region of Ghana, 2022: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2022, 19, 10449. [Google Scholar] [CrossRef]
- Babar, Z.U.; Dodani, S.K.; Nasim, A. Treatment outcome and adverse effects of colistin in adult patients with carbapenem-resistant gram-negative bacteremia from Pakistan. Int. J. Infect. Dis. 2021, 106, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Bilal, H.; Khan, M.N.; Rehman, T.; Hameed, M.F.; Yang, X. Antibiotic resistance in Pakistan: A systematic review of past decade. BMC Infect. Dis. 2021, 21, 244. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, M.; Huang, J.; Rahman, S.U.; Shah, J.M.; Chen, L.; Gao, Y.; Wang, M.; Wang, L. High incidence of multidrug-resistant Escherichia coli coharboring mcr-1 and blaCTX-M-15 recovered from pigs. Infect. Drug Resist. 2019, 12, 2135. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, M.; Huang, J.; Shah, J.M.; Ali, I.; Rahman, S.U.; Wang, L. Characterization and resistant determinants linked to mobile elements of ESBL-producing and mcr-1-positive Escherichia coli recovered from the chicken origin. Microb. Pathog. 2021, 150, 104722. [Google Scholar] [CrossRef]
- Shafiq, M.; Huang, J.; Shah, J.; Wang, X.; Rahman, S.; Ali, I.; Chen, L.; Wang, L. Characterization and virulence factors distribution of bla CTX-M and mcr-1 carrying Escherichia coli isolates from bovine mastitis. J. Appl. Microbiol. 2021, 131, 634–646. [Google Scholar] [CrossRef]
- Wang, R.; van Dorp, L.; Shaw, L.P.; Bradley, P.; Wang, Q.; Wang, X.; Jin, L.; Zhang, Q.; Liu, Y.; Rieux, A.; et al. The global distribution and spread of the mobilized colistin resistance gene mcr-1. Nat. Commun. 2018, 9, 1179. [Google Scholar] [CrossRef]
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef]
- Sile, I.; Romane, E.; Reinsone, S.; Maurina, B.; Tirzite, D.; Dambrova, M. Medicinal plants and their uses recorded in the Archives of Latvian Folklore from the 19th century. J. Ethnopharmacol. 2020, 249, 112378. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.X.; Jiang, M.X. Effects of tetrandrine on cardiovascular electrophysiologic properties. Acta Pharmacol. Sin. 2002, 23, 1069–1074. [Google Scholar] [PubMed]
- Lee, Y.S.; Han, S.H.; Lee, S.H.; Kim, Y.G.; Park, C.B.; Kang, O.H.; Keum, J.H.; Kim, S.B.; Mun, S.H.; Seo, Y.S.; et al. The mechanism of antibacterial activity of tetrandrine against Staphylococcus aureus. Foodborne Pathog. Dis. 2012, 9, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Xie, S.M.; Li, S.X.; Song, Y.J.; Lv, X.L.; Zhang, H. Synergistic mechanism for tetrandrine on fluconazole against Candida albicans through the mitochondrial aerobic respiratory metabolism pathway. J. Med. Microbiol. 2014, 63, 988–996. [Google Scholar] [CrossRef]
- Ragland, S.A.; Criss, A.K. From bacterial killing to immune modulation: Recent insights into the functions of lysozyme. PLoS Pathog. 2017, 13, e1006512. [Google Scholar] [CrossRef]
- Son, S.J.; Huang, R.; Squire, C.J.; Leung, I.K.H. MCR-1: A promising target for structure-based design of inhibitors to tackle polymyxin resistance. Drug Discov. Today 2019, 24, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, M.; Rahman, S.U.; Bilal, H.; Ullah, A.; Noman, S.M.; Zeng, M.; Yuan, Y.; Xie, Q.; Li, X.; Jiao, X. Incidence and molecular characterization of ESBL-producing and colistin-resistant Escherichia coli isolates recovered from healthy food-producing animals in Pakistan. J. Appl. Microbiol. 2022, 133, 1169–1182. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing. EUCAST Clinical Breakpoints; European Society of Clinical Microbiology and Infectious Diseases: Basil, Switzerland, 2016. [Google Scholar]
- Lopez-Carrizales, M.; Velasco, K.I.; Castillo, C.; Flores, A.; Magaña, M.; Martinez-Castanon, G.A.; Martinez-Gutierrez, F. In vitro synergism of silver nanoparticles with antibiotics as an alternative treatment in multiresistant uropathogens. Antibiotics 2018, 7, 50. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).