Halogenase-Targeted Genome Mining Leads to the Discovery of (±) Pestalachlorides A1a, A2a, and Their Atropisomers
Abstract
1. Introduction
2. Results
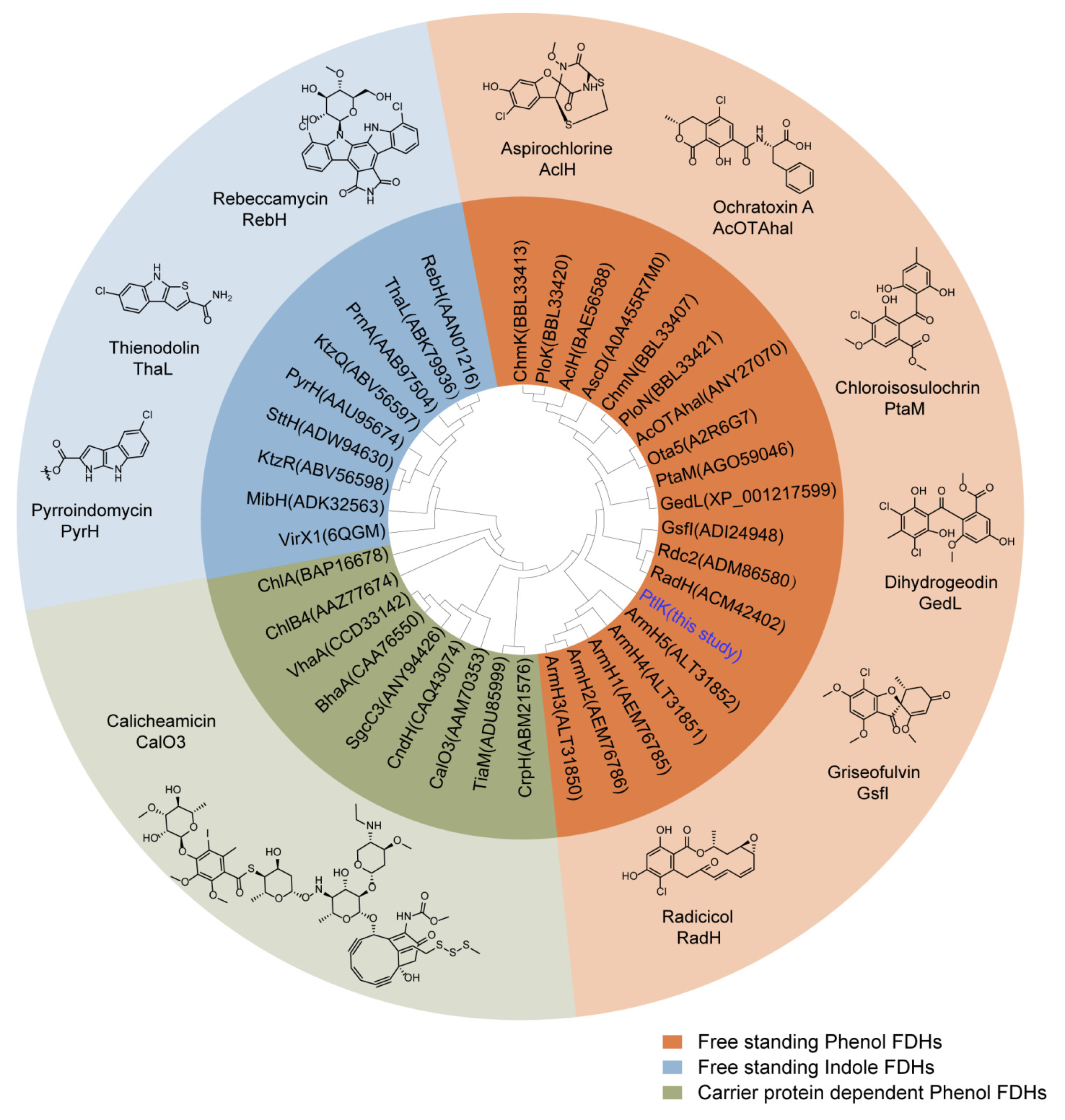
2.1. Genome Mining of the Halogenase-Containing Biosynthesis Gene Cluster
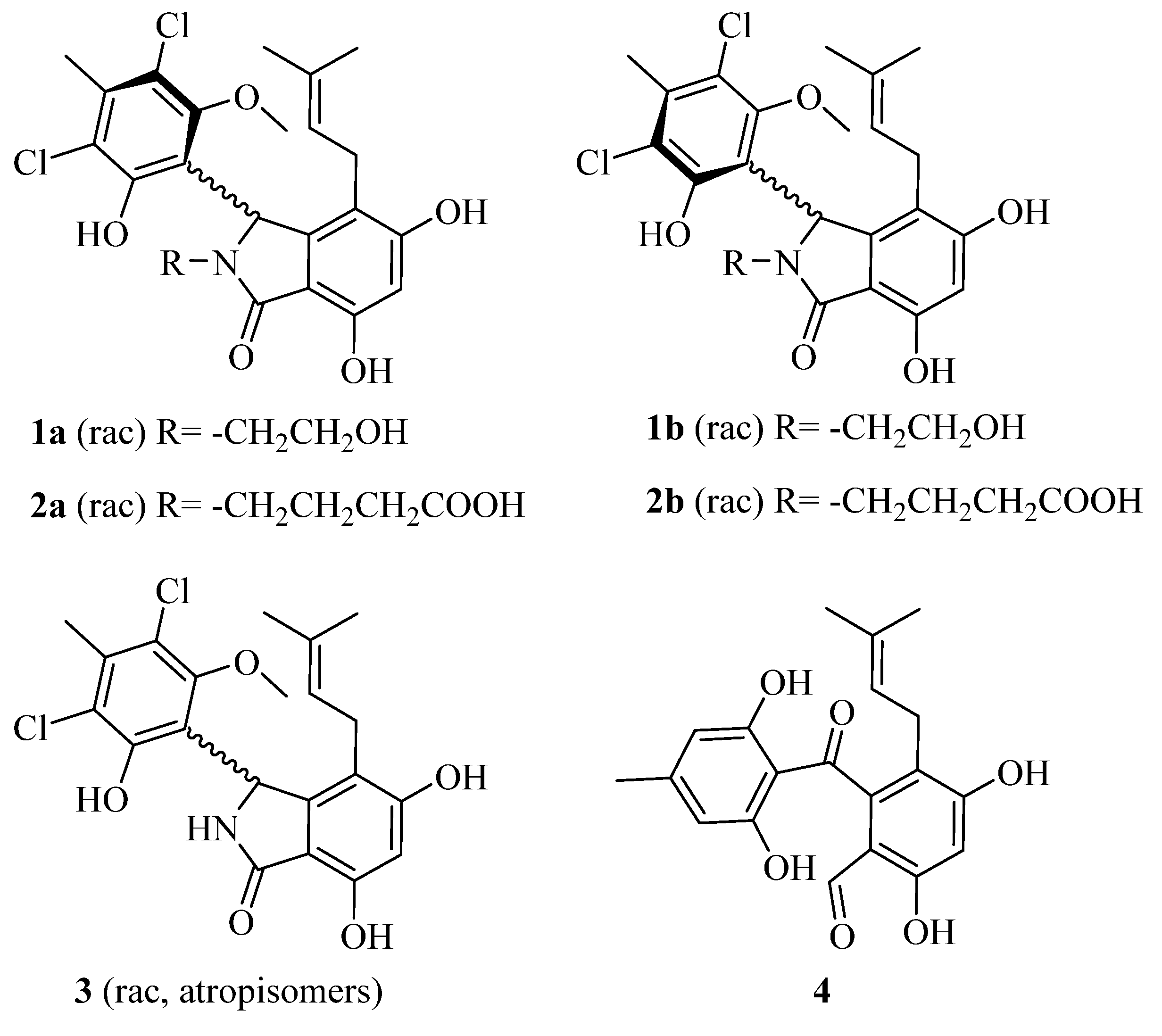
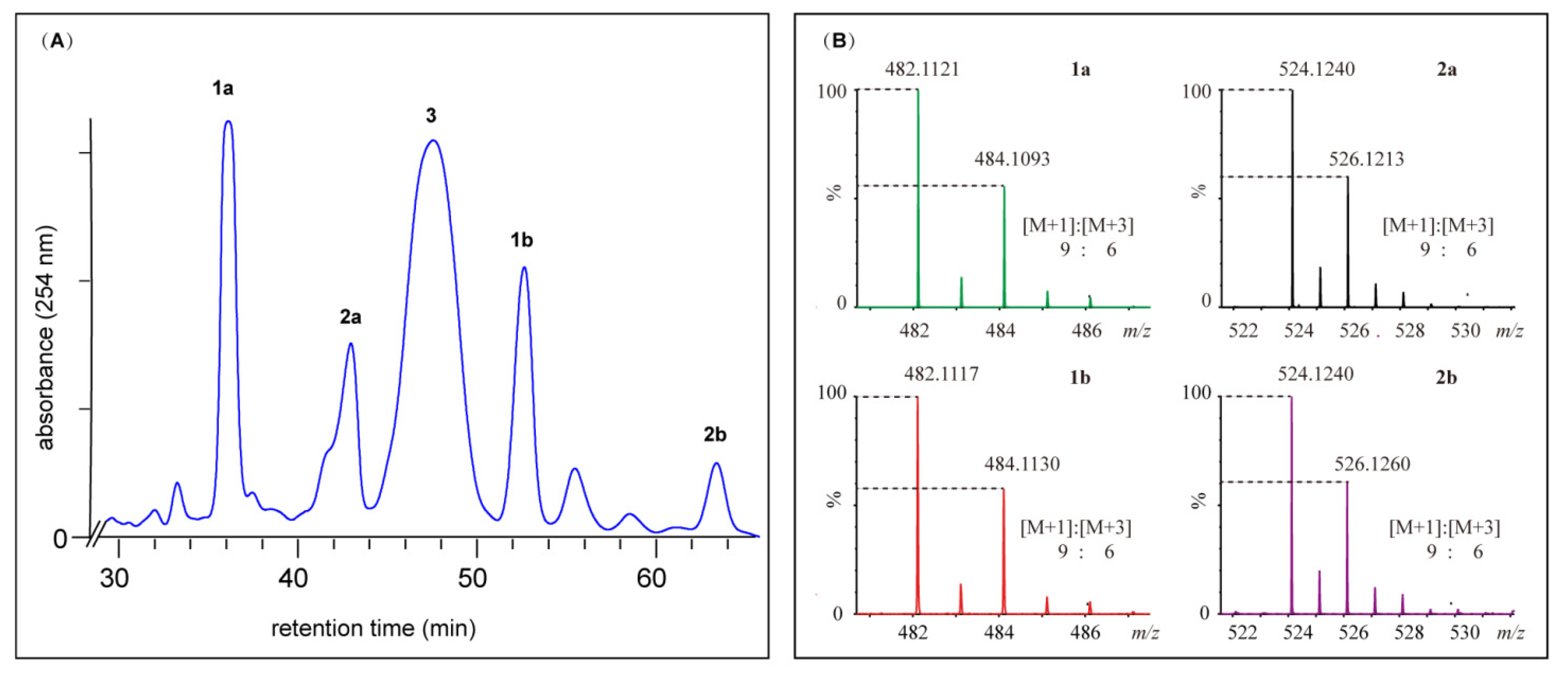
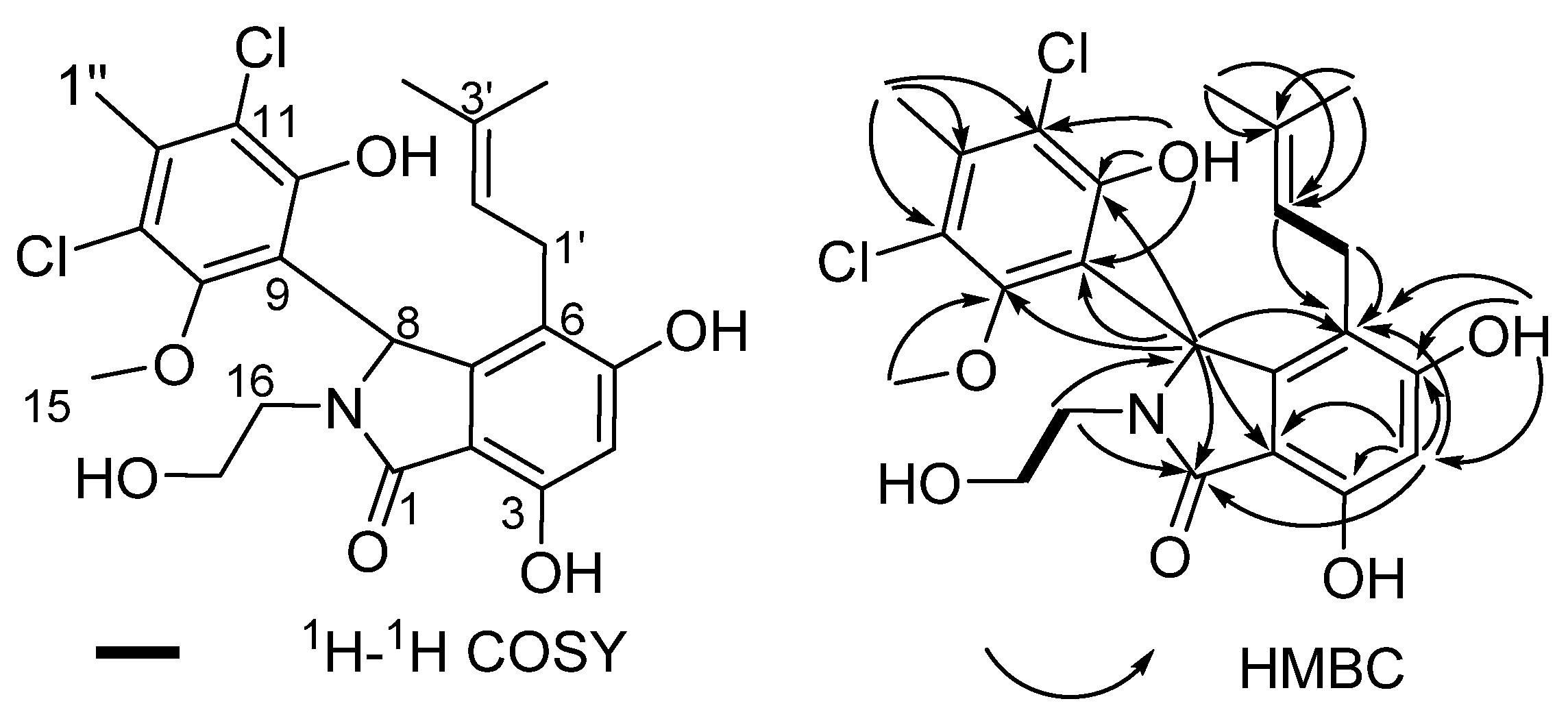
2.2. Structural Elucidation for (±) Pestalachlorides A1a, A1b, A2a, and A2b
2.3. Proposed Biosynthetic Pathway for Pestalachlorides
2.4. Antimicrobial Activities of Pestalachlorides
3. Discussion
4. Materials and Methods
4.1. General Experimental Details
4.2. Genome Mining of the Halogenase-Containing Biosynthesis Gene Clusters
4.3. Culture Condition Prioritization for the Production of Chlorinated Compounds
4.4. Fermentation and Isolation
4.5. The Calculation of the Relative Gibbs Energy Barriers
4.6. Antibacterial Bioassay
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parisini, E.; Metrangolo, P.; Pilati, T.; Resnati, G.; Terraneo, G. Halogen bonding in halocarbon-protein complexes: A structural survey. Chem. Soc. Rev. 2011, 40, 2267–2278. [Google Scholar] [CrossRef]
- Crowe, C.; Molyneux, S.; Sharma, S.V.; Zhang, Y.; Gkotsi, D.S.; Connaris, H.; Goss, R.J.M. Halogenases: A palette of emerging opportunities for synthetic biology–synthetic chemistry and C–H functionalisation. Chem. Soc. Rev. 2021, 50, 9443–9481. [Google Scholar] [CrossRef]
- Latham, J.; Brandenburger, E.; Shepherd, S.A.; Menon, B.R.K.; Micklefield, J. Development of Halogenase Enzymes for Use in Synthesis. Chem. Rev. 2018, 118, 232–269. [Google Scholar] [CrossRef]
- Whitfield, R.; Brown, F. The Benefits of Chlorine Chemistry in Pharmaceuticals in the United States and Canada. IHS Econ. 2016. [Google Scholar]
- Gribble, G.W. A recent survey of naturally occurring organohalogen compounds. Environ. Chem. 2015, 12, 396–405. [Google Scholar] [CrossRef]
- van Santen, J.A.; Poynton, E.F.; Iskakova, D.; McMann, E.; Alsup, T.A.; Clark, T.N.; Fergusson, C.H.; Fewer, D.P.; Hughes, A.H.; McCadden, C.A.; et al. The Natural Products Atlas 2.0: A database of microbially-derived natural products. Nucleic Acids Res. 2022, 50, D1317–D1323. [Google Scholar] [CrossRef] [PubMed]
- Cochereau, B.; Meslet-Cladière, L.; Pouchus, Y.F.; Grovel, O.; Roullier, C. Halogenation in Fungi: What Do We Know and What Remains to Be Discovered? Molecules 2022, 27, 3157. [Google Scholar] [CrossRef] [PubMed]
- Ludewig, H.; Molyneux, S.; Ferrinho, S.; Guo, K.; Lynch, R.; Gkotsi, D.S.; Goss, R.J.M. Halogenases: Structures and functions. Curr. Opin. Struct. Biol. 2020, 65, 51–60. [Google Scholar] [CrossRef]
- Menon, B.R.K.; Richmond, D.; Menon, N. Halogenases for biosynthetic pathway engineering: Toward new routes to naturals and non-naturals. Catal. Rev. 2022, 64, 533–591. [Google Scholar] [CrossRef]
- Zeng, J.; Zhan, J. Chlorinated Natural Products and Related Halogenases. Isr. J. Chem. 2019, 59, 387–402. [Google Scholar] [CrossRef]
- Agarwal, V.; Miles, Z.D.; Winter, J.M.; Eustáquio, A.S.; El Gamal, A.A.; Moore, B.S. Enzymatic Halogenation and Dehalogenation Reactions: Pervasive and Mechanistically Diverse. Chem. Rev. 2017, 117, 5619–5674. [Google Scholar] [CrossRef] [PubMed]
- Phintha, A.; Prakinee, K.; Chaiyen, P. Chapter Eleven—Structures, mechanisms and applications of flavin-dependent halogenases. In The Enzymes; Chaiyen, P., Tamanoi, F., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 47, pp. 327–364. [Google Scholar]
- Dong, C.; Flecks, S.; Unversucht, S.; Haupt, C.; van Pée, K.H.; Naismith, J.H. Tryptophan 7-halogenase (PrnA) structure suggests a mechanism for regioselective chlorination. Science 2005, 309, 2216–2219. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; De Laurentis, W.; Leang, K.; Herrmann, J.; Ihlefeld, K.; van Pée, K.-H.; Naismith, J.H. Structural Insights into Regioselectivity in the Enzymatic Chlorination of Tryptophan. J. Mol. Biol. 2009, 391, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Scherlach, K.; Hertweck, C. Mining and unearthing hidden biosynthetic potential. Nat. Commun. 2021, 12, 3864. [Google Scholar] [CrossRef] [PubMed]
- Rajwani, R.; Ohlemacher, S.I.; Zhao, G.; Liu, H.B.; Bewley, C.A. Genome-Guided Discovery of Natural Products through Multiplexed Low-Coverage Whole-Genome Sequencing of Soil Actinomycetes on Oxford Nanopore Flongle. mSystems 2021, 6, e0102021. [Google Scholar] [CrossRef]
- Miethke, M.; Pieroni, M.; Weber, T.; Bronstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B.; et al. Towards the sustainable discovery and development of new antibiotics. Nat. Rev. Chem. 2021, 726–749. [Google Scholar] [CrossRef]
- Claesen, J.; Bibb, M. Genome mining and genetic analysis of cypemycin biosynthesis reveal an unusual class of posttranslationally modified peptides. Proc. Natl. Acad. Sci. USA 2010, 107, 16297–16302. [Google Scholar] [CrossRef]
- Bergmann, S.; Schümann, J.; Scherlach, K.; Lange, C.; Brakhage, A.A.; Hertweck, C. Genomics-driven discovery of PKS-NRPS hybrid metabolites from Aspergillus nidulans. Nat. Chem. Biol. 2007, 3, 213–217. [Google Scholar] [CrossRef]
- Franke, J.; Ishida, K.; Hertweck, C. Genomics-Driven Discovery of Burkholderic Acid, a Noncanonical, Cryptic Polyketide from Human Pathogenic Burkholderia Species. Angew. Chem. Int. Ed. 2012, 51, 11611–11615. [Google Scholar] [CrossRef]
- Luo, M.; Chang, S.; Li, Y.; Xi, X.; Chen, M.; He, N.; Wang, M.; Zhao, W.; Xie, Y. Molecular Networking-Based Screening Led to the Discovery of a Cyclic Heptadepsipeptide from an Endolichenic Xylaria sp. J. Nat. Prod. 2022, 85, 972–979. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Zhang, G.; He, N.; Guo, W.; Hong, B.; Xie, Y. Potashchelins, a Suite of Lipid Siderophores Bearing Both L-threo and L-erythro Beta-Hydroxyaspartic Acids, Acquired From the Potash-Salt-Ore-Derived Extremophile Halomonas sp. MG34. Front. Chem. 2020, 8, 197. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, N.; Luo, M.; Hong, B.; Xie, Y. Application of untargeted tandem mass spectrometry with molecular networking for detection of enniatins and beauvericins from complex samples. J. Chromatogr. A 2020, 1634, 461626. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, X.; He, N.; Xie, Y.; Hong, B. Rescrutiny of the sansanmycin biosynthetic gene cluster leads to the discovery of a novel sansanmycin analogue with more potency against Mycobacterium tuberculosis. J. Antibiot. 2019, 72, 769–774. [Google Scholar] [CrossRef]
- Shi, Y.; Gu, R.; Li, Y.; Wang, X.; Ren, W.; Li, X.; Wang, L.; Xie, Y.; Hong, B. Exploring novel herbicidin analogues by transcriptional regulator overexpression and MS/MS molecular networking. Microb. Cell Factories 2019, 18, 175. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.T.; Nielsen, J.B.; Anyaogu, D.C.; Holm, D.K.; Nielsen, K.F.; Larsen, T.O.; Mortensen, U.H. Heterologous reconstitution of the intact geodin gene cluster in Aspergillus nidulans through a simple and versatile PCR based approach. PLoS ONE 2013, 8, e72871. [Google Scholar] [CrossRef]
- Li, E.; Jiang, L.; Guo, L.; Zhang, H.; Che, Y. Pestalachlorides A–C, antifungal metabolites from the plant endophytic fungus Pestalotiopsis adusta. Bioorg. Med. Chem. 2008, 16, 7894–7899. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Wu, P.; Li, H.; Xue, J.; Wei, X. Pestalotinones A-D, new benzophenone antibiotics from endophytic fungus Pestalotiopsis trachicarpicola SC-J551. J. Antibiot. 2022, 75, 207–212. [Google Scholar] [CrossRef] [PubMed]
- LaPlante, S.R.; Edwards, P.J.; Fader, L.D.; Jakalian, A.; Hucke, O. Revealing atropisomer axial chirality in drug discovery. ChemMedChem 2011, 6, 505–513. [Google Scholar] [CrossRef]
- Xu, X.; Liu, L.; Zhang, F.; Wang, W.; Li, J.; Guo, L.; Che, Y.; Liu, G. Identification of the first diphenyl ether gene cluster for pestheic acid biosynthesis in plant endophyte Pestalotiopsis fici. ChemBioChem 2014, 15, 284–292. [Google Scholar] [CrossRef]
- Chiang, Y.M.; Szewczyk, E.; Davidson, A.D.; Entwistle, R.; Keller, N.P.; Wang, C.C.; Oakley, B.R. Characterization of the Aspergillus nidulans monodictyphenone gene cluster. Appl. Environ. Microbiol. 2010, 76, 2067–2074. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, E.; Chiang, Y.M.; Oakley, C.E.; Davidson, A.D.; Wang, C.C.; Oakley, B.R. Identification and characterization of the asperthecin gene cluster of Aspergillus nidulans. Appl. Environ. Microbiol. 2008, 74, 7607–7612. [Google Scholar] [CrossRef] [PubMed]
- Szwalbe, A.J.; Williams, K.; Song, Z.; de Mattos-Shipley, K.; Vincent, J.L.; Bailey, A.M.; Willis, C.L.; Cox, R.J.; Simpson, T.J. Characterisation of the biosynthetic pathway to agnestins A and B reveals the reductive route to chrysophanol in fungi. Chem. Sci. 2019, 10, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Simpson, T.J. Genetic and Biosynthetic Studies of the Fungal Prenylated Xanthone Shamixanthone and Related Metabolites in Aspergillus spp. Revisited. ChemBioChem 2012, 13, 1680–1688. [Google Scholar] [CrossRef]
- Sanchez, J.F.; Entwistle, R.; Hung, J.H.; Yaegashi, J.; Jain, S.; Chiang, Y.M.; Wang, C.C.; Oakley, B.R. Genome-based deletion analysis reveals the prenyl xanthone biosynthesis pathway in Aspergillus nidulans. J. Am. Chem. Soc. 2011, 133, 4010–4017. [Google Scholar] [CrossRef]
- Slavov, N.; Cvengros, J.; Neudorfl, J.M.; Schmalz, H.G. Total synthesis of the marine antibiotic pestalone and its surprisingly facile conversion into pestalalactone and pestalachloride A. Angew. Chem. Int. Ed. 2010, 49, 7588–7591. [Google Scholar] [CrossRef] [PubMed]
- Augner, D.; Gerbino, D.C.; Slavov, N.; Neudörfl, J.M.; Schmalz, H.G. N-Capping of primary amines with 2-acyl-benzaldehydes to give isoindolinones. Org. Lett. 2011, 13, 5374–5377. [Google Scholar] [CrossRef] [PubMed]
- Wachi, Y.; Yamashita, T.; Komatsu, K.; Yoshida, S. JP Patent JKXXAF JP 07061950 A2 19950307, 1995.
- Cueto, M.; Jensen, P.R.; Kauffman, C.; Fenical, W.; Lobkovsky, E.; Clardy, J. Pestalone, a New Antibiotic Produced by a Marine Fungus in Response to Bacterial Challenge. J. Nat. Prod. 2001, 64, 1444–1446. [Google Scholar] [CrossRef]
- Wei, M.-Y.; Li, D.; Shao, C.-L.; Deng, D.-S.; Wang, C.-Y. (±)−Pestalachloride D, an Antibacterial Racemate of Chlorinated Benzophenone Derivative from a Soft Coral-Derived Fungus Pestalotiopsis sp. Mar. Drugs 2013, 11, 1050–1060. [Google Scholar] [CrossRef]
- Xing, Q.; Gan, L.-S.; Mou, X.-F.; Wang, W.; Wang, C.-Y.; Wei, M.-Y.; Shao, C.-L. Isolation, resolution and biological evaluation of pestalachlorides E and F containing both point and axial chirality. RSC Adv. 2016, 6, 22653–22658. [Google Scholar] [CrossRef]
- Wang, W.; Park, C.; Oh, E.; Sung, Y.; Lee, J.; Park, K.-H.; Kang, H. Benzophenone Compounds, from a Marine-Derived Strain of the Fungus Pestalotiopsis neglecta, Inhibit Proliferation of Pancreatic Cancer Cells by Targeting the MEK/ERK Pathway. J. Nat. Prod. 2019, 82, 3357–3365. [Google Scholar] [CrossRef]
- Payne, J.T.; Andorfer, M.C.; Lewis, J.C. Regioselective arene halogenation using the FAD-dependent halogenase RebH. Angew. Chem. Int. Ed. 2013, 52, 5271–5274. [Google Scholar] [CrossRef] [PubMed]
- Bitto, E.; Huang, Y.; Bingman, C.A.; Singh, S.; Thorson, J.S.; Phillips, G.N., Jr. The structure of flavin-dependent tryptophan 7-halogenase RebH. Proteins 2008, 70, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Moritzer, A.C.; Minges, H.; Prior, T.; Frese, M.; Sewald, N.; Niemann, H.H. Structure-based switch of regioselectivity in the flavin-dependent tryptophan 6-halogenase Thal. J. Biol. Chem. 2019, 294, 2529–2542. [Google Scholar] [CrossRef]
- Frese, M.; Sewald, N. Enzymatic halogenation of tryptophan on a gram scale. Angew. Chem. Int. Ed. 2015, 54, 298–301. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Sneath, P.H.; Sokal, R.R. Numerical taxonomy. Nature 1962, 193, 855–860. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically—Tenth Edition, M7-A10; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
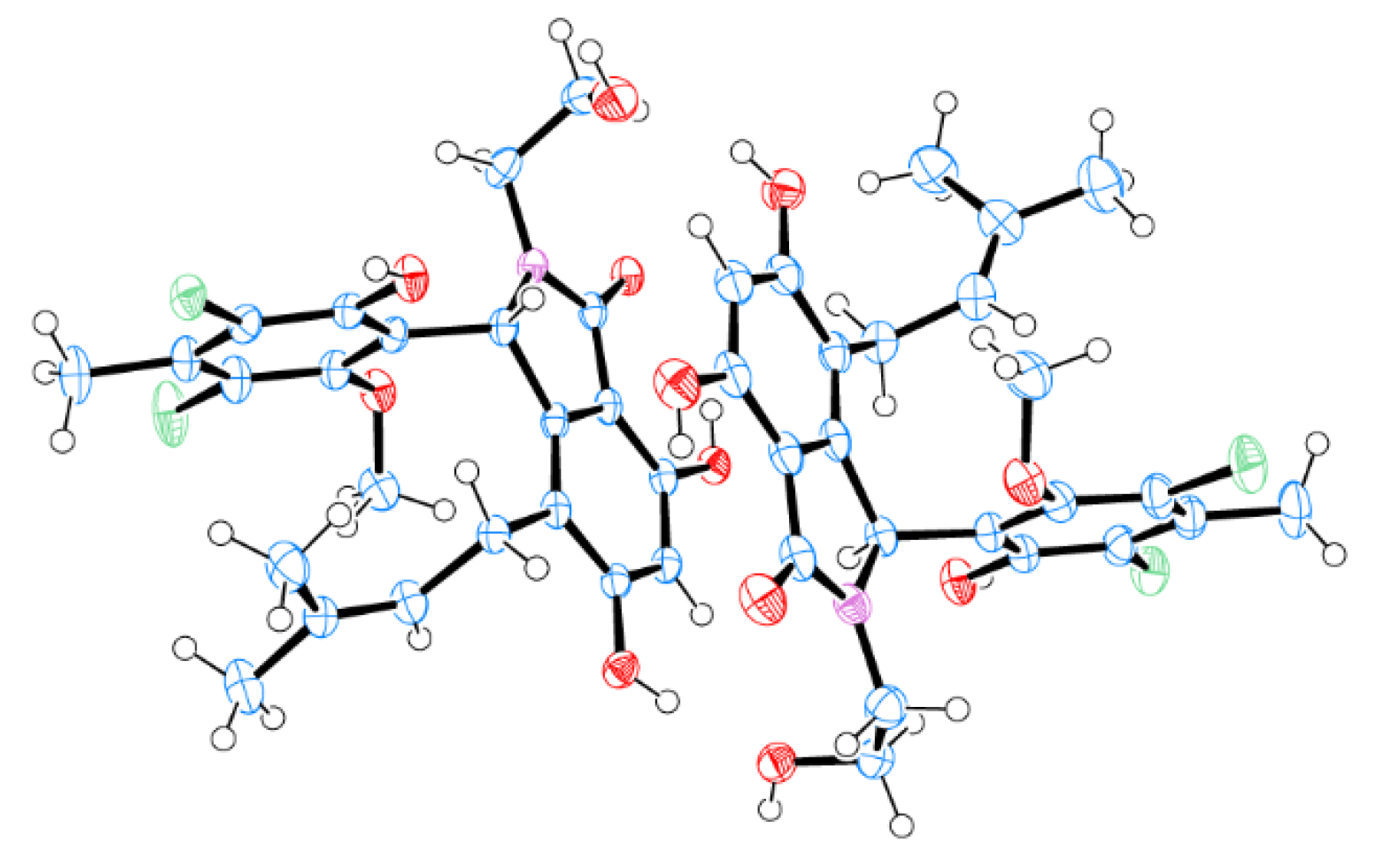

| No. | 1a | 1b * | 2a | 2b * | |||
|---|---|---|---|---|---|---|---|
| δC, Type | δH, Multi. (J in Hz) | δC, Type | δH, Multi. (J in Hz) | δC, Type | δH, Multi. (J in Hz) | δH, Multi. (J in Hz) | |
| 1 | 168.6, C | 169.2, C | 168.5, C | ||||
| 2 | 109.2, C | 109.7, C | 109.0, C | ||||
| 3 | 153.9, C | 153.6, C | 153.9, C | ||||
| 4 | 101.3, CH | 6.32, s | 101.2, CH | 6.28, s | 101.5, CH | 6.32, s | 6.29, s |
| 5 | 159.7, C | 159.6, C | 159.7, C | ||||
| 6 | 114.1, C | 114.3, C | 114.2, C | ||||
| 7 | 146.5, C | 145.7, C | 146.5, C | ||||
| 8 | 55.1, CH | 6.13, s | 57.1, CH | 6.02, s | 54.5, CH | 6.09, s | 5.96, s |
| 9 | 117.8, C | 117.0, C | 119.1, C | ||||
| 10 | 151.1, C | 151.6, C | 151.2, C | ||||
| 11 | 117.8, C | 117.7, C | 117.9, C | ||||
| 12 | 134.9, C | 134.8, C | 134.9, C | ||||
| 13 | 119.1, C | 118.5, C | 117.7, C | ||||
| 14 | 154.7, C | 154.3, C | 154.7, C | ||||
| 15 | 59.8, -OCH3 | 3.05, s | 61.5, -OCH3 | 3.97, s | 59.8, -OCH3 | 3.04, s | 3.97, s |
| 16 | 41.8, CH2 | 2.58, dt (13.3, 5.4) | 42.3, CH2 | 2.85–2.78, m | 38.4, CH2 | 2.56, dt (13.9, 5.9) | 2.84, dt (13.6, 6.6) |
| 3.70, dt (13.9, 6.9) | 3.40–3.35, m | 3.63, dt (14.7, 7.6) | 3.54–3.46, m | ||||
| 17 | 58.3, CH2 | 3.45, dt (11.5, 5.1) | 58.8, CH2 | 3.59–3.50, m | 23.2, CH2 | 1.69, m | 1.69, m |
| 18 | - | - | - | - | 31.1, CH2 | 2.16, t (7.4) | 2.18, t (6.6) |
| 1’ | 23.8, CH2 | 2.78, dd (15.1, 6.7) | 23.6, CH2 | 2.73, dd, (15.4, 6.2) | 23.9, CH2 | 2.77, dd (15.1, 6.7) | 2.72, dd (15.7, 5.6) |
| 2.93, dd (15.4, 5.2) | 2.96, dd, (15.5, 5.5) | 2.94, dd (15.4, 5.0) | 2.94, dd, (15.4, 5.0) | ||||
| 2’ | 122.3, CH | 4.37, t (5.4) | 122.6, CH | 4.16, t (5.8) | 122.3, CH | 4.36, m | 4.16, m |
| 3’ | 129.7, C | 129.5, C | 129.7, C | ||||
| 4’ | 25.1, CH3 | 1.31, s | 25.1, CH3 | 1.29, s | 25.1, CH3 | 1.31, s | 1.29, s |
| 5’ | 17.4, CH3 | 1.39, s | 17.5, CH3 | 1.40, s | 17.4, CH3 | 1.38, s | 1.39, s |
| 1” | 18.1, CH3 | 2.39, s | 18.1, CH3 | 2.38, s | 18.1, CH3 | 2.39, s | 2.38, s |
| OH-3 | 9.08, s | 8.98, s | 9.09, s | 8.98, s | |||
| OH-5 | 9.86, s | 9.25, s | 9.88, s | 9.25, s | |||
| OH-10 | 10.03, s | 9.79, s | 10.06, s | 9.81, s | |||
| OH-17 | 3.36, s | 4.78, s | - | - | - | ||
| COOH | - | - | - | - | 174.0, C | 11.99, s | 11.99, s |
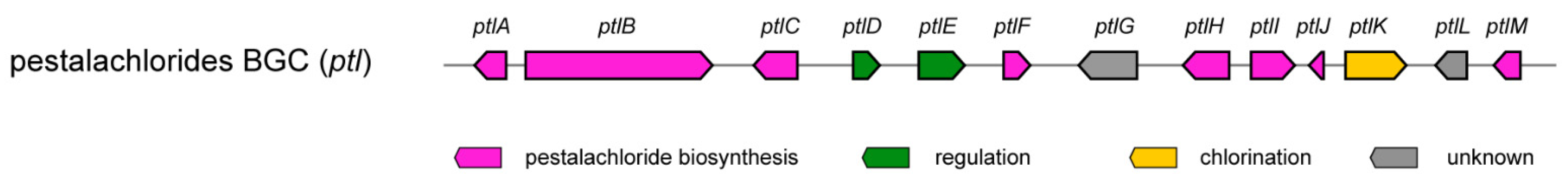 | ||||
|---|---|---|---|---|
| Genes | Putative Function | Ged Homolog (% id.) | Pta Homolog (% id.) | Mdp Homolog (% id.) |
| ptlA | Lactamase B | GedB (70) | PtaB (92) | MdpF (67) |
| ptlB | non-reducing PKS | GedC (63) | PtaA (87) | MdpG (66) |
| ptlC | Baeyer-Villiger oxidase | GedK (45) | PtaJ (47) | MdpL (43) |
| ptlD | transcriptional regulator | GedD (39) | PtaR1 (36) | MdpA (38) |
| ptlE | transcriptional regulator | GedR (61) | PtaR2 (29) | MdpE (29) |
| ptlF | Glutathione S-transferase | - | - | MdpJ (39) |
| ptlG | Pyranose dehydrogenase | - | - | - |
| ptlH | Xanthone prenyltransferase | - | - | - |
| ptlI | O-methyltransferase | - | - | - |
| ptlJ | Anthrone oxygenase | GedH (44) | PtaC (41) | MdpH2 (43) |
| ptlK | Flavine halogenase | GedL (51) | PtaM (47) | - |
| ptlL | Short-chain dehydrogenase | - | - | MdpC (25) |
| ptlM | Oxidoreductase | GedF (48) | PtaF (49) | MdpK (51) |
| Antibacterial Activity (MIC, μg/mL) | ||||||
|---|---|---|---|---|---|---|
| Compounds | Positive Controls | |||||
| 1a | 2a | 3 | Fluconazole | Vancomycin | Meropenem | |
| S. Aureusa | 32 | > 32 | 8 | ND | 0.5 | ND |
| MRSA b | 32 | > 32 | 4 | ND | 0.5 | 16 |
| E. faeciumc | 32 | > 32 | 16 | ND | 0.25 | ND |
| VRE d | 32 | > 32 | 8 | ND | 16 | ND |
| C. albicanse | > 32 | > 32 | > 32 | 0.5 | ND | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, M.; Wang, M.; Chang, S.; He, N.; Shan, G.; Xie, Y. Halogenase-Targeted Genome Mining Leads to the Discovery of (±) Pestalachlorides A1a, A2a, and Their Atropisomers. Antibiotics 2022, 11, 1304. https://doi.org/10.3390/antibiotics11101304
Luo M, Wang M, Chang S, He N, Shan G, Xie Y. Halogenase-Targeted Genome Mining Leads to the Discovery of (±) Pestalachlorides A1a, A2a, and Their Atropisomers. Antibiotics. 2022; 11(10):1304. https://doi.org/10.3390/antibiotics11101304
Chicago/Turabian StyleLuo, Mengna, Mengyuan Wang, Shanshan Chang, Ning He, Guangzhi Shan, and Yunying Xie. 2022. "Halogenase-Targeted Genome Mining Leads to the Discovery of (±) Pestalachlorides A1a, A2a, and Their Atropisomers" Antibiotics 11, no. 10: 1304. https://doi.org/10.3390/antibiotics11101304
APA StyleLuo, M., Wang, M., Chang, S., He, N., Shan, G., & Xie, Y. (2022). Halogenase-Targeted Genome Mining Leads to the Discovery of (±) Pestalachlorides A1a, A2a, and Their Atropisomers. Antibiotics, 11(10), 1304. https://doi.org/10.3390/antibiotics11101304







