Antibiotic Choices for Pediatric Periorbital Cellulitis—A 20-Year Retrospective Study from Taiwan
Abstract
:1. Introduction
2. Results
2.1. Demographics and Characteristics of Patients
2.2. The Culture Results from Different Culture Sites
2.3. The Cultured Bacteria in Preseptal and Orbital Cellulitis
3. Discussion
4. Materials and Methods
4.1. Participants and Ethics
4.2. Data Retrieval and Processing
4.3. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murphy, D.C.; Meghji, S.; Alfiky, M.; Bath, A.P. Paediatric periorbital cellulitis: A 10-year retrospective case series review. J Paediatr. Child Health 2021, 57, 227–233. [Google Scholar] [CrossRef]
- Santos, J.C.; Pinto, S.; Ferreira, S.; Maia, C.; Alves, S.; da Silva, V. Pediatric preseptal and orbital cellulitis: A 10-year experience. Int. J. Pediatr. Otorhinolaryngol. 2019, 120, 82–88. [Google Scholar] [CrossRef]
- Rashed, F.; Cannon, A.; Heaton, P.A.; Paul, S.P. Diagnosis, management and treatment of orbital and periorbital cellulitis in children. Emerg Nurse 2016, 24, 30–35, quiz 37. [Google Scholar] [CrossRef]
- Goncalves, R.; Menezes, C.; Machado, R.; Ribeiro, I.; Lemos, J.A. Periorbital cellulitis in children: Analysis of outcome of intravenous antibiotic therapy. Orbit 2016, 35, 175–180. [Google Scholar] [CrossRef]
- Ekhlassi, T.; Becker, N. Preseptal and orbital cellulitis. Dis. A-Mon. DM 2017, 63, 30–32. [Google Scholar] [CrossRef]
- Wong, S.J.; Levi, J. Management of pediatric orbital cellulitis: A systematic review. Int. J. Pediatr. Otorhinolaryngol. 2018, 110, 123–129. [Google Scholar] [CrossRef]
- Oxford, L.E.; McClay, J. Complications of acute sinusitis in children. Otolaryngol. Head Neck Surg. 2005, 133, 32–37. [Google Scholar] [CrossRef]
- Liu, I.T.; Kao, S.C.; Wang, A.G.; Tsai, C.C.; Liang, C.K.; Hsu, W.M. Preseptal and orbital cellulitis: A 10-year review of hospitalized patients. J. Chin. Med. Assoc. 2006, 69, 415–422. [Google Scholar] [CrossRef]
- Bagheri, A.; Tavakoli, M.; Aletaha, M.; Salour, H.; Ghaderpanah, M. Orbital and preseptal cellulitis: A 10-year survey of hospitalized patients in a tertiary eye hospital in Iran. Int. Ophthalmol. 2012, 32, 361–367. [Google Scholar] [CrossRef]
- Amin, N.; Syed, I.; Osborne, S. Assessment and management of orbital cellulitis. Br. J. Hosp. Med. 2016, 77, 216–220. [Google Scholar] [CrossRef]
- Botting, A.M.; McIntosh, D.; Mahadevan, M. Paediatric pre- and post-septal peri-orbital infections are different diseases. A retrospective review of 262 cases. Int. J. Pediatr. Otorhinolaryngol. 2008, 72, 377–383. [Google Scholar] [CrossRef]
- Napierkowski, D. Uncovering common bacterial skin infections. Nurse Pract. 2013, 38, 30–37, quiz 37-38. [Google Scholar] [CrossRef]
- Mathias, M.T.; Horsley, M.B.; Mawn, L.A.; Laquis, S.J.; Cahill, K.V.; Foster, J.; Amato, M.M.; Durairaj, V.D. Atypical presentations of orbital cellulitis caused by methicillin-resistant Staphylococcus aureus. Ophthalmology 2012, 119, 1238–1243. [Google Scholar] [CrossRef]
- Beech, T.; Robinson, A.; McDermott, A.; Sinha, A. Paediatric periorbital cellulitis and its management. Rhinology 2007, 45, 47–49. [Google Scholar]
- Nageswaran, S.; Woods, C.R.; Benjamin, D.K., Jr.; Givner, L.B.; Shetty, A.K. Orbital cellulitis in children. Pediatric Infect. Dis. J. 2006, 25, 695–699. [Google Scholar] [CrossRef]
- Yang, M.; Quah, B.L.; Seah, L.L.; Looi, A. Orbital cellulitis in children-medical treatment versus surgical management. Orbit 2009, 28, 124–136. [Google Scholar] [CrossRef]
- Fanella, S.; Singer, A.; Embree, J. Presentation and management of pediatric orbital cellulitis. Can. J. Infect. Dis. Med. Microbiol. 2011, 22, 97–100. [Google Scholar] [CrossRef]
- Sharma, A.; Liu, E.S.; Le, T.D.; Adatia, F.A.; Buncic, J.R.; Blaser, S.; Richardson, S. Pediatric orbital cellulitis in the Haemophilus influenzae vaccine era. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2015, 19, 206–210. [Google Scholar] [CrossRef]
- Seltz, L.B.; Smith, J.; Durairaj, V.D.; Enzenauer, R.; Todd, J. Microbiology and antibiotic management of orbital cellulitis. Pediatrics 2011, 127, e566–e572. [Google Scholar] [CrossRef]
- Giletto, J.B.; Scherr, S.A.; Mikaelian, D.O. Orbital complications of acute sinusitis in children. Trans. -Pa. Acad. Ophthalmol. Otolaryngol. 1981, 34, 60–64. [Google Scholar]
- Le, T.D.; Liu, E.S.; Adatia, F.A.; Buncic, J.R.; Blaser, S. The effect of adding orbital computed tomography findings to the Chandler criteria for classifying pediatric orbital cellulitis in predicting which patients will require surgical intervention. J. Am. Assoc. Pediatric Ophthalmol. Strabismus 2014, 18, 271–277. [Google Scholar] [CrossRef]
- Babar, T.F.; Zaman, M.; Khan, M.N.; Khan, M.D. Risk factors of preseptal and orbital cellulitis. J. Coll. Physicians Surg. Pak. 2009, 19, 39–42. [Google Scholar]
- Georgakopoulos, C.D.; Eliopoulou, M.I.; Stasinos, S.; Exarchou, A.; Pharmakakis, N.; Varvarigou, A. Periorbital and orbital cellulitis: A 10-year review of hospitalized children. Eur. J. Ophthalmol. 2010, 20, 1066–1072. [Google Scholar] [CrossRef]
- Givner, L.B. Periorbital versus orbital cellulitis. Pediatric Infect. Dis. J. 2002, 21, 1157–1158. [Google Scholar] [CrossRef]
- Shih, E.J.; Chen, J.K.; Tsai, P.J.; Bee, Y.S. Differences in characteristics, aetiologies, isolated pathogens, and the efficacy of antibiotics in adult patients with preseptal cellulitis and orbital cellulitis between 2000–2009 and 2010–2019. Br. J. Ophthalmol. 2021; Online ahead of print. [Google Scholar] [CrossRef]
- McKinley, S.H.; Yen, M.T.; Miller, A.M.; Yen, K.G. Microbiology of pediatric orbital cellulitis. Am. J. Ophthalmol. 2007, 144, 497–501. [Google Scholar] [CrossRef]
- Sadow, K.B.; Chamberlain, J.M. Blood cultures in the evaluation of children with cellulitis. Pediatrics 1998, 101, E4. [Google Scholar] [CrossRef]
- Harris, G.J. Subperiosteal abscess of the orbit. Age as a factor in the bacteriology and response to treatment. Ophthalmology 1994, 101, 585–595. [Google Scholar] [CrossRef]
- Wald, E.R. Periorbital and orbital infections. Infect. Dis. Clin. N. Am. 2007, 21, 393–408, vi. [Google Scholar] [CrossRef]
- Wang, J.T.; Fang, C.T.; Chen, Y.C.; Wu, C.L.; Chen, M.L.; Chang, S.C. Staphylococcal cassette chromosome mec in MRSA, Taiwan. Emerg. Infect. Dis. 2007, 13, 494–497. [Google Scholar] [CrossRef]
- Hsueh, P.R.; Chen, M.L.; Sun, C.C.; Chen, W.H.; Pan, H.J.; Yang, L.S.; Chang, S.C.; Ho, S.W.; Lee, C.Y.; Hsieh, W.C.; et al. Antimicrobial drug resistance in pathogens causing nosocomial infections at a university hospital in Taiwan, 1981–1999. Emerg. Infect. Dis. 2002, 8, 63–68. [Google Scholar] [CrossRef]
- Chang, Y.S.; Chen, P.L.; Hung, J.H.; Chen, H.Y.; Lai, C.C.; Ou, C.Y.; Chang, C.M.; Wang, C.K.; Cheng, H.C.; Tseng, S.H. Orbital complications of paranasal sinusitis in Taiwan, 1988 through 2015: Acute ophthalmological manifestations, diagnosis, and management. PLoS ONE 2017, 12, e0184477. [Google Scholar] [CrossRef]
- Huang, S.F.; Lee, T.J.; Lee, Y.S.; Chen, C.C.; Chin, S.C.; Wang, N.C. Acute rhinosinusitis-related orbital infection in pediatric patients: A retrospective analysis. Ann. Otol. Rhinol. Laryngol. 2011, 120, 185–190. [Google Scholar] [CrossRef]
- Lin, S.W.; Wang, Y.H.; Lee, M.Y.; Ku, M.S.; Sun, H.L.; Lu, K.H.; Lue, K.H. Clinical spectrum of acute rhinosinusitis among atopic and nonatopic children in Taiwan. Int. J. Pediatr. Otorhinolaryngol. 2012, 76, 70–75. [Google Scholar] [CrossRef]
- Bedwell, J.; Bauman, N.M. Management of pediatric orbital cellulitis and abscess. Curr. Opin. Otolaryngol. Head Neck Surg. 2011, 19, 467–473. [Google Scholar] [CrossRef]
- Bamberger, D.M.; Boyd, S.E. Management of Staphylococcus aureus infections. Am. Fam. Physician 2005, 72, 2474–2481. [Google Scholar]
- Lee, A.S.; De Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Primers 2018, 4, 18033. [Google Scholar] [CrossRef]
- Adamson, J.; Waterfield, T. Fifteen-minute consultation: Preseptal and orbital cellulitis. Arch. Dis. Child. Educ. Pract. 2019, 104, 79–83. [Google Scholar] [CrossRef]
- Ho, T.S.; Wang, S.M.; Shen, C.F.; Lee, K.H.; Liu, C.C. Clinical perspectives of childhood tuberculosis in Taiwan. J. Formos. Med. Assoc. 2011, 110, 737–743. [Google Scholar] [CrossRef]
- Chalumeau, M.; Tonnelier, S.; D’Athis, P.; Tréluyer, J.M.; Gendrel, D.; Bréart, G.; Pons, G. Fluoroquinolone safety in pediatric patients: A prospective, multicenter, comparative cohort study in France. Pediatrics 2003, 111, e714–e719. [Google Scholar] [CrossRef]
- Lee, S.; Yen, M.T. Management of preseptal and orbital cellulitis. Saudi J. Ophthalmol. 2011, 25, 21–29. [Google Scholar] [CrossRef]
- Brook, I. Microbiology and antimicrobial treatment of orbital and intracranial complications of sinusitis in children and their management. Int. J. Pediatric Otorhinolaryngol. 2009, 73, 1183–1186. [Google Scholar] [CrossRef]
- Mahalingam, S.; Hone, R.; Lloyd, G.; Grounds, R.; Shamil, E.; Wong, G.; Al-Lami, A.; Pervez, A.; Rudd, J.; Poon, J.S.; et al. The management of periorbital cellulitis secondary to sinonasal infection: A multicenter prospective study in the United Kingdom. Int. Forum Allergy Rhinol. 2020, 10, 726–737. [Google Scholar] [CrossRef]
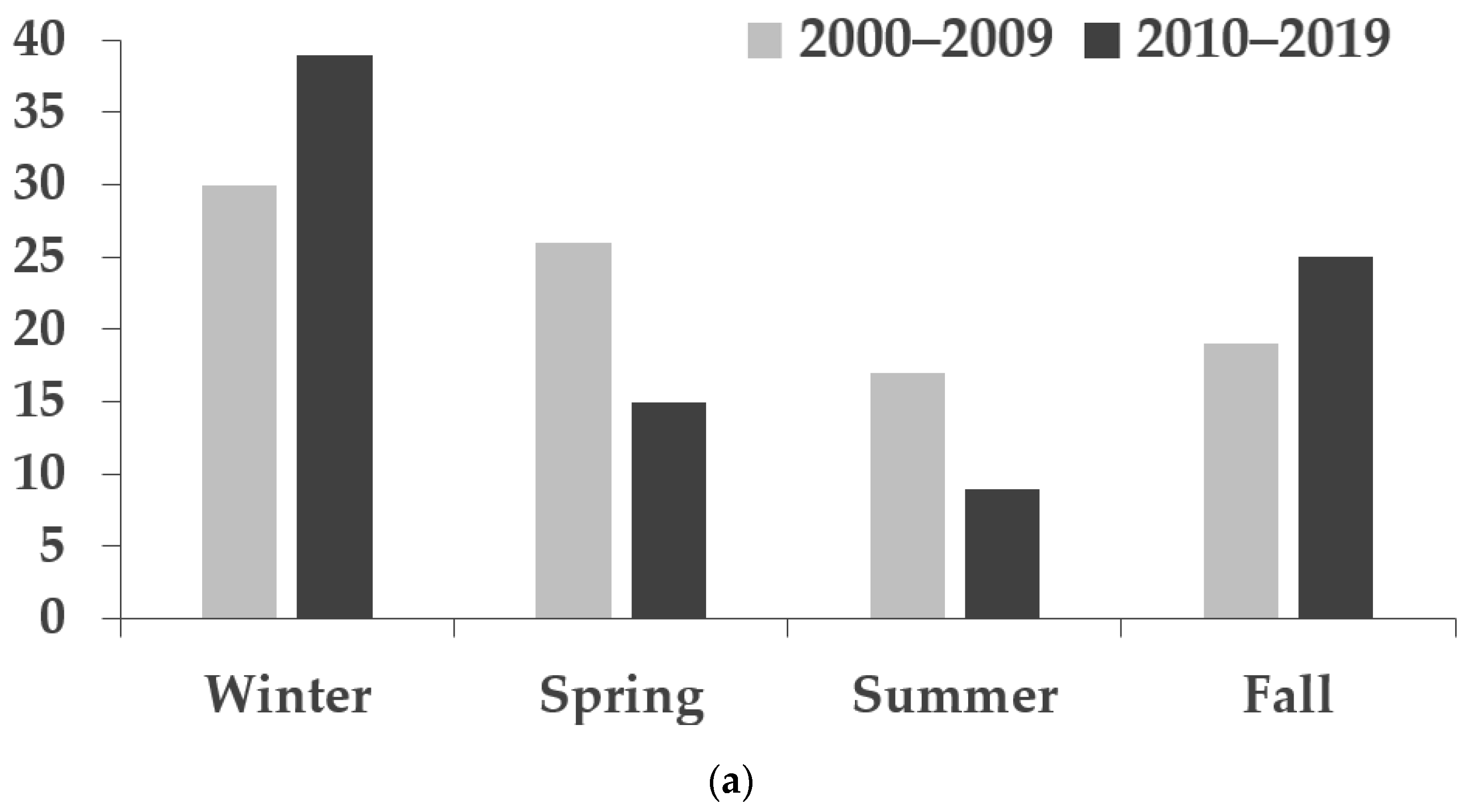
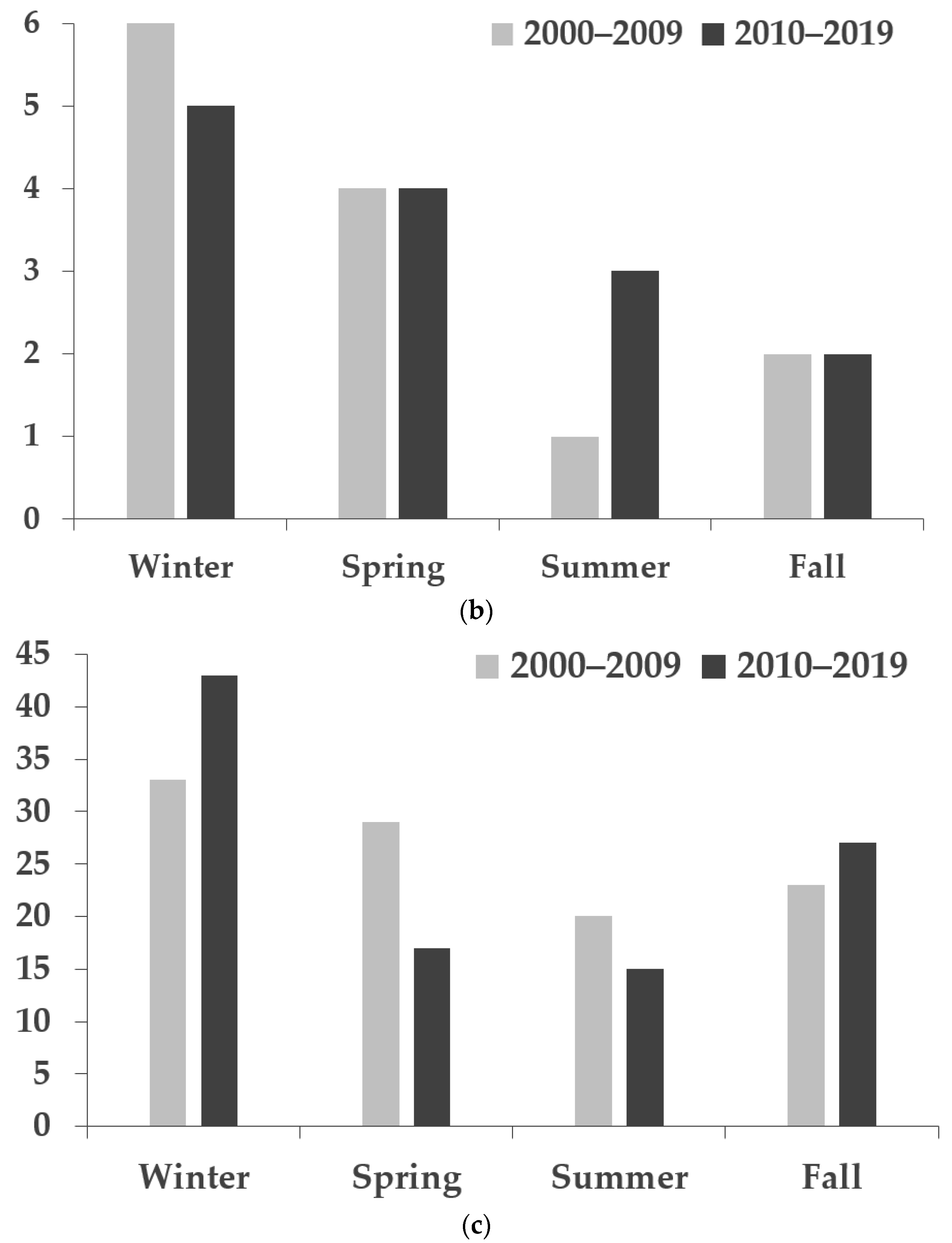
| 2000–2009 | 2010–2019 | ||||||
|---|---|---|---|---|---|---|---|
| Preseptal Cellulitis (n = 92) | Orbital Cellulitis (n = 13) | Total (n = 105) | Preseptal Cellulitis (n = 88) | Orbital Cellulitis (n = 14) | Total (n = 102) | p-Value | |
| Sex (%) a | |||||||
| Male | 48 (52.2) | 7 (53.9) | 55 (52.4) | 34 (38.6) | 9 (64.3) | 43 (42.2) | 0.141 |
| Female | 44 (47.8) | 6 (46.2) | 50 (47.6) | 54 (61.4) | 5 (35.7) | 59 (57.8) | |
| Eye (%) a | |||||||
| Right | 37 (40.2) | 3 (23.1) | 40 (38.1) | 30 (34.1) | 8 (57.1) | 38 (37.3) | 0.942 |
| Left | 50 (54.3) | 9 (69.2) | 59 (56.2) | 52 (59.1) | 5 (35.7) | 57 (55.9) | |
| Both | 5 (5.4) | 1 (7.7) | 6 (5.7) | 6 (6.8) | 1 (7.1) | 7 (6.9) | |
| Age (%) b | |||||||
| Mean ± SD | 3.5 ± 3.8 | 5.0 ± 4.0 | 3.7 ± 3.8 | 4.1 ± 4.3 | 6.3 ± 5.6 | 4.4 ± 4.5 | 0.212 |
| Median (IQR) | 2 (1.0–4.8) | 6 (0.5–8.5) | 2 (1.0–6.0) | 2.3 (1.3–4.8) | 5 (1.5–10.3) | 2.5 (1.4–6.0) | |
| Etiology (%) a | |||||||
| Dacryocystitis | 2 (2.2) | 0 (0.0) | 2 (1.9) | 1 (1.1) | 0 (0.0) | 1 (1.0) | 1.000 |
| Dacryoadenitis | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (7.1) | 1 (1.0) | 0.493 |
| Hordeolum | 16 (17.4) | 1 (7.7) | 17 (16.2) | 13 (14.8) | 1 (7.1) | 14 (13.7) | 0.619 |
| Conjunctivitis | 3 (3.3) | 0 (0.0) | 3 (2.9) | 5 (5.7) | 2 (14.3) | 7 (6.9) | 0.210 |
| Insect/animal bite | 16 (17.4) | 0 (0.0) | 16 (15.2) | 17 (19.3) | 6 (42.9) | 23 (22.5) | 0.084 |
| URI | 17 (18.5) | 0 (0.0) | 17 (16.2) | 16 (18.2) | 2 (14.3) | 18 (17.6) | 0.780 |
| Skin infection | 9 (9.8) | 0 (0.0) | 9 (8.6) | 8 (9.1) | 1 (7.1) | 9 (8.8) | 0.849 |
| Sinusitis | 10 (10.9) | 4 (30.8) | 14 (13.3) | 5 (5.7) | 0 (0.0) | 5 (4.9) | 0.016 |
| Odontogenic origin | 2 (2.2) | 2 (15.4) | 4 (3.8) | 4 (4.5) | 0 (0.0) | 4 (3.9) | 1.000 |
| Periocular trauma | 3 (3.3) | 0 (0.0) | 3 (2.9) | 2 (2.3) | 0 (0.0) | 2 (2.0) | 1.000 |
| Acute tonsillitis | 1 (1.1) | 0 (0.0) | 1 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1.000 |
| Dermoid cyst rupture | 0 (0.0) | 1 (7.7) | 1 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1.000 |
| Scarlet fever | 0 (0.0) | 1 (7.7) | 1 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1.000 |
| Malignancy | 0 (0.0) | 1 (7.7) | 1 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1.000 |
| Unknown causes | 13 (14.1) | 3 (23.1) | 16 (15.2) | 17 (19.3) | 1 (7.1) | 18 (17.6) | 0.640 |
| 2000–2009 | 2010–2019 | |||
|---|---|---|---|---|
| Preseptal Cellulitis | Orbital Cellulitis | Preseptal Cellulitis | Orbital Cellulitis | |
| Local culture (%) | ||||
| Conjunctival swab | 9/14 (64.3) | 3/5 (60.0) | 8/12 (66.7) | 2/3 (66.7) |
| Abscess | 16/18 (88.9) | 0 (0.0) | 9/12 (75.0) | 4/4 (100.0) |
| Nasopharyngeal swab | 2/2 (100.0) | 1/1 (100.0) | 0 (0.0) | 0 (0.0) |
| Total | 27/34 (79.4) | 4/6 (66.7) | 17/24 (70.8) | 6/7 (85.7) |
| Systemic culture (%) | ||||
| Blood | 2/70 (2.9) | 0/8 (0.0) | 1/78 (1.3) | 0/13 (0.0) |
| 2000–2009 | 2010–2019 | ||||||
|---|---|---|---|---|---|---|---|
| Preseptal Cellulitis (n = 31) | Orbital Cellulitis (n = 5) | Total (n = 36) | Preseptal Cellulitis (n = 19) | Orbital Cellulitis (n = 6) | Total (n = 25) | p-Value a | |
| Pathogen (%) | |||||||
| MSSA | 11 (35.5) | 0 | 11 (30.6) | 1 (5.3) | 1 (16.7) | 2 (8.0) | 0.055 |
| MRSA | 10 (32.3) | 1 (20.0) | 11 (30.6) | 11 (57.9) | 5 (83.3) | 16 (64.0) | 0.010 |
| Other staphylococcus species b | 5 (16.1) | 0 | 5 (13.9) | 3 (15.8) | 0 | 3 (12.0) | 1.000 |
| Streptococcus species c | 2 (6.5) | 2 (40.0) | 4 (11.1) | 0 | 0 | 0 | 0.137 |
| Others | 3 (9.7) | 2 (40.0) | 5 (13.9) | 4 (21.1) | 0 | 4 (16.0) | 0.727 |
| 2000–2009 | 2010–2019 | |||||
|---|---|---|---|---|---|---|
| Pre-Septal (%) | Orbital (%) | Total (%) | Pre-Septal (%) | Orbital (%) | Total (%) | |
| (n = 31) | (n = 5) | (n = 36) | (n = 19) | (n = 6) | (n = 25) | |
| Pathogen (%) | ||||||
| Gram positive | ||||||
| MSSA | 11 * (35.5) | 11 * (30.6) | 1 (5.3) | 1 (16.7) | 2 (8.0) | |
| MRSA | 10 (32.3) | 1 (20.0) | 11 (30.6) | 11 (57.9) | 5 (83.3) | 16 (64.0) |
| CoNS | 3 a* (9.7) | 3 a* (8.3) | 3 a* (15.8) | 3 a (12.0) | ||
| Staphylococcus epidermidis | 1 (3.2) | 1 (2.8) | ||||
| Staphylococcus hominis | 1 a (3.2) | 1 a (2.8) | ||||
| Streptococcus constellatus | 1* (20.0) | 1* (2.8) | ||||
| Streptococcus viridans | 1 * (3.2) | 1* (2.8) | ||||
| Streptococcus pneumoniae | 1 (3.2) | 1 (2.8) | ||||
| Group A Streptococcus | 1 (20.0) | 1 (2.8) | ||||
| Gram-positive bacilli | 2 (10.5) | 2 (8.0) | ||||
| Gram negative | ||||||
| Nesseria gonorrhea | 1 (20.0) | 1 (2.8) | ||||
| Aeromonas salmonicida | 1 (5.3) | 1 (4.0) | ||||
| Propionibacterium acnes | 1 (3.2) | 1 (2.8) | ||||
| Haemophilus influenzae | 2 * (6.5) | 2 * (5.6) | ||||
| Citrobacter koseri | 1 * (5.3) | 1 (4.0) | ||||
| Eikenella corrodens | 1 * (20.0) | 1 * (2.8) | ||||
| 2000–2009 | 2010–2019 | |||||
|---|---|---|---|---|---|---|
| Antibiotics | Sensitive No. | Resistant No. | Sensitivity Rate (%) | Sensitive No. | Resistant No. | Sensitivity Rate (%) |
| Imipenem | 2 | 0 | 100 | 17 | 0 | 100 |
| Levofloxacin | 20 | 0 | 100 | 17 | 0 | 100 |
| Rifampin | 21 | 0 | 100 | 16 | 0 | 100 |
| Vancomycin | 21 | 0 | 100 | 17 | 0 | 100 |
| Trimethoprim/ sulfamethoxazole | 19 | 3 | 86.4 | 17 | 0 | 100 |
| Minocycline | 11 | 5 | 68.8 | 6 | 0 | 100 |
| Chloramphenicol | 7 | 13 | 35.0 | 10 | 7 | 58.8 |
| Clindamycin | 5 | 16 | 23.8 | 5 | 12 | 29.4 |
| Erythromycin | 4 | 18 | 18.2 | 5 | 12 | 29.4 |
| Oxacillin | 10 | 11 | 47.6 | 2 | 15 | 11.8 |
| Penicillin | 1 | 18 | 5.3 | 0 | 4 | 0 |
| Sensitivity Rate (%) = sensitive no./(sensitive no. + resistant no.) × 100 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shih, E.-J.; Chen, J.-K.; Tsai, P.-J.; Lin, M.-C.; Bee, Y.-S. Antibiotic Choices for Pediatric Periorbital Cellulitis—A 20-Year Retrospective Study from Taiwan. Antibiotics 2022, 11, 1288. https://doi.org/10.3390/antibiotics11101288
Shih E-J, Chen J-K, Tsai P-J, Lin M-C, Bee Y-S. Antibiotic Choices for Pediatric Periorbital Cellulitis—A 20-Year Retrospective Study from Taiwan. Antibiotics. 2022; 11(10):1288. https://doi.org/10.3390/antibiotics11101288
Chicago/Turabian StyleShih, En-Jie, Jui-Kuang Chen, Pei-Jhen Tsai, Muh-Chiou Lin, and Youn-Shen Bee. 2022. "Antibiotic Choices for Pediatric Periorbital Cellulitis—A 20-Year Retrospective Study from Taiwan" Antibiotics 11, no. 10: 1288. https://doi.org/10.3390/antibiotics11101288
APA StyleShih, E.-J., Chen, J.-K., Tsai, P.-J., Lin, M.-C., & Bee, Y.-S. (2022). Antibiotic Choices for Pediatric Periorbital Cellulitis—A 20-Year Retrospective Study from Taiwan. Antibiotics, 11(10), 1288. https://doi.org/10.3390/antibiotics11101288





