Impact of a Novel Anticoccidial Analogue on Systemic Staphylococcus aureus Infection in a Bioluminescent Mouse Model
Abstract
:1. Introduction
2. Materials and Methods
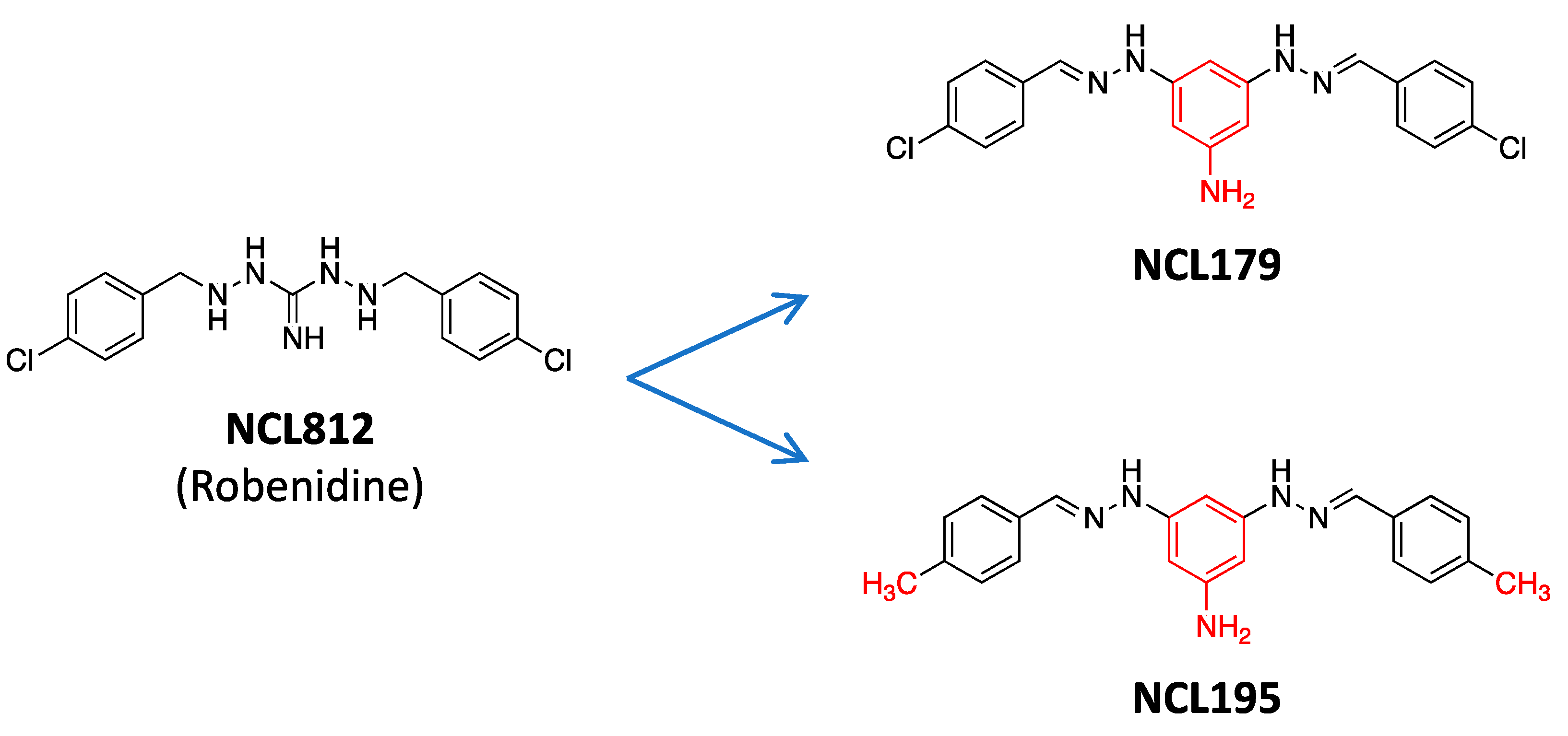
2.1. Antimicrobials and Chemicals
2.2. Organisms and Growth Conditions
2.3. Antimicrobial Susceptibility Testing
2.4. Time-Dependent Growth Inhibitory Assay
2.5. In Vitro Cytotoxicity Assays
2.6. Hemolysis Assay
2.7. Ethics Statements
2.8. Safety Testing of NCL179 Following Oral Administration to Mice
2.9. Agar Well Diffusion Method
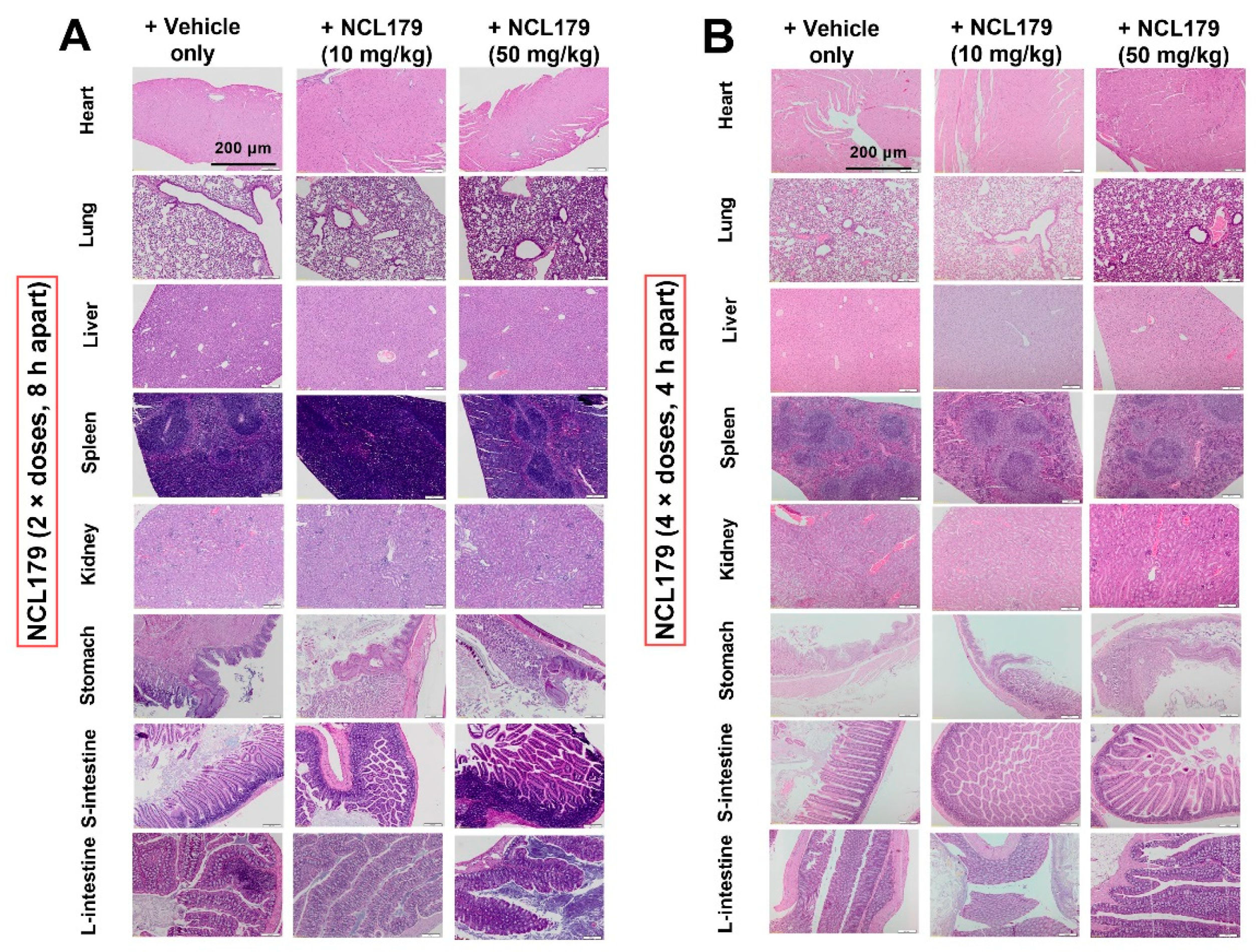
2.10. Histopathological Examination
2.11. Oral Efficacy Testing of NCL179 Following Systemic Challenge of Mice with Bioluminescent S. aureus
3. Results
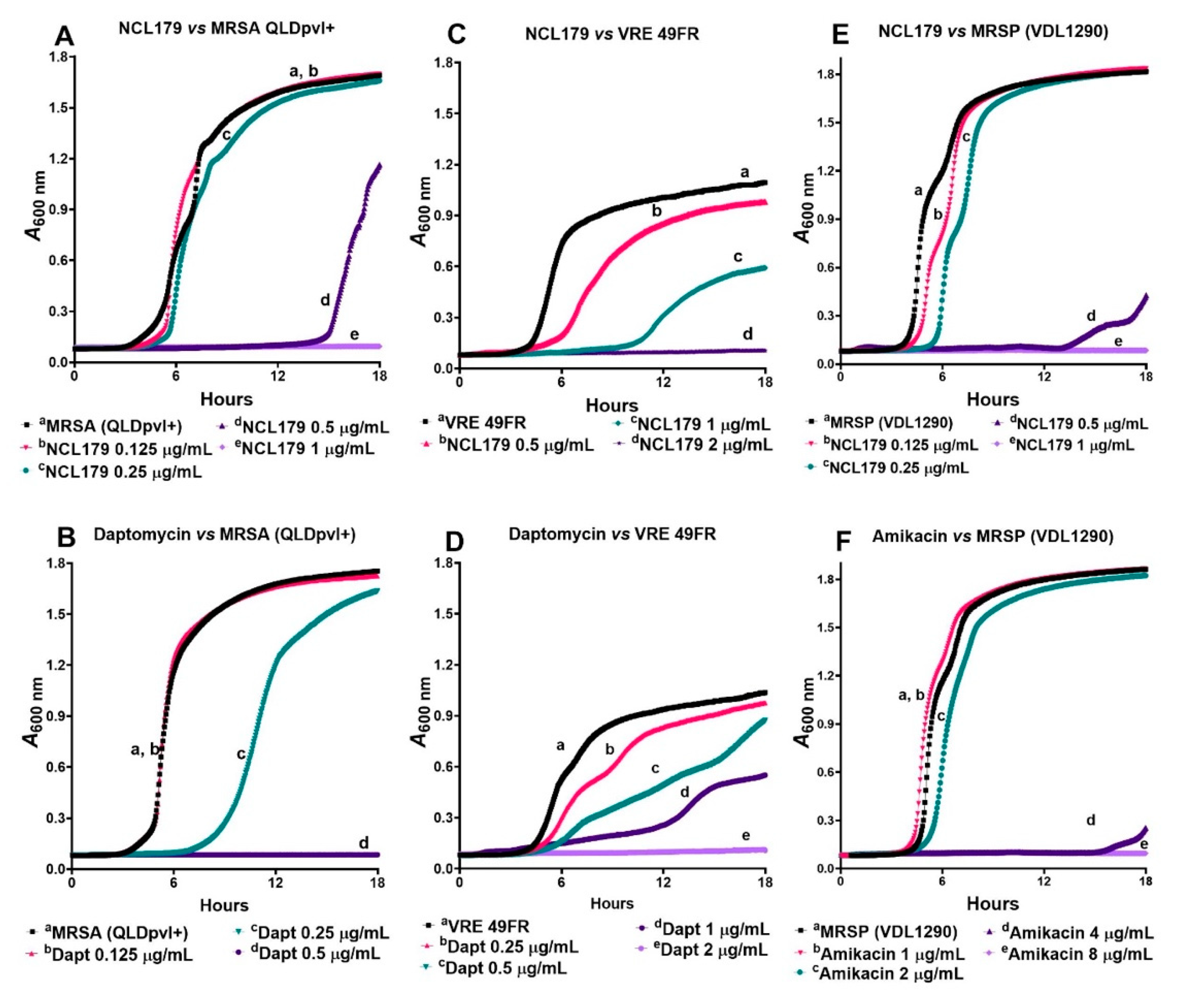
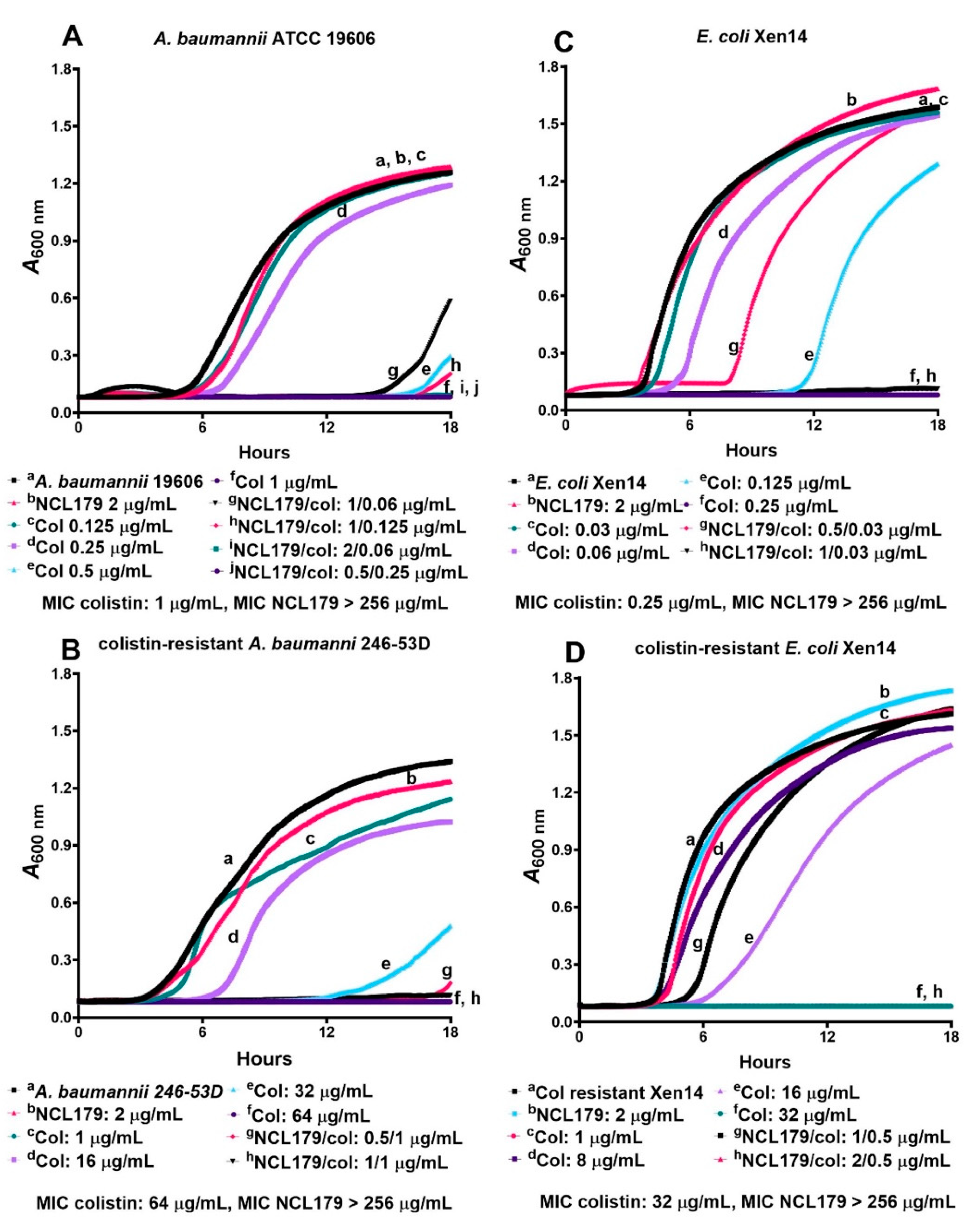
3.1. NCL179 Shows In Vitro Activity against Gram-Positive Pathogens and also against Gram-Negative Pathogens in the Presence of Sub-Inhibitory Concentrations of Colistin
3.2. NCL179 Exhibits Time- and Concentration-Dependent Kill Kinetics of Bacterial Growth
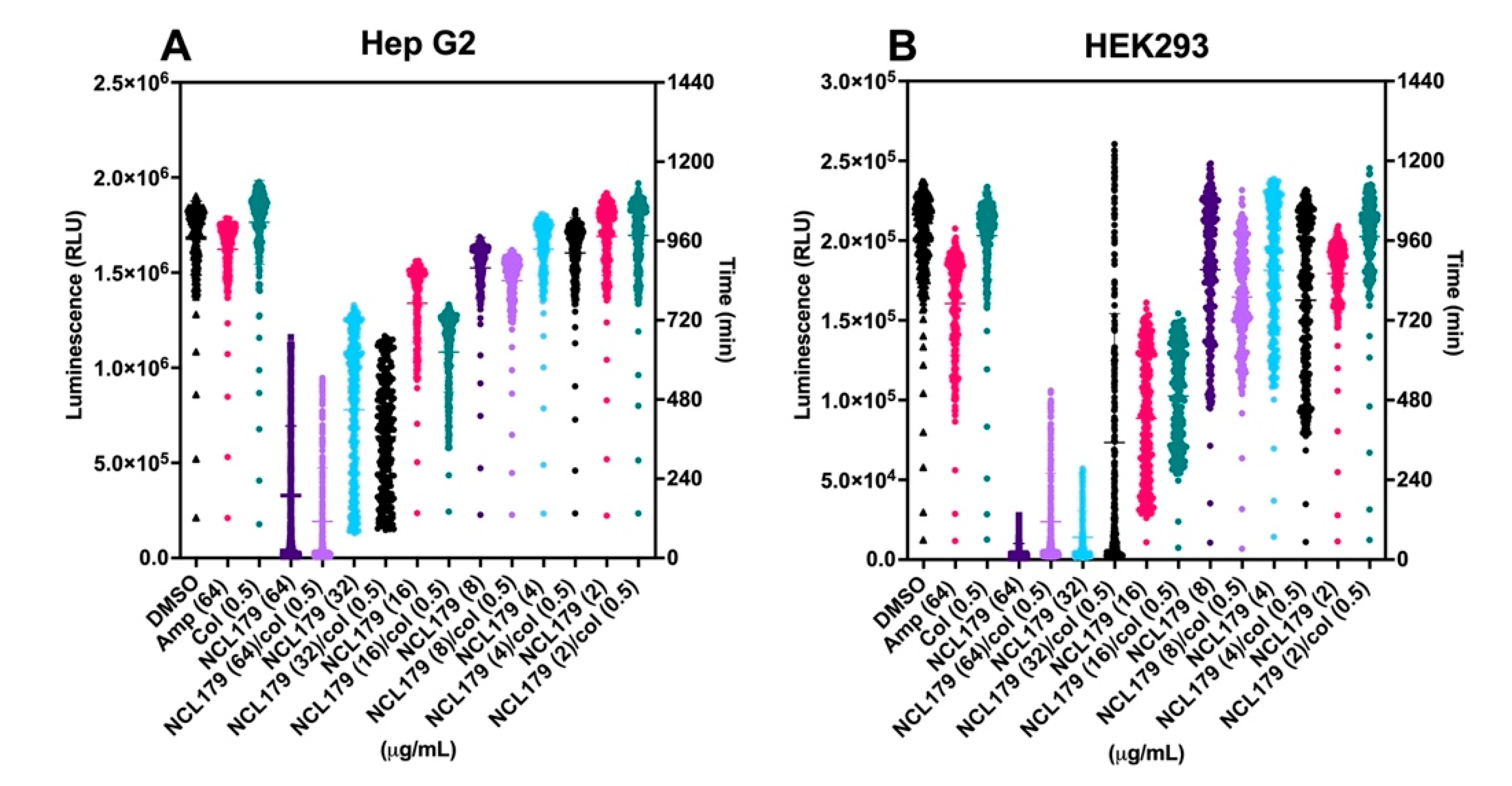
3.3. Combination of NCL179 and Colistin Shows Limited Cytotoxicity to Mammalian Cell Lines
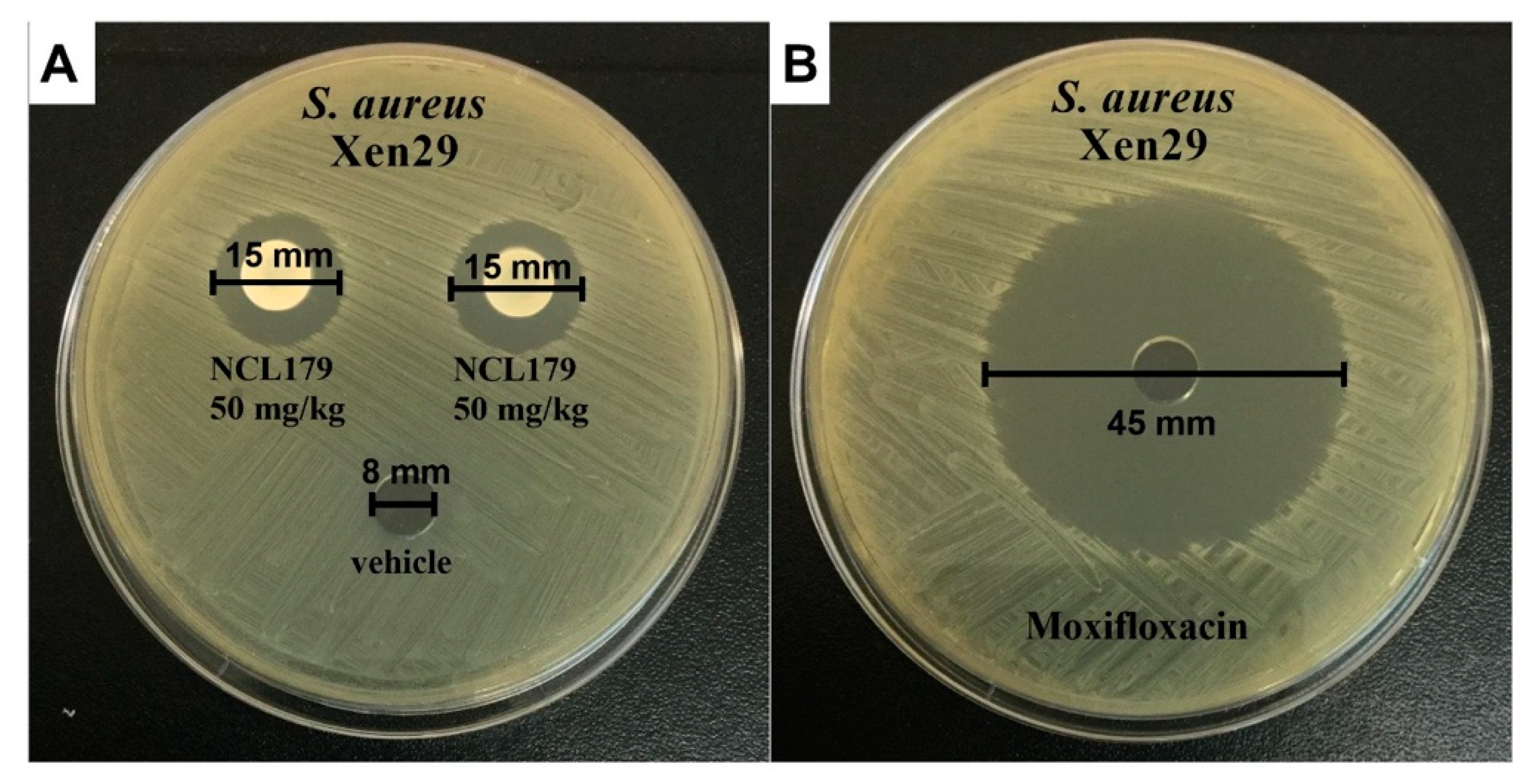
3.4. Agar Well Diffusion Shows NCL179 Remains Active in Formulations
3.5. Oral Administration of NCL179 Shows Systemic Safety in Mice
3.6. Oral Treatment of Mice with NCL179 Reduces S. aureus Populations and Significantly Prolongs Survival Times
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Michael, C.A.; Dominey-Howes, D.; Labbate, M. The antimicrobial resistance crisis: Causes, consequences, and management. Front. Public Health 2014, 2, 145. [Google Scholar] [CrossRef] [PubMed]
- Merker, M.; Tueffers, L.; Vallier, M.; Groth, E.E.; Sonnenkalb, L.; Unterweger, D.; Baines, J.F.; Niemann, S.; Schulenburg, H. Evolutionary Approaches to Combat Antibiotic Resistance: Opportunities and Challenges for Precision Medicine. Front. Immunol. 2020, 11, 1938. [Google Scholar] [CrossRef] [PubMed]
- Pendleton, J.N.; Gorman, S.P.; Gilmore, B.F. Clinical relevance of the ESKAPE pathogens. Expert Rev. Anti-Infect. Ther. 2013, 11, 297–308. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Venter, H.; Veltman, T.; Williams, R.; O’Donovan, L.A.; Russell, C.C.; McCluskey, A.; Page, S.W.; Ogunniyi, A.D.; Trott, D.J. In vitro synergistic activity of NCL195 in combination with colistin against Gram-negative bacterial pathogens. Int. J. Antimicrob. Agents 2021, 57, 106323. [Google Scholar] [CrossRef] [PubMed]
- Pi, H.; Nguyen, H.T.; Venter, H.; Boileau, A.R.; Woolford, L.; Garg, S.; Page, S.W.; Russell, C.C.; Baker, J.R.; McCluskey, A.; et al. In vitro Activity of Robenidine Analog NCL195 in Combination with Outer Membrane Permeabilizers against Gram-Negative Bacterial Pathogens and Impact on Systemic Gram-Positive Bacterial Infection in Mice. Front. Microbiol. 2020, 11, 1556. [Google Scholar] [CrossRef]
- Talbot, G.H.; Jezek, A.; Murray, B.E.; Jones, R.N.; Ebright, R.H.; Nau, G.J.; Rodvold, K.A.; Newland, J.G.; Boucher, H.W. The Infectious Diseases Society of America’s 10 x ‘20 Initiative (10 New Systemic Antibacterial Agents US Food and Drug Administration Approved by 2020): Is 20 x ‘20 a Possibility? Clin. Infect. Dis. 2019, 69, 1–11. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Review on Antimicrobial Resistance: London, UK, 2016; pp. 1–84. [Google Scholar]
- Ma, Y.X.; Wang, C.Y.; Li, Y.Y.; Li, J.; Wan, Q.Q.; Chen, J.H.; Tay, F.R.; Niu, L.N. Considerations and Caveats in Combating ESKAPE Pathogens against Nosocomial Infections. Adv. Sci. 2020, 7, 1901872. [Google Scholar] [CrossRef] [Green Version]
- Bassetti, M.; Peghin, M.; Vena, A.; Giacobbe, D.R. Treatment of Infections Due to MDR Gram-Negative Bacteria. Front. Med. 2019, 6, 74. [Google Scholar] [CrossRef]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef]
- Sheu, C.-C.; Chang, Y.-T.; Lin, S.-Y.; Chen, Y.-H.; Hsueh, P.-R. Infections Caused by Carbapenem-Resistant Enterobacteriaceae: An Update on Therapeutic Options. Front. Microbiol. 2019, 10, 80. [Google Scholar] [CrossRef] [Green Version]
- Zgurskaya, H.I.; Lopez, C.A.; Gnanakaran, S. Permeability barrier of Gram-negative cell envelopes and approaches to bypass it. ACS Infect. Dis. 2015, 1, 512–522. [Google Scholar] [CrossRef] [Green Version]
- Sims, M.; Mariyanovski, V.; McLeroth, P.; Akers, W.; Lee, Y.C.; Brown, M.L.; Du, J.; Pedley, A.; Kartsonis, N.A.; Paschke, A. Prospective, randomized, double-blind, Phase 2 dose-ranging study comparing efficacy and safety of imipenem/cilastatin plus relebactam with imipenem/cilastatin alone in patients with complicated urinary tract infections. J. Antimicrob. Chemother. 2017, 72, 2616–2626. [Google Scholar] [CrossRef] [Green Version]
- Powles, M.A.; Galgoci, A.; Misura, A.; Colwell, L.; Dingley, K.H.; Tang, W.; Wu, J.; Blizzard, T.; Motyl, M.; Young, K. In Vivo Efficacy of Relebactam (MK-7655) in Combination with Imipenem-Cilastatin in Murine Infection Models. Antimicrob. Agents Chemother. 2018, 62, e02577-17. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.R.; Rybak, J.M.; Claeys, K.C. Imipenem-Cilastatin-Relebactam: A Novel β-Lactam-β-Lactamase Inhibitor Combination for the Treatment of Multidrug-Resistant Gram-Negative Infections. Pharmacotherapy 2020, 40, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Titov, I.; Wunderink, R.G.; Roquilly, A.; Rodríguez Gonzalez, D.; David-Wang, A.; Boucher, H.W.; Kaye, K.S.; Losada, M.C.; Du, J.; Tipping, R.; et al. A Randomized, Double-blind, Multicenter Trial Comparing Efficacy and Safety of Imipenem/Cilastatin/Relebactam Versus Piperacillin/Tazobactam in Adults with Hospital-acquired or Ventilator-associated Bacterial Pneumonia (RESTORE-IMI 2 Study). Clin. Infect. Dis. 2020, 73, e4539–e4548. [Google Scholar] [CrossRef]
- Clark, J.A.; Burgess, D.S. Plazomicin: A new aminoglycoside in the fight against antimicrobial resistance. Ther. Adv. Infect. Dis. 2020, 7, 2049936120952604. [Google Scholar] [CrossRef] [PubMed]
- Årdal, C.; Balasegaram, M.; Laxminarayan, R.; McAdams, D.; Outterson, K.; Rex, J.H.; Sumpradit, N. Antibiotic development—Economic, regulatory and societal challenges. Nat. Rev. Microbiol. 2020, 18, 267–274. [Google Scholar] [CrossRef]
- Bergen, P.J.; Bulman, Z.P.; Saju, S.; Bulitta, J.B.; Landersdorfer, C.; Forrest, A.; Li, J.; Nation, R.L.; Tsuji, B.T. Polymyxin combinations: Pharmacokinetics and pharmacodynamics for rationale use. J. Hum. Pharmacol. Drug Ther. 2015, 35, 34–42. [Google Scholar] [CrossRef]
- Mohapatra, S.S.; Dwibedy, S.K.; Padhy, I. Polymyxins, the last-resort antibiotics: Mode of action, resistance emergence, and potential solutions. J. Biosci. 2021, 46, 85. [Google Scholar] [CrossRef] [PubMed]
- Theuretzbacher, U. Global antimicrobial resistance in Gram-negative pathogens and clinical need. Curr. Opin. Microbiol. 2017, 39, 106–112. [Google Scholar] [CrossRef]
- Baron, S.; Hadjadj, L.; Rolain, J.M.; Olaitan, A.O. Molecular mechanisms of polymyxin resistance: Knowns and unknowns. Int. J. Antimicrob. Agents 2016, 48, 583–591. [Google Scholar] [CrossRef]
- Justo, J.A.; Bosso, J.A. Adverse reactions associated with systemic polymyxin therapy. J. Hum. Pharmacol. Drug Ther. 2015, 35, 28–33. [Google Scholar] [CrossRef]
- Theriault, N.; Tillotson, G.; Sandrock, C.E. Global travel and Gram-negative bacterial resistance; implications on clinical management. Expert Rev. Anti-Infect. Ther. 2021, 19, 181–196. [Google Scholar] [CrossRef]
- Willyard, C. The drug-resistant bacteria that pose the greatest health threats. Nature 2017, 543, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tagliabue, A.; Rappuoli, R. Changing Priorities in Vaccinology: Antibiotic Resistance Moving to the Top. Front. Immunol. 2018, 9, 1068. [Google Scholar] [CrossRef]
- Abraham, R.J.; Stevens, A.J.; Young, K.A.; Russell, C.; Qvist, A.; Khazandi, M.; Wong, H.S.; Abraham, S.; Ogunniyi, A.D.; Page, S.W.; et al. Robenidine Analogues as Gram-Positive Antibacterial Agents. J. Med. Chem. 2016, 59, 2126–2138. [Google Scholar] [CrossRef] [PubMed]
- Ogunniyi, A.D.; Khazandi, M.; Stevens, A.J.; Sims, S.K.; Page, S.W.; Garg, S.; Venter, H.; Powell, A.; White, K.; Petrovski, K.R. Evaluation of robenidine analog NCL195 as a novel broad-spectrum antibacterial agent. PLoS ONE 2017, 12, e0183457. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.T.; O’Donovan, L.A.; Venter, H.; Russell, C.C.; McCluskey, A.; Page, S.W.; Trott, D.J.; Ogunniyi, A.D. Comparison of Two Transmission Electron Microscopy Methods to Visualize Drug-Induced Alterations of Gram-Negative Bacterial Morphology. Antibiotics 2021, 10, 307. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests For Bacteria Isolated from Animals, 4th ed.; CLSI Supplement VET08; CLSI: Wayne, PA, USA, 2018. [Google Scholar]
- Clinical and Laboratory Standards Institute. Methods for Determining Bactericidal Activity of Antimicrobial Agents; Approved Guideline; CLSI: Wayne, PA, USA, 1999. [Google Scholar]
- Elemam, A.; Rahimian, J.; Doymaz, M. In vitro evaluation of antibiotic synergy for polymyxin B-resistant carbapenemase-producing Klebsiella pneumoniae. J. Clin. Microbiol. 2010, 48, 3558–3562. [Google Scholar] [CrossRef] [Green Version]
- Khazandi, M.; Pi, H.; Chan, W.Y.; Ogunniyi, A.D.; Sim, J.X.F.; Venter, H.; Garg, S.; Page, S.W.; Hill, P.B.; McCluskey, A. In vitro Antimicrobial Activity of Robenidine, Ethylenediaminetetraacetic Acid and Polymyxin B Nonapeptide Against Important Human and Veterinary Pathogens. Front. Microbiol. 2019, 10, 837. [Google Scholar] [CrossRef] [Green Version]
- Hwang, I.S.; Hwang, J.H.; Choi, H.; Kim, K.J.; Lee, D.G. Synergistic effects between silver nanoparticles and antibiotics and the mechanisms involved. J. Med. Microbiol. 2012, 61, 1719–1726. [Google Scholar] [CrossRef] [Green Version]
- Asokan, G.V.; Ramadhan, T.; Ahmed, E.; Sanad, H. WHO Global Priority Pathogens List: A Bibliometric Analysis of Medline-PubMed for Knowledge Mobilization to Infection Prevention and Control Practices in Bahrain. Oman Med. J. 2019, 34, 184–193. [Google Scholar] [CrossRef]
- Zhen, X.; Lundborg, C.S.; Sun, X.; Hu, X.; Dong, H. Economic burden of antibiotic resistance in ESKAPE organisms: A systematic review. Antimicrob. Resist. Infect. Control 2019, 8, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- van der Eerden, M.M.; Vlaspolder, F.; de Graaff, C.S.; Groot, T.; Bronsveld, W.; Jansen, H.M.; Boersma, W.G. Comparison between pathogen directed antibiotic treatment and empirical broad spectrum antibiotic treatment in patients with community acquired pneumonia: A prospective randomised study. Thorax 2005, 60, 672–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. New Report Calls for Urgent Action to Avert Antimicrobial Resistance Crisis; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Petrosillo, N.; Ioannidou, E.; Falagas, M.E. Colistin monotherapy vs. combination therapy: Evidence from microbiological, animal and clinical studies. Clin. Microbiol. Infect. 2008, 14, 816–827. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Li, R.; Xiao, X.; Wang, Z. Antibiotic adjuvants: An alternative approach to overcome multi-drug resistant Gram-negative bacteria. Crit. Rev. Microbiol. 2019, 45, 301–314. [Google Scholar] [CrossRef]
- Doi, Y. Treatment Options for Carbapenem-resistant Gram-negative Bacterial Infections. Clin. Infect. Dis. 2019, 69, S565–S575. [Google Scholar] [CrossRef] [Green Version]
- Brennan-Krohn, T.; Pironti, A.; Kirby, J.E. Synergistic activity of colistin-containing combinations against colistin-resistant Enterobacteriaceae. Antimicrob. Agents Chemother. 2018, 62, e00873-18. [Google Scholar] [CrossRef] [Green Version]
- Witherell, K.S.; Price, J.; Bandaranayake, A.D.; Olson, J.; Call, D.R. Circumventing colistin resistance by combining colistin and antimicrobial peptides to kill colistin-resistant and multidrug-resistant Gram-negative bacteria. J. Glob. Antimicrob. Resist. 2020, 22, 706–712. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Xie, X.; Wu, X.; Li, X.; Zhao, Z.; Luo, S.; Wan, Z.; Liu, J.; Fu, L.; et al. In vitro and in vivo assessment of the antibacterial activity of colistin alone and in combination with other antibiotics against Acinetobacter baumannii and Escherichia coli. J. Glob. Antimicrob. Resist. 2020, 20, 351–359. [Google Scholar] [CrossRef]
- Kim, W.Y.; Moon, J.Y.; Huh, J.W.; Choi, S.H.; Lim, C.M.; Koh, Y.; Chong, Y.P.; Hong, S.B. Comparable Efficacy of Tigecycline versus Colistin Therapy for Multidrug-Resistant and Extensively Drug-Resistant Acinetobacter baumannii Pneumonia in Critically Ill Patients. PLoS ONE 2016, 11, e0150642. [Google Scholar] [CrossRef] [Green Version]
- Katip, W.; Uitrakul, S.; Oberdorfer, P. A Comparison of Colistin versus Colistin Plus Meropenem for the Treatment of Carbapenem-Resistant Acinetobacter baumannii in Critically Ill Patients: A Propensity Score-Matched Analysis. Antibiotics 2020, 9, 647. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, G.; Yang, L.; Wu, H.; Song, Z.; Wang, H.; Høiby, N.; Ulrich, M.; Molin, S.; Riethmüller, J.; Döring, G. Colistin-Tobramycin Combinations Are Superior to Monotherapy Concerning the Killing of Biofilm Pseudomonas aeruginosa. J. Infect. Dis. 2010, 202, 1585–1592. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, Z.A.; Paterson, D.L.; Potoski, B.A.; Kilayko, M.C.; Sandovsky, G.; Sordillo, E.; Polsky, B.; Adams-Haduch, J.M.; Doi, Y. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: Superiority of combination antimicrobial regimens. Antimicrob. Agents Chemother. 2012, 56, 2108–2113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ontong, J.C.; Ozioma, N.F.; Voravuthikunchai, S.P.; Chusri, S. Synergistic antibacterial effects of colistin in combination with aminoglycoside, carbapenems, cephalosporins, fluoroquinolones, tetracyclines, fosfomycin, and piperacillin on multidrug resistant Klebsiella pneumoniae isolates. PLoS ONE 2021, 16, e0244673. [Google Scholar] [CrossRef]
- Nation, R.L.; Garonzik, S.M.; Thamlikitkul, V.; Giamarellos-Bourboulis, E.J.; Forrest, A.; Paterson, D.L.; Li, J.; Silveira, F.P. Dosing guidance for intravenous colistin in critically-ill patients. Clin. Infect. Dis. 2017, 64, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.-Y.; Wu, D.; Feng, X.-H. Polymyxin monotherapy versus polymyxin-based combination therapy against carbapenem-resistant Klebsiella pneumoniae: A systematic review and meta-analysis. J. Glob. Antimicrob. Resist. 2020, 23, 197–202. [Google Scholar] [CrossRef]
- Li, Y.Y.; Wang, J.; Wang, R.; Cai, Y. Double-carbapenem therapy in the treatment of multidrug resistant Gram-negative bacterial infections: A systematic review and meta-analysis. BMC Infect. Dis. 2020, 20, 408. [Google Scholar] [CrossRef]
- Angst, D.C.; Tepekule, B.; Sun, L.; Bogos, B.; Bonhoeffer, S. Comparing treatment strategies to reduce antibiotic resistance in an in vitro epidemiological setting. Proc. Natl. Acad. Sci. USA 2021, 118, e2023467118. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.; Wolfensberger, A.; Nemeth, J.; Schreiber, P.W.; Sax, H.; Kuster, S.P. Monotherapy versus combination therapy for multidrug-resistant Gram-negative infections: Systematic Review and Meta-Analysis. Sci. Rep. 2019, 9, 15290. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.S.; Dzara, K.; Downing, N.S. Efficacy and safety concerns are important reasons why the FDA requires multiple reviews before approval of new drugs. Health Aff. 2015, 34, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, D.H.; Page, S.W. Antimicrobial stewardship in veterinary medicine. ASM Microbiol. Spectr. 2018, 6, 3–6. [Google Scholar] [CrossRef] [Green Version]

| Strain/Isolate | 1 MIC and 2 MBC Range (µg/mL) | ||
|---|---|---|---|
| NCL179 | Daptomycin | Amikacin | |
| 3 MRSA (n = 20) | 1–2 | 0.5–2 | ND |
| 4 MSSA (n = 2) | 1 | 0.5 | ND |
| 5 MRSP (n = 20) | 1–2 | 7 ND | 8–16 |
| 6 VRE (n = 20) | 2–4 | 0.5–2 | ND |
| Strain/Isolate | 1 MIC Range (μg/mL) | 2 FICI | 3 DRI | ||||
|---|---|---|---|---|---|---|---|
| Single Antibiotic | Combination | ||||||
| Colistin | NCL179 | Colistin | NCL179 | Colistin | NCL179 | ||
| A. baumannii (n = 14) | 0.5–2 | >256 | 0.008–0.5 | 0.5–4 | 0.016–0.25 * | 4–64 | 64–512 |
| Colistin-resistant A. baumannii (n = 4) | 64–128 | >256 | 0.5–1 | 1–4 | 0.008 * | 64-128 | 64–256 |
| E. coli (n = 23) | 0.125–0.5 | >256 | 0.008–0.125 | 0.5–4 | 0.064–0.25 * | 4–16 | 64–512 |
| Colistin-resistant E. coli (n = 1) | 32 | >256 | 0.5 | 2 | 0.016 * | 64 | 128 |
| K. pneumoniae (n = 22) | 0.25–2 | >256 | 0.015–0.5 | 0.5–4 | 0.06–0.25 * | 4–16 | 64–512 |
| P. aeruginosa (n = 25) | 0.25–2 | >256 | 0.015–0.5 | 0.5–4 | 0.06–0.25 * | 4–16 | 64–512 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, H.T.; Venter, H.; Woolford, L.; Young, K.; McCluskey, A.; Garg, S.; Page, S.W.; Trott, D.J.; Ogunniyi, A.D. Impact of a Novel Anticoccidial Analogue on Systemic Staphylococcus aureus Infection in a Bioluminescent Mouse Model. Antibiotics 2022, 11, 65. https://doi.org/10.3390/antibiotics11010065
Nguyen HT, Venter H, Woolford L, Young K, McCluskey A, Garg S, Page SW, Trott DJ, Ogunniyi AD. Impact of a Novel Anticoccidial Analogue on Systemic Staphylococcus aureus Infection in a Bioluminescent Mouse Model. Antibiotics. 2022; 11(1):65. https://doi.org/10.3390/antibiotics11010065
Chicago/Turabian StyleNguyen, Hang Thi, Henrietta Venter, Lucy Woolford, Kelly Young, Adam McCluskey, Sanjay Garg, Stephen W. Page, Darren J. Trott, and Abiodun David Ogunniyi. 2022. "Impact of a Novel Anticoccidial Analogue on Systemic Staphylococcus aureus Infection in a Bioluminescent Mouse Model" Antibiotics 11, no. 1: 65. https://doi.org/10.3390/antibiotics11010065
APA StyleNguyen, H. T., Venter, H., Woolford, L., Young, K., McCluskey, A., Garg, S., Page, S. W., Trott, D. J., & Ogunniyi, A. D. (2022). Impact of a Novel Anticoccidial Analogue on Systemic Staphylococcus aureus Infection in a Bioluminescent Mouse Model. Antibiotics, 11(1), 65. https://doi.org/10.3390/antibiotics11010065








