Characteristics, Influencing Factors, Predictive Scoring System, and Outcomes of the Patients with Nephrotoxicity Associated with Administration of Intravenous Colistin
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Characteristics of Patients with Nephrotoxicity
2.3. Outcomes and Prognosis of Patients Receiving Intravenous Colistin with Acute Kidney Injury
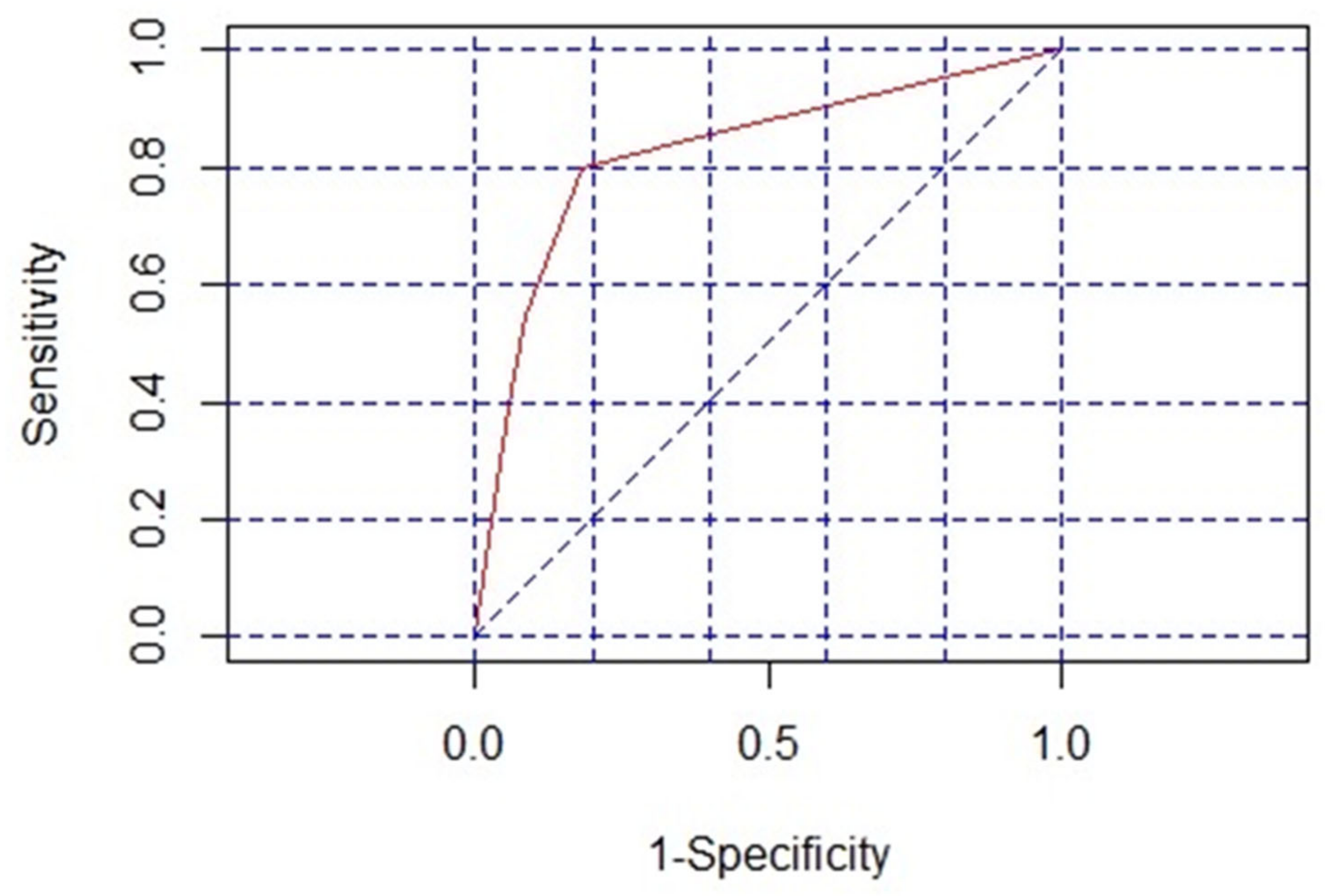
2.4. Risk Factors and Prediction Model for Colistin-Associated Nephrotoxicity
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Data Collection and Variables
4.3. Colistin Preparation and Dosing
4.4. Definitions of Outcomes
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morris, S.; Cerceo, E. Trends, epidemiology, and management of multi-drug resistant gram-negative bacterial infections in the hospitalized setting. Antibiotics 2020, 9, 196. [Google Scholar] [CrossRef]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- Giske, C.G.; Monnet, D.L.; Cars, O.; Carmeli, Y. Clinical and economic impact of common multidrug-resistant gram-negative bacilli. Antimicrob. Agents Chemother. 2008, 52, 813–821. [Google Scholar] [CrossRef]
- Ja Ko, H.; Hyok Jeon, M.; Ju Choo, E.; Jung Lee, E.; Hyong Kim, T.; Jun, J.B.; Gil, H.W. Early acute kidney injury is a risk factor that predicts mortality in patients treated with colistin. Nephron Clin. Pract. 2011, 117, c284–c288. [Google Scholar]
- Michalopoulos, A.; Tsiodras, S.; Rellos, K.; Mentzelopoulos, S.; Falagas, M. Colistin treatment in patients with ICU-acquired infections caused by multiresistant Gram-negative bacteria: The renaissance of an old antibiotic. Clin. Microbiol. Infect. 2005, 11, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Nørgaard, S.M.; Jensen, C.S.; Aalestrup, J.; Vandenbroucke-Grauls, C.M.; de Boer, M.G.; Pedersen, A.B. Choice of therapeutic interventions and outcomes for the treatment of infections caused by multidrug-resistant gram-negative pathogens: A systematic review. Antimicrob. Resist. Infect. Control 2019, 8, 170. [Google Scholar] [CrossRef] [PubMed]
- Hawkey, P.M.; Warren, R.E.; Livermore, D.M.; McNulty, C.A.; Enoch, D.A.; Otter, J.A.; Wilson, A.P.R. Treatment of infections caused by multidrug-resistant gram-negative bacteria: Report of the British society for antimicrobial chemothera-py/healthcare infection society/british infection association joint working party. J. Antimicrob. Chemother. 2018, 73, iii2–iii78. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Kasiakou, S.K.; Saravolatz, L.D. Colistin: The revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin. Infect. Dis. 2005, 40, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Doi, Y. Treatment options for carbapenem-resistant gram-negative bacterial infections. Clin. Infect. Dis. 2019, 69, S565–S575. [Google Scholar] [CrossRef] [PubMed]
- Zusman, O.; Altunin, S.; Koppel, F.; Dishon Benattar, Y.; Gedik, H.; Paul, M. Polymyxin monotherapy or in combination against carbapenem-resistant bacteria: Systematic review and meta-analysis. J. Antimicrob. Chemother. 2016, 72, 29–39. [Google Scholar] [CrossRef]
- Tsuji, B.T.; Pogue, J.M.; Zavascki, A.P.; Paul, M.; Daikos, G.L.; Forrest, A.; Giacobbe, D.R.; Viscoli, C.; Giamarellou, H.; Karaiskos, I. International consensus guidelines for the optimal use of the polymyxins: Endorsed by the American college of clinical pharmacy (ACCP), European society of clinical microbiology and infectious diseases (ESCMID), infectious diseases society of America (IDSA), international society for anti-infective Pharmacology (ISAP), society of critical care medicine (SCCM), and society of infectious diseases pharmacists (SIDP). Pharmacother. J. Hum. Pharmacol. Drug Ther. 2019, 39, 10–39. [Google Scholar]
- Jung, S.Y.; Lee, S.H.; Lee, S.Y.; Yang, S.; Noh, H.; Chung, E.K.; Lee, J.I. Antimicrobials for the treatment of drug-resistant Acinetobacter baumannii pneumonia in critically ill patients: A systemic review and Bayesian network meta-analysis. Crit. Care 2017, 21, 319. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Tagliabue, C.; Bosis, S.; Principi, N. Levofloxacin for the treatment of Mycoplasma pneumoniae-associated me-ningoencephalitis in childhood. Int. J. Antimicrob. Agents 2011, 37, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Omrani, A.S.; Alfahad, W.A.; Shoukri, M.M.; Baadani, A.M.; Aldalbahi, S.; Almitwazi, A.A.; Albarrak, A.M. High dose in-travenous colistin methanesulfonate therapy is associated with high rates of nephrotoxicity; a prospective cohort study from Saudi Arabia. Ann. Clin. Microbiol. Antimicrob. 2015, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Sorlí, L.; Luque, S.; Grau, S.; Berenguer, N.; Segura, C.; Montero, M.M.; Álvarez-Lerma, F.; Knobel, H.; Benito, N.; Horcajada, J.P. Trough colistin plasma level is an independent risk factor for nephrotoxicity: A prospective observational cohort study. BMC Infect. Dis. 2013, 13, 380. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.M.; Gaifer, Z.; Al-Zakwani, I.S. Incidence and risk factors of nephrotoxicity in patients on colistimethate sodium. Int. J. Clin. Pharm. 2018, 40, 444–449. [Google Scholar] [CrossRef]
- Koomanachai, P.; Tiengrim, S.; Kiratisin, P.; Thamlikitkul, V. Efficacy and safety of colistin (colistimethate sodium) for therapy of infections caused by multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii in Siriraj Hospital, Bangkok, Thailand. Int. J. Infect. Dis. 2007, 11, 402–406. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miano, T.A.; Lautenbach, E.; Wilson, F.P.; Guo, W.; Borovskiy, Y.; Hennessy, S. Attributable risk and time course of colistin-associated acute kidney injury. Clin. J. Am. Soc. Nephrol. 2018, 13, 542–550. [Google Scholar] [CrossRef]
- Javan, A.O.; Shokouhi, S.; Sahraei, Z. A review on colistin nephrotoxicity. Eur. J. Clin. Pharmacol. 2015, 71, 801–810. [Google Scholar] [CrossRef]
- Ozkan, G.; Ulusoy, S.; Orem, A.; Alkanat, M.; Mungan, S.; Yulug, E.; Yucesan, F.B. How does colistin-induced nephropathy develop and can it be treated? Antimicrob. Agents Chemother. 2013, 57, 3463–3469. [Google Scholar] [CrossRef]
- Wiedermann, C.J.; Wiedermann, W.; Joannidis, M. Hypoalbuminemia and acute kidney injury: A meta-analysis of observa-tional clinical studies. Intensive Care Med. 2010, 36, 1657–1665. [Google Scholar] [CrossRef]
- Nation, R.L.; Garonzik, S.M.; Thamlikitkul, V.; Giamarellos-Bourboulis, E.J.; Forrest, A.; Paterson, D.L.; Li, J.; Silveira, F.P. Dosing guidance for intravenous colistin in critically ill patients. Clin. Infect. Dis. 2017, 64, 565–571. [Google Scholar] [CrossRef]
- Grégoire, N.; Aranzana-Climent, V.; Magréault, S.; Marchand, S.; Couet, W. Clinical pharmacokinetics and pharmacody-namics of colistin. Clin. Pharmacokinet. 2017, 56, 1441–1460. [Google Scholar] [CrossRef] [PubMed]
- Zavascki, A.P. Polymyxins for the treatment of extensively-drug-resistant Gram-negative bacteria: From pharmacokinetics to bedside. Expert Rev. Anti-Infect. Ther. 2014, 12, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Gordon, O.; Vig Slenters, T.N.; Brunetto, P.S.; Villaruz, A.E.; Sturdevant, D.E.; Otto, M.; Landmann, R.; Fromm, K.M. Silver coordination polymers for prevention of implant infection: Thiol interaction, impact on respiratory chain enzymes, and hy-droxyl radical induction. Antimicrob. Agents Chemother. 2010, 54, 4208–4218. [Google Scholar] [CrossRef]
- Bozkurt, I.; Sharma, A.; Esen, S. Colistin-induced nephrotoxicity and the role of N-acetylcysteine: A retrospective cohort study. J. Infect. Dev. Ctries. 2017, 11, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Rocco, M.; Montini, L.; Alessandri, E.; Venditti, M.; Laderchi, A.; De Gennaro, P.; Raponi, G.; Vitale, M.; Pietropaoli, P.; An-tonelli, M. Risk factors for acute kidney injury in critically ill patients receiving high intravenous doses of colistin me-thanesulfonate and/or other nephrotoxic antibiotics: A retrospective cohort study. Crit. Care 2013, 17, R174. [Google Scholar] [CrossRef] [PubMed]
- Hartzell, J.D.; Neff, R.; Ake, J.; Howard, R.; Olson, S.; Paolino, K.; Vishnepolsky, M.; Weintrob, A.; Wortmann, G. Ne-phrotoxicity associated with intravenous colistin (colistimethate sodium) treatment at a tertiary care medical center. Clin. Infect. Dis. 2009, 48, 1724–1728. [Google Scholar] [CrossRef] [PubMed]
- Taejaroenkul, P.; Khamlek, S.; Khienprasit, N.; Lucksiri, A. Incidence and Risk Factors of Acute Kidney Failure in Patients Receiving Colistin in a Provincial Hospital. Naresuan Univ. J. Sci. Technol. 2019, 27, 77–86. [Google Scholar]
- Temocin, F.; Erdinc, S.; Tulek, N.; Demirelli, M.; Bulut, C.; Ertem, G. Incidence and risk factors for colistin-associated ne-phrotoxicity. Jpn. J. Infect. Dis. 2015, 68, 318–320. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, T.P.; Wolowich, W.R.; Reddy, A.; Cano, E.; Abbo, L.; Smith, L.B. Incidence and predictors of nephrotoxicity associ-ated with intravenous colistin in overweight and obese patients. Antimicrob. Agents Chemother. 2012, 56, 2392–2396. [Google Scholar] [CrossRef] [PubMed]
- Phe, K.; Lee, Y.; McDaneld, P.M.; Prasad, N.; Yin, T.; Figueroa, D.A.; Musick, W.L.; Cottreau, J.M.; Hu, M.; Tam, V.H. In vitro assessment and multicenter cohort study of comparative nephrotoxicity rates associated with colistimethate versus poly-myxin B therapy. Antimicrob. Agents Chemother. 2014, 58, 2740–2746. [Google Scholar] [CrossRef] [PubMed]
- Balkan, I.I.; Dogan, M.; Durdu, B.; Batirel, A.; Hakyemez, I.N.; Cetin, B.; Karabay, O.; Gonen, I.; Ozkan, A.S.; Uzun, S. Colistin nephrotoxicity increases with age. Scand. J. Infect. Dis. 2014, 46, 678–685. [Google Scholar] [CrossRef]
- Durante-Mangoni, E.; Andini, R.; Signoriello, S.; Cavezza, G.; Murino, P.; Buono, S.; De Cristofaro, M.; Taglialatela, C.; Bassetti, M.; Malacarne, P. Acute kidney injury during colistin therapy: A prospective study in patients with extensively-drug resistant Acinetobacter baumannii infections. Clin. Microbiol. Infect. 2016, 22, 984–989. [Google Scholar] [CrossRef]
- Lameire, N.H.; Bagga, A.; Cruz, D.; De Maeseneer, J.; Endre, Z.; Kellum, J.A.; Liu, K.D.; Mehta, R.L.; Pannu, N.; Van Biesen, W. Acute kidney injury: An increasing global concern. Lancet 2013, 382, 170–179. [Google Scholar] [CrossRef]
- Kwon, K.H.; Oh, J.Y.; Yoon, Y.-S.; Jeong, Y.-J.; Kim, K.S.; Shin, S.J.; Chung, J.W.; Huh, H.J.; Chae, S.L.; Park, S.Y. Colistin treatment in carbapenem-resistant Acinetobacter baumannii pneumonia patients: Incidence of nephrotoxicity and outcomes. Int. J. Antimicrob. Agents 2015, 45, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.-A.; Lee, J.E.; Huh, W.; Peck, K.R.; Kim, Y.-G.; Kim, D.J.; Oh, H.Y. Predictors of acute kidney injury associated with intravenous colistin treatment. Int. J. Antimicrob. Agents 2010, 35, 473–477. [Google Scholar] [CrossRef]
- Giacobbe, D.R.; di Masi, A.; Leboffe, L.; Del Bono, V.; Rossi, M.; Cappiello, D.; Coppo, E.; Marchese, A.; Casulli, A.; Signori, A. Hypoalbuminemia as a predictor of acute kidney injury during colistin treatment. Sci. Rep. 2018, 8, 11968. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, K.-H.; Yoo, S.; Pai, H. Clinical characteristics and risk factors of colistin-induced nephrotoxicity. Int. J. Antimicrob. Agents 2009, 34, 434–438. [Google Scholar] [CrossRef]
- Nicholson, J.; Wolmarans, M.; Park, G. The role of albumin in critical illness. Br. J. Anaesth. 2000, 85, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, J.; Abernethy, V.E.; Wang, Z.; Lieberthal, W.; Koh, J.S.; Levine, J.S. Albumin is a major serum survival factor for renal tubular cells and macrophages through scavenging of ROS. Am. J. Physiol. Renal Physiol. 1999, 277, F711–F722. [Google Scholar] [CrossRef]
- Doshi, N.M.; Mount, K.L.; Murphy, C.V. Nephrotoxicity associated with intravenous colistin in critically ill patients. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2011, 31, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Pogue, J.M.; Lee, J.; Marchaim, D.; Yee, V.; Zhao, J.J.; Chopra, T.; Lephart, P.; Kaye, K.S. Incidence of and risk factors for col-istin-associated nephrotoxicity in a large academic health system. Clin. Infect. Dis. 2011, 53, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.M.; Haynes, K.; Gallagher, J.C. Emergent renal dysfunction with colistin pharmacotherapy. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2013, 33, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Rattanaumpawan, P.; Ungprasert, P.; Thamlikitkul, V. Risk factors for colistin-associated nephrotoxicity. J. Infect. 2011, 62, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Tuon, F.F.; Rigatto, M.H.; Lopes, C.K.; Kamei, L.K.; Rocha, J.L.; Zavascki, A.P. Risk factors for acute kidney injury in patients treated with polymyxin B or colistin methanesulfonate sodium. Int. J. Antimicrob. Agents 2014, 43, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Phe, K.; Johnson, M.L.; Palmer, H.R.; Tam, V.H. Validation of a model to predict the risk of nephrotoxicity in patients receiving colistin. Antimicrob. Agents Chemother. 2014, 58, 6946–6948. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.B.; Prytherch, D.R.; Jarvis, S.; Kovacs, C.; Meredith, P.; Schmidt, P.E.; Briggs, J. A comparison of the ability of the physiologic components of medical emergency team criteria and the UK National Early Warning Score to discriminate patients at risk of a range of adverse clinical outcomes. Crit. Care Med. 2016, 44, 2171–2181. [Google Scholar] [CrossRef]
- Levey, A.; Coresh, J.; Greene, T.; Stevens, L.; Zhang, Y.; Hendriksen, S.; Kusek, J.; Van Lente, F. Chronic kidney disease epi-demiology collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann. Intern. Med. 2009, 145, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Lameire, N.H.; Levin, A.; Kellum, J.A.; Cheung, M.; Jadoul, M.; Winkelmayer, W.C.; Stevens, P.E. Harmonizing acute and chronic kidney disease definition and classification: Report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int. 2021, 100, 516–526. [Google Scholar] [CrossRef]
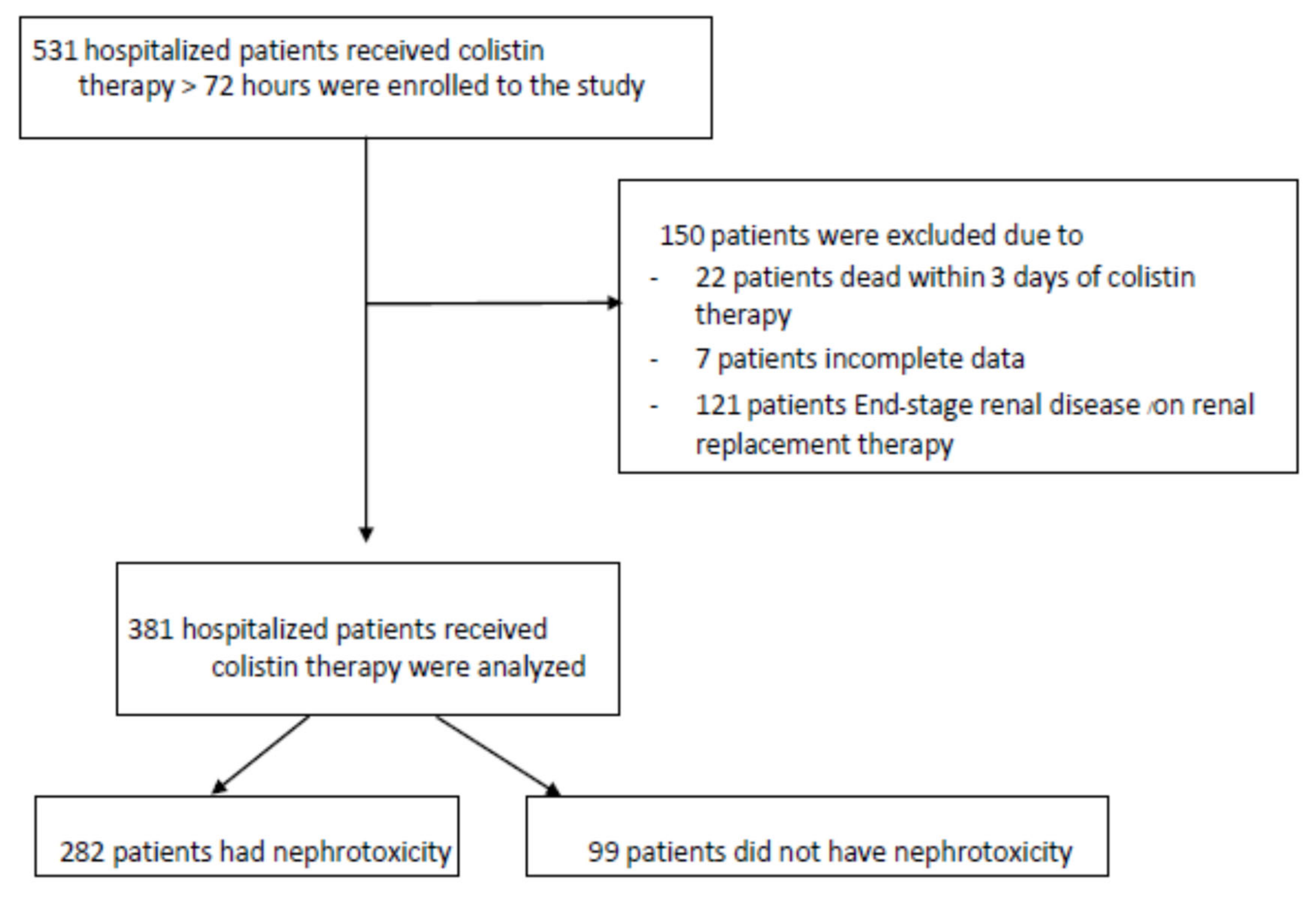
| Parameters | Value(s) for Patients a (N = 381) |
|---|---|
| Demographic data | |
| Age (years), median (IQR b) | 64 (51–62) |
| Male sex | 233 (61) |
| Body mass index (kg/m2), median (IQR) | 23 (21–24) |
| Comorbidities | |
| Coronary artery disease(s) | 12 (3) |
| Congestive heart failure | 33 (9) |
| Hypertension | 156 (41) |
| Diabetes mellitus | 79 (21) |
| Pulmonary disease(s) | 178 (47) |
| Chronic liver disease(s) | 70 (18) |
| Chorionic kidney disease(s) | 88 (23) |
| Cerebrovascular disease(s) | 76 (20) |
| Hematologic/solid organ malignancy | 136 (36) |
| HIV infection | 9 (2) |
| Charlson comorbidity index, median (IQR) | 6 (5–8) |
| Clinical characteristics | |
| Emergency indication of admission | 152 (40) |
| Initial admission (not mutually exclusive) in intensive care unit | 132 (35) |
| Severity score, median (IQR) | 6 (4–9) |
| Site(s) of infection | |
| Bloodstream | 54 (14) |
| Respiratory tract | 260 (68) |
| Urinary tract | 53 (14) |
| Musculoskeletal | 18 (5) |
| Gastrointestinal tract | 29 (8) |
| Multiple | 30 (8) |
| Unknown | 7 (2) |
| Causative pathogen(s) | |
| Acinetobacter baumannii | 228 (60) |
| Pseudomonas aeruginosa | 30 (8) |
| Klebsiella pneumoniae | 65(17) |
| Escherichia coli | 21 (6) |
| Concomitant | 34 (9) |
| Unknown | 70 (18) |
| Administration of intravenous colistin | |
| With loading dosage | 335 (88) |
| Dosage per ABW c (mg/kg/day), median (IQR) | 4.89 (3.78–5.94) |
| Dosage per IBW d (mg/kg/day), median (IQR) | 4.26 (3.49–5.16) |
| Cumulative dosage (mg), median (IQR) | 2012 (1451–3015) |
| Duration(days), median (IQR) | 11 (6–14) |
| Concurrent medication | |
| Inotropic agent/vasopressor | 93 (24) |
| Non-steroidal anti-inflammatory drug | 10 (3) |
| Diuretic agent | 148 (39) |
| Aminoglycoside(s) | 10 (3) |
| Β-lactam antibiotic(s) | 376 (98) |
| Fluoroquinolone(s) | 45 (12) |
| Vancomycin | 55 (14) |
| Tigecycline | 6 (2) |
| Clindamycin | 12 (3) |
| Cotrimoxazole | 30 (8) |
| Amphotericin B | 27 (7) |
| Characteristics | Values (N = 282) |
|---|---|
| The Kidney Disease: Improving Global Outcomes (KDIGO) classification, n (%) | |
| Acute kidney disease (AKD) without acute kidney injury (AKI) | 40 (14) |
| Acute kidney disease (AKD) with acute kidney injury (AKI) | |
| Stage 1 | 146 (52) |
| Stage 2 | 63 (22) |
| Stage 3 | 18 (6) |
| Chronic kidney disease (CKD) | 6 (2) |
| Electrolyte and acid-base disturbance, n (%) | |
| Hyperkalemia | 78 (28) |
| Hypocalcemia | 29 (10) |
| Hyperphosphatemia | 18 (6) |
| Acidosis | 50 (18) |
| Systemic complication, n (%) | |
| Volume overload | 86 (30) |
| Respiratory failure | 24 (9) |
| Uremic symptoms | 19 (7) |
| Recovery with eGFR > 60 mL/min/1.73 m2 within 3 months, n (%) | 182 (65) |
| Outcome | Values for the Patients with Nephrotoxicity (N = 282) | Values for the Patients without Nephrotoxicity (N = 99) | p Value c |
|---|---|---|---|
| Mortality, no. (%) of patients | |||
| In-hospital | 157 (56) | 24 (24) | <0.001 |
| 14 day | 82 (52) | 14 (58) | 0.735 |
| 30 day | 152 (54) | 78 (79) | <0.001 |
| Admission to the intensive care unit | 218 (77) | 75 (76) | 0.861 |
| Length of stay in the intensive care unit | 10 (4,20) | 10 (5,18) | 0.995 |
| Length of hospital stay after survival (days), median (IQR) | 59 (45,68) | 40 (26,51) | <0.001 |
| Cost (baht b), median (IQR) | 484,926 (233,266–706,125) | 364,956 (213,325–650,683) | 0.048 |
| Parameter | Values b | Crude OR (95% CI) | Adjusted OR (95% CI) | p Value c | |
|---|---|---|---|---|---|
| Patients with Nephrotoxicity a (N = 282) | Patients without Nephrotoxicity (N = 99) | ||||
| Age > 60 years | 200 (71) | 49 (49) | 2.49 (1.55–3.98) | 1.92 (1.20–2.21) | 0.031 |
| Male sex | 175 (61) | 58 (59) | 1.16 (0.73–1.84) | ||
| Body mass index > 25 | 150 (53) | 38 (38) | 1.82 (1.14–2.91) | 1.12 (0.91–2.01) | 0.057 |
| Charlson comorbidity index > 6 | 102 (36) | 15 (15) | 3.17 (1.74–5.89) | 2.01 (1.34–2.89) | 0.004 |
| Severity score, median (IQR) d | 6 (4,9) | 6 (4,8) | 1.15 (0.78–2.02) | ||
| Initial admission (not mutually exclusive) Intensive care unit | 104 (37) | 28 (28) | 1.48 (0.90–2.44) | 1.16 (0.79–2.01) | 0.325 |
| Bloodstream infection | 46 (16) | 8 (8) | 2.23 (1.02–4.92) | 1.82 (0.76–3.01) | 0.189 |
| Respiratory tract infection | 200 (71) | 60 (61) | 1.59 (0.98–2.56) | 1.15 (0.74–1.77) | 0.365 |
| Anemia | 132 (47) | 44 (44) | 1.10 (0.69–1.74) | ||
| Elevated transaminase enzyme(s) | 107 (38) | 39 (39) | 0.94 (0.59–1.50) | ||
| Serum albumin < 3.5 g/dL | 198 (70) | 48 (48) | 2.50 (1.57–4.01) | 1.59 (1.03–2.71) | 0.042 |
| Inotropic agent/vasopressor use | 70 (25) | 23 (23) | 1.09 (0.84–1.67) | ||
| Diuretic use | 120 (43) | 28 (28) | 1.88 (1.14–3.09) | 1.11 (0.89–2.02) | 0.074 |
| Concomitant nephrotoxic agent use | 175 (62) | 40 (40) | 2.41 (1.51–3.85) | 1.70 (1.08–2.75) | 0.040 |
| With loading dosage of colistin | 251 (89) | 84 (85) | 1.45 (0.74–2.81) | ||
| Dosage of colistin per ABW e (mg/kg/day), median (IQR) d | 4.96 (3.88–6.02) | 4.81 (3.75–5.89) | 1.09 (0.77–1.78) | ||
| Dosage of colistin per IBW f (mg/kg/day), median (IQR) d | 4.42 (3.81–5.29) | 4.20 (3.38–5.00) | 1.11 (0.91–1.97) | 1.03 (0.82–1.81) | 0.097 |
| Cumulative dosage of colistin (mg), median (IQR) d | 2097 (1502–3124) | 1998 (1402–2995) | 1.05 (0.74–1.67) | ||
| Duration of colistin administration (day), median (IQR) d | 11 (7–15) | 10 (6–13) | 1.01 (0.58–1.42) | ||
| Parameter | Diagnostic Index |
|---|---|
| Age > 60 years | |
| Yes | 1 |
| No | 0 |
| Charlson comorbidity index > 6 | |
| Yes | 1 |
| No | 0 |
| Serum albumin < 3.5 g/dL | |
| Yes | 1 |
| No | 0 |
| Concomitant nephrotoxic agent use | |
| Yes | 1 |
| No | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sangthawan, P.; Geater, A.F.; Naorungroj, S.; Nikomrat, P.; Nwabor, O.F.; Chusri, S. Characteristics, Influencing Factors, Predictive Scoring System, and Outcomes of the Patients with Nephrotoxicity Associated with Administration of Intravenous Colistin. Antibiotics 2022, 11, 2. https://doi.org/10.3390/antibiotics11010002
Sangthawan P, Geater AF, Naorungroj S, Nikomrat P, Nwabor OF, Chusri S. Characteristics, Influencing Factors, Predictive Scoring System, and Outcomes of the Patients with Nephrotoxicity Associated with Administration of Intravenous Colistin. Antibiotics. 2022; 11(1):2. https://doi.org/10.3390/antibiotics11010002
Chicago/Turabian StyleSangthawan, Pornpen, Alan Frederick Geater, Surarit Naorungroj, Piyarat Nikomrat, Ozioma Forstinus Nwabor, and Sarunyou Chusri. 2022. "Characteristics, Influencing Factors, Predictive Scoring System, and Outcomes of the Patients with Nephrotoxicity Associated with Administration of Intravenous Colistin" Antibiotics 11, no. 1: 2. https://doi.org/10.3390/antibiotics11010002
APA StyleSangthawan, P., Geater, A. F., Naorungroj, S., Nikomrat, P., Nwabor, O. F., & Chusri, S. (2022). Characteristics, Influencing Factors, Predictive Scoring System, and Outcomes of the Patients with Nephrotoxicity Associated with Administration of Intravenous Colistin. Antibiotics, 11(1), 2. https://doi.org/10.3390/antibiotics11010002






