Anti-Mycobacterial Drug Resistance in Japan: How to Approach This Problem?
Abstract
1. Introduction
1.1. Epidemiological Reality of Mycobacterial Infections in Japan
1.1.1. Tuberculosis
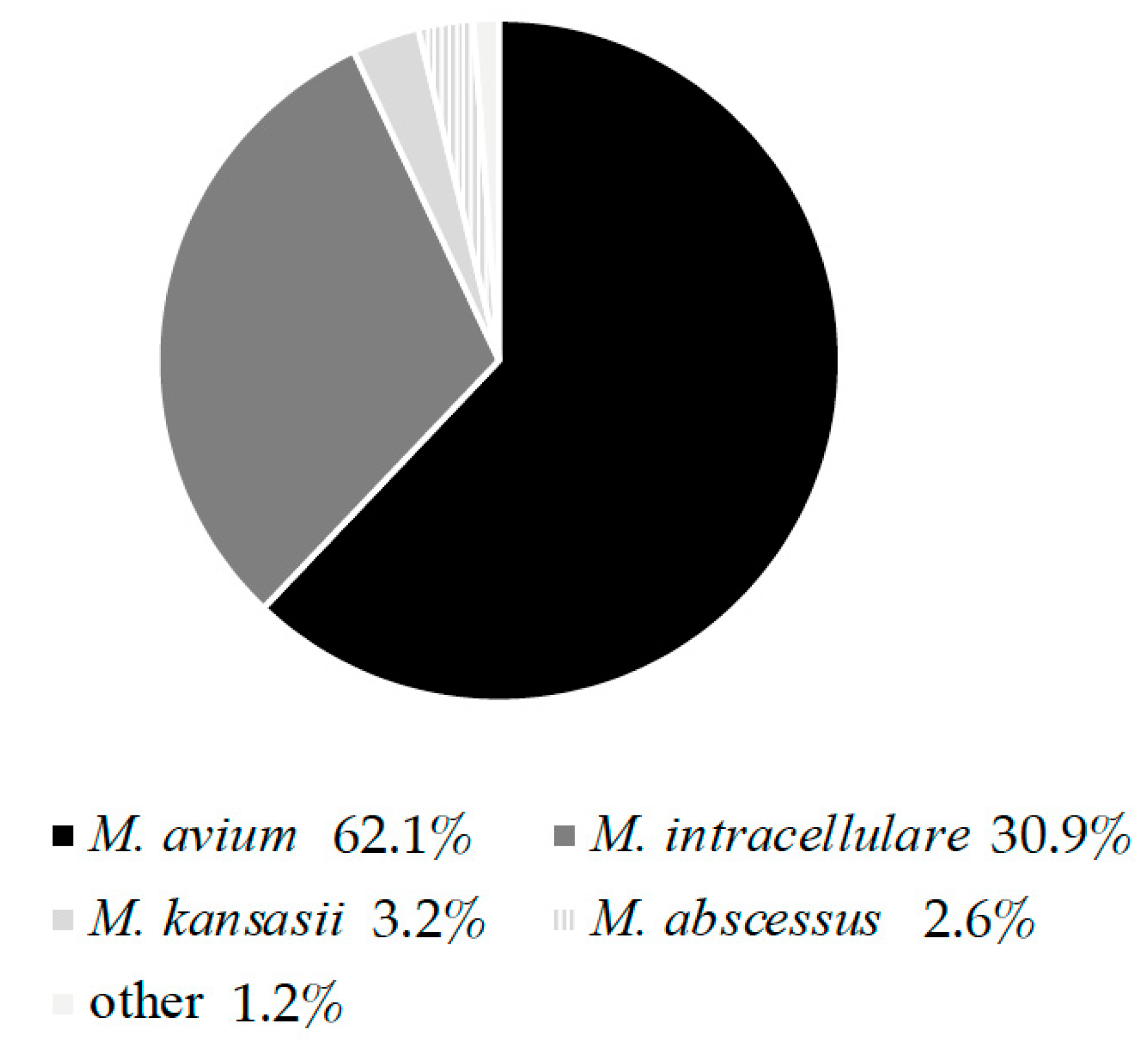
1.1.2. Non-Tuberculous Mycobacteria
1.2. Current Treatment Strategies and Problems
1.2.1. Tuberculosis
1.2.2. Mycobacterium avium-intracellulare Complex
1.2.3. Mycobacterium abscessus Species
2. Drug Susceptibility Testing and Limitations
2.1. Principle of Drug Susceptibility Testing: Why This Process Is Necessary
2.2. Mycobacterium tuberculosis
2.2.1. Phenotypic Drug Susceptibility Testing
2.2.2. Genotypic Drug Susceptibility Testing
2.3. Slowly Growing Mycobacteria (MAC)
2.4. Rapidly Growing Mycobacteria (MABS)
3. Drug Resistant Status of Major Mycobacterial Pathogens
3.1. M. tuberculosis
3.1.1. Characteristics of Drug-Resistant Tuberculosis in Japan (Drug Resistance, Prognosis)
3.1.2. Treatment Regimen: Tailor-Made Treatment
3.2. Mycobacterium avium-intracellulare Complex
3.2.1. Characteristics of Pulmonary Mycobacterium avium-intracellulare Complex Disease in Japan (Drug Resistance, Prognosis)
3.2.2. Drug Resistance of Mycobacterium avium-intracellulare Complex
3.2.3. Current Slowly Growing Mycobacteria (MAC) Drug Susceptibility Testing Problems in Japan
3.3. Mycobacterium abscessus Species
3.3.1. Characteristics of Mycobacterium abscessus Species Pulmonary Disease in Japan (Drug Resistance, Prognosis)
3.3.2. Current Rapidly Growing Mycobacteria Drug Susceptibility Testing Problems in Japan
4. New Antimicrobial Therapeutic Candidates
4.1. M. tuberculosis
4.1.1. Oxazolidinones: Stezolid
4.1.2. DprE1 Inhibitors: PBTZ169, OPC-167832, TBA-7371
4.1.3. Diarylquinolines: TBAJ-587, TBAJ-876
4.2. M. avium-intracellulare Complex
4.2.1. Bedaquiline
4.2.2. Benzimidazoles: SPR719
4.3. M. abscessus Species
Omadacycline
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| ALIS | amikacin liposome inhalation suspension |
| AMK | amikacin |
| BDQ | bedaquiline |
| CAMHB | cation-adjusted Mueller-Hinton broth |
| CLO | clofazimine |
| CLR | clarithromycin |
| CLSI | Clinical and Laboratory Standards Institute |
| DCS | D-cycloserine |
| DMD | delamanid |
| DOX | doxycycline |
| DprE1 | Decaprenylphosphoryl-β-D-ribose 2′-epimerase |
| DST | drug susceptibility testing |
| EMB | ethambutol |
| ETO | ethionamide |
| EVM | enviomycin sulfate |
| FOX | cefoxitin |
| gDST | genotypic drug susceptibility testing |
| GLI | Global Laboratory Initiative |
| HIV | human immunodeficiency virus |
| IPM | imipenem |
| INH | isoniazid |
| JSTB | The Japanese Society for Tuberculosis and NTM |
| KAN | kanamycin |
| LVX | levofloxacin |
| LZD | linezolid |
| MDR | multidrug resistant |
| MIC | minimum inhibitory concentration |
| MABS | Mycobacterium abscessus species |
| MAB | Mycobacterium abscessus subsp. abscessus |
| MAC | Mycobacterium avium–intracellulare complex |
| MFLX | moxifloxacin |
| MMA | Mycobacterium abscessus subsp. massiliense |
| Mtb | Mycobacterium tuberculosis |
| NTM | non-tuberculosis mycobacteria |
| OMC | omadacycline |
| PAS | calcium para-aminosalicylate hydrate |
| PD | pulmonary disease |
| pDST | phenotypic drug susceptibility testing |
| PTO | pretomanid |
| PZA | pyrazinamide |
| RGM | rapidly growing mycobacteria |
| RFB | rifabutin |
| RIF | rifampin |
| SGM | slowly growing mycobacteria |
| STR | streptomycin |
| STX | sitafloxacin |
| SXT | trimethoprim-sulfamethoxazole |
| SZD | stezolid |
| TB | tuberculosis |
| WHO | World Health Organization |
| XDR | extensively drug resistant |
References
- Tuberculosis Surveillance Center, Research Institute of Tuberculosis, Japan Anti-Tuberculosis Association. TUBERCULOSIS IN JAPAN ANNUAL REPORT 2021. Available online: https://jata-ekigaku.jp/wp-content/uploads/2021/11/TB-in-Japan_2021.pdf (accessed on 13 December 2021).
- Namkoong, H.; Kurashima, A.; Morimoto, K.; Hoshino, Y.; Hasegawa, N.; Ato, M.; Mitarai, S. Epidemiology of Pulmonary Nontuberculous Mycobacterial Disease, Japan. Emerg. Infect. Dis. 2016, 22, 1116–1117. [Google Scholar] [CrossRef]
- Morimoto, K.; Iwai, K.; Uchimura, K.; Okumura, M.; Yoshiyama, T.; Yoshimori, K.; Ogata, H.; Kurashima, A.; Gemma, A.; Kudoh, S. A steady increase in nontuberculous mycobacteriosis mortality and estimated prevalence in Japan. Ann. Am. Thorac. Soc. 2014, 11, 1–8. [Google Scholar] [CrossRef]
- Morimoto, K.; Hasegawa, N.; Izumi, K.; Namkoong, H.; Uchimura, K.; Yoshiyama, T.; Hoshino, Y.; Kurashima, A.; Sokunaga, J.; Shibuya, S.; et al. A Laboratory-based Analysis of Nontuberculous Mycobacterial Lung Disease in Japan from 2012 to 2013. Ann. Am. Thorac. Soc. 2017, 14, 49–56. [Google Scholar] [CrossRef]
- Kamada, K.; Yoshida, A.; Iguchi, S.; Arai, Y.; Uzawa, Y.; Konno, S.; Shimojima, M.; Kikuchi, K. Geographical distribution and regional differences in 532 clinical isolates of rapidly growing mycobacterial species in Japan. Sci. Rep. 2021, 11, 4960. [Google Scholar] [CrossRef] [PubMed]
- Cowman, S.; van Ingen, J.; Griffith, D.E.; Loebinger, M.R. Non-tuberculous mycobacterial pulmonary disease. Eur. Respir. J. 2019, 54, 1900250. [Google Scholar] [CrossRef] [PubMed]
- Hoefsloot, W.; van Ingen, J.; Andrejak, C.; Angeby, K.; Bauriaud, R.; Bemer, P.; Beylis, N.; Boeree, M.J.; Cacho, J.; Chihota, V.; et al. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: An NTM-NET collaborative study. Eur. Respir. J. 2013, 42, 1604–1613. [Google Scholar] [CrossRef] [PubMed]
- Nahid, P.; Dorman, S.E.; Alipanah, N.; Barry, P.M.; Brozek, J.L.; Cattamanchi, A.; Chaisson, L.H.; Chaisson, R.E.; Daley, C.L.; Grzemska, M.; et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: Treatment of Drug-Susceptible Tuberculosis. Clin. Infect. Dis. 2016, 63, e147–e195. [Google Scholar] [CrossRef]
- Nahid, P.; Mase, S.R.; Migliori, G.B.; Sotgiu, G.; Bothamley, G.H.; Brozek, J.L.; Cattamanchi, A.; Cegielski, J.P.; Chen, L.; Daley, C.L.; et al. Treatment of Drug-Resistant Tuberculosis. An Official ATS/CDC/ERS/IDSA Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2019, 200, e93–e142. [Google Scholar] [CrossRef]
- World Health Organization. WHO Consolidated Guidelines on Tuberculosis, Module 4: Treatment—Drug-Resistant Tuberculosis Treatment. Available online: https://www.who.int/publications/i/item/9789240007048 (accessed on 17 November 2021).
- Mirzayev, F.; Viney, K.; Linh, N.N.; Gonzalez-Angulo, L.; Gegia, M.; Jaramillo, E.; Zignol, M.; Kasaeva, T. World Health Organization recommendations on the treatment of drug-resistant tuberculosis, 2020 update. Eur. Respir. J. 2021, 57, 2003300. [Google Scholar] [CrossRef] [PubMed]
- The Treatment Committee of the Japanese Society for Tuberculosis and Non-Tuberculous Mycobacteriosis. Approach to MDR TB treatment in Japan. Kekkaku 2020, 95, 79–84. (In Japanese) [Google Scholar]
- Conradie, F.; Diacon, A.H.; Ngubane, N.; Howell, P.; Everitt, D.; Crook, A.M.; Mendel, C.M.; Egizi, E.; Moreira, J.; Timm, J.; et al. Treatment of Highly Drug-Resistant Pulmonary Tubserculosis. N. Engl. J. Med. 2020, 382, 893–902. [Google Scholar] [CrossRef]
- Viney, K.; Linh, N.N.; Gegia, M.; Zignol, M.; Glaziou, P.; Ismail, N.; Kasaeva, T.; Mirzayev, F. New definitions of pre-extensively and extensively drug-resistant tuberculosis: Update from the World Health Organization. Eur. Respir. J. 2021, 57, 2100361. [Google Scholar] [CrossRef]
- Dorman, S.E.; Nahid, P.; Kurbatova, E.V.; Phillips, P.P.J.; Bryant, K.; Dooley, K.E.; Engle, M.; Goldberg, S.V.; Phan, H.T.T.; Hakim, J.; et al. Four-Month Rifapentine Regimens with or without Moxifloxacin for Tuberculosis. N. Engl. J. Med. 2021, 384, 1705–1718. [Google Scholar] [CrossRef]
- Daley, C.L.; Iaccarino, J.M.; Lange, C.; Cambau, E.; Wallace, R.J.; Andrejak, C.; Bottger, E.C.; Brozek, J.; Griffith, D.E.; Guglielmetti, L.; et al. Treatment of Nontuberculous Mycobacterial Pulmonary Disease: An Official ATS/ERS/ESCMID/IDSA Clinical Practice Guideline. Clin. Infect. Dis. 2020, 71, 905–913. [Google Scholar] [CrossRef]
- Griffith, D.E.; Aksamit, T.R. Managing Mycobacterium avium Complex Lung Disease with a Little Help from My Friend. Chest 2021, 159, 1372–1381. [Google Scholar] [CrossRef]
- Diel, R.; Nienhaus, A.; Ringshausen, F.C.; Richter, E.; Welte, T.; Rabe, K.F.; Loddenkemper, R. Microbiologic Outcome of Interventions Against Mycobacterium avium Complex Pulmonary Disease: A Systematic Review. Chest 2018, 153, 888–921. [Google Scholar] [CrossRef]
- Kadota, J.I.; Kurashima, A.; Suzuki, K. The clinical efficacy of a clarithromycin-based regimen for Mycobacterium avium complex disease: A nationwide post-marketing study. J. Infect. Chemother. 2017, 23, 293–300. [Google Scholar] [CrossRef]
- Furuuchi, K.; Morimoto, K.; Kurashima, A.; Fujiwara, K.; Nakamoto, K.; Tanaka, Y.; Tachibana, H.; Yoshimori, K.; Sasaki, Y.; Ohta, K. Treatment Duration and Disease Recurrence Following the Successful Treatment of Patients with Mycobacterium avium Complex Lung Disease. Chest 2020, 157, 1442–1445. [Google Scholar] [CrossRef]
- Griffith, D.E.; Brown-Elliott, B.A.; Langsjoen, B.; Zhang, Y.; Pan, X.; Girard, W.; Nelson, K.; Caccitolo, J.; Alvarez, J.; Shepherd, S.; et al. Clinical and molecular analysis of macrolide resistance in Mycobacterium avium complex lung disease. Am. J. Respir. Crit. Care Med. 2006, 174, 928–934. [Google Scholar] [CrossRef]
- Morimoto, K.; Namkoong, H.; Hasegawa, N.; Nakagawa, T.; Morino, E.; Shiraishi, Y.; Ogawa, K.; Izumi, K.; Takasaki, J.; Yoshiyama, T.; et al. Macrolide-Resistant Mycobacterium avium Complex Lung Disease: Analysis of 102 Consecutive Cases. Ann. Am. Thorac. Soc. 2016, 13, 1904–1911. [Google Scholar] [CrossRef]
- Griffith, D.E.; Eagle, G.; Thomson, R.; Aksamit, T.R.; Hasegawa, N.; Morimoto, K.; Addrizzo-Harris, D.J.; O’Donnell, A.E.; Marras, T.K.; Flume, P.A.; et al. Amikacin Liposome Inhalation Suspension for Treatment-Refractory Lung Disease Caused by Mycobacterium avium Complex (CONVERT). A Prospective, Open-Label, Randomized Study. Am. J. Respir. Crit. Care Med. 2018, 198, 1559–1569. [Google Scholar] [CrossRef]
- Winthrop, K.L.; Flume, P.A.; Thomson, R.; Mange, K.C.; Yuen, D.W.; Ciesielska, M.; Morimoto, K.; Ruoss, S.J.; Codecasa, L.R.; Yim, J.J.; et al. Amikacin Liposome Inhalation Suspension for Mycobacterium avium Complex Lung Disease: A 12-Month Open-Label Extension Clinical Trial. Ann. Am. Thorac. Soc. 2021, 18, 1147–1157. [Google Scholar] [CrossRef]
- Martiniano, S.L.; Wagner, B.D.; Levin, A.; Nick, J.A.; Sagel, S.D.; Daley, C.L. Safety and Effectiveness of Clofazimine for Primary and Refractory Nontuberculous Mycobacterial Infection. Chest 2017, 152, 800–809. [Google Scholar] [CrossRef]
- Kwak, N.; Whang, J.; Yang, J.S.; Kim, T.S.; Kim, S.A.; Yim, J.J. Minimal Inhibitory Concentration of Clofazimine Among Clinical Isolates of Nontuberculous Mycobacteria and Its Impact on Treatment Outcome. Chest 2021, 159, 517–523. [Google Scholar] [CrossRef]
- Asakura, T.; Suzuki, S.; Fukano, H.; Okamori, S.; Kusumoto, T.; Uwamino, Y.; Ogawa, T.; So, M.; Uno, S.; Namkoong, H.; et al. Sitafloxacin-Containing Regimen for the Treatment of Refractory Mycobacterium avium Complex Lung Disease. Open Forum Infect. Dis. 2019, 6, ofz108. [Google Scholar] [CrossRef]
- Pasipanodya, J.G.; Ogbonna, D.; Ferro, B.E.; Magombedze, G.; Srivastava, S.; Deshpande, D.; Gumbo, T. Systematic Review and Meta-analyses of the Effect of Chemotherapy on Pulmonary Mycobacterium abscessus Outcomes and Disease Recurrence. Antimicrob. Agents Chemother. 2017, 61, e01206–e01217. [Google Scholar] [CrossRef]
- Kwak, N.; Dalcolmo, M.P.; Daley, C.L.; Eather, G.; Gayoso, R.; Hasegawa, N.; Jhun, B.W.; Koh, W.J.; Namkoong, H.; Park, J.; et al. M ycobacterium abscessus pulmonary disease: Individual patient data meta-analysis. Eur. Respir. J. 2019, 54, 1801991. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, L.; Mao, Y.; Ye, M.; Guo, Q.; Zhang, Y.; Xu, L.; Zhang, Z.; Li, B.; Chu, H. Clinical Efficacy and Adverse Effects of Antibiotics Used to Treat Mycobacterium abscessus Pulmonary Disease. Front. Microbiol. 2019, 10, 1977. [Google Scholar] [CrossRef]
- Park, Y.; Park, Y.E.; Jhun, B.W.; Park, J.; Kwak, N.; Jo, K.W.; Yim, J.J.; Shim, T.S.; Kang, Y.A. Impact of Susceptibility to Injectable Antibiotics on the Treatment Outcomes of Mycobacterium abscessus Pulmonary Disease. Open Forum Infect. Dis. 2021, 8, ofab215. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Susceptibility Testing of Mycobacteria, Nocardiae and Other Aerobic Actinomycetes. Approved Standard. CLSI Document M24 3rd Edition (CLSI 2018). Available online: https://clsi.org/standards/products/microbiology/documents/m24/ (accessed on 21 December 2021).
- Raju, R.M.; Raju, S.M.; Zhao, Y.; Rubin, E.J. Leveraging Advances in Tuberculosis Diagnosis and Treatment to Address Nontuberculous Mycobacterial Disease. Emerg Infect. Dis. 2016, 22, 365–369. [Google Scholar] [CrossRef]
- Farooq, H.Z.; Cirillo, D.M.; Hillemann, D.; Wyllie, D.; van der Werf, M.J.; Kodmon, C.; Nikolayevskyy, V. Limited Capability for Testing Mycobacterium tuberculosis for Susceptibility to New Drugs. Emerg. Infect. Dis. 2021, 27, 985–987. [Google Scholar] [CrossRef] [PubMed]
- Telenti, A.; Imboden, P.; Marchesi, F.; Lowrie, D.; Cole, S.; Colston, M.J.; Matter, L.; Schopfer, K.; Bodmer, T. Detection of rifampicin-resistance mutations in Mycobacterium tuberculosis. Lancet 1993, 341, 647–650. [Google Scholar] [CrossRef]
- Global Laboratory Initiative. GLI Model TB Diagnostic Algorithms. Available online: https://stoptb.org/wg/gli/assets/documents/gli_algorithms.pdf (accessed on 17 November 2021).
- Kadura, S.; King, N.; Nakhoul, M.; Zhu, H.; Theron, G.; Koser, C.U.; Farhat, M. Systematic review of mutations associated with resistance to the new and repurposed Mycobacterium tuberculosis drugs bedaquiline, clofazimine, linezolid, delamanid and pretomanid. J. Antimicrob. Chemother. 2020, 75, 2031–2043. [Google Scholar] [CrossRef] [PubMed]
- Feuerriegel, S.; Kohl, T.A.; Utpatel, C.; Andres, S.; Maurer, F.P.; Heyckendorf, J.; Jouet, A.; Badalato, N.; Foray, L.; Fouad Kamara, R.; et al. Rapid genomic first- and second-line drug resistance prediction from clinical Mycobacterium tuberculosis specimens using Deeplex-MycTB. Eur. Respir. J. 2021, 57, 2001796. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.; Halse, T.A.; Shea, J.; Modestil, H.; Fowler, R.C.; Musser, K.A.; Escuyer, V.; Lapierre, P. Assessing Nanopore Sequencing for Clinical Diagnostics: A Comparison of Next-Generation Sequencing (NGS) Methods for Mycobacterium tuberculosis. J. Clin. Microbiol. 2020, 59, e00583-20. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Susceptibility Testing of Mycobacteria, Nocardiae and Other Aerobic Actinomycetes. Approved Standard. CLSI Document M62 (CLSI 2018). Available online: https://clsi.org/standards/products/microbiology/documents/m24-supplement/ (accessed on 21 December 2021).
- Moon, S.M.; Park, H.Y.; Kim, S.Y.; Jhun, B.W.; Lee, H.; Jeon, K.; Kim, D.H.; Huh, H.J.; Ki, C.S.; Lee, N.Y.; et al. Clinical Characteristics, Treatment Outcomes, and Resistance Mutations Associated with Macrolide-Resistant Mycobacterium avium Complex Lung Disease. Antimicrob. Agents Chemother. 2016, 60, 6758–6765. [Google Scholar] [CrossRef] [PubMed]
- Brown-Elliott, B.A.; Iakhiaeva, E.; Griffith, D.E.; Woods, G.L.; Stout, J.E.; Wolfe, C.R.; Turenne, C.Y.; Wallace, R.J., Jr. In vitro activity of amikacin against isolates of Mycobacterium avium complex with proposed MIC breakpoints and finding of a 16S rRNA gene mutation in treated isolates. J. Clin. Microbiol. 2013, 51, 3389–3394. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, B.J.; Kook, Y.; Yun, Y.J.; Shin, J.H.; Kim, B.J.; Kook, Y.H. Mycobacterium massiliense is differentiated from Mycobacterium abscessus and Mycobacterium bolletii by erythromycin ribosome methyltransferase gene (erm) and clarithromycin susceptibility patterns. Microbiol. Immunol. 2010, 54, 347–353. [Google Scholar] [CrossRef]
- Brown-Elliott, B.A.; Vasireddy, S.; Vasireddy, R.; Iakhiaeva, E.; Howard, S.T.; Nash, K.; Parodi, N.; Strong, A.; Gee, M.; Smith, T.; et al. Utility of sequencing the erm(41) gene in isolates of Mycobacterium abscessus subsp. abscessus with low and intermediate clarithromycin MICs. J. Clin. Microbiol. 2015, 53, 1211–1215. [Google Scholar] [CrossRef]
- Wallace, R.J.; Meier, A., Jr.; Brown, B.A.; Zhang, Y.; Sander, P.; Onyi, G.O.; Böttger, E.C. Genetic basis for clarithromycin resistance among isolates of Mycobacterium chelonae and Mycobacterium abscessus. Antimicrob. Agents Chemother. 1996, 40, 1676–1681. [Google Scholar] [CrossRef]
- Mougari, F.; Amarsy, R.; Veziris, N.; Bastian, S.; Brossier, F.; Bercot, B.; Raskine, L.; Cambau, E. Standardized interpretation of antibiotic susceptibility testing and resistance genotyping for Mycobacterium abscessus with regard to subspecies and erm41 sequevar. J. Antimicrob. Chemother. 2016, 71, 2208–2212. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, D.H.; Moon, S.M.; Song, J.Y.; Huh, H.J.; Lee, N.Y.; Shin, S.J.; Koh, W.J.; Jhun, B.W. Association between 16S rRNA gene mutations and susceptibility to amikacin in Mycobacterium avium Complex and Mycobacterium abscessus clinical isolates. Sci. Rep. 2021, 11, 6108. [Google Scholar] [CrossRef]
- Tuberculosis Research Committee (RYOKEN), Tokyo, Japan. Nationwide survey of anti-tuberculosis drug resistance in Japan. Int. J. Tuberc. Lung Dis. 2015, 19, 157–162. [Google Scholar] [CrossRef]
- Kawatsu, L.; Uchimura, K.; Izumi, K.; Ohkado, A.; Yoshiyama, T. Treatment outcome of multidrug-resistant tuberculosis in Japan - the first cross-sectional study of Japan tuberculosis surveillance data. BMC Infect. Dis. 2018, 18, 445. [Google Scholar] [CrossRef]
- Drug-resistant Mycobacterium tuberculosis in Japan: A nationwide survey, 2002. Int. J. Tuberc. Lung Dis. 2007, 11, 1129–1135.
- Lifan, Z.; Sainan, B.; Feng, S.; Siyan, Z.; Xiaoqing, L. Linezolid for the treatment of extensively drug-resistant tuberculosis: A systematic review and meta-analysis. Int. J. Tuberc. Lung Dis. 2019, 23, 1293–1307. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Position Statement on Innovative Clinical Trial Design for Development of New TB Treatments. Available online: https://www.who.int/publications/i/item/9789240030800 (accessed on 17 November 2021).
- Kawasaki, M.; Echiverri, C.; Raymond, L.; Cadena, E.; Reside, E.; Gler, M.T.; Oda, T.; Ito, R.; Higashiyama, R.; Katsuragi, K.; et al. Lipoarabinomannan in sputum to detect bacterial load and treatment response in patients with pulmonary tuberculosis: Analytic validation and evaluation in two cohorts. PLoS Med. 2019, 16, e1002780. [Google Scholar] [CrossRef] [PubMed]
- Sakashita, K.; Takeuchi, R.; Takeda, K.; Takamori, M.; Ito, K.; Igarashi, Y.; Hayashi, E.; Iguchi, M.; Ono, M.; Kashiyama, T.; et al. Ultrasensitive enzyme-linked immunosorbent assay for the detection of MPT64 secretory antigen to evaluate Mycobacterium tuberculosis viability in sputum. Int. J. Infect. Dis. 2020, 96, 244–253. [Google Scholar] [CrossRef]
- Hayashi, M.; Takayanagi, N.; Kanauchi, T.; Miyahara, Y.; Yanagisawa, T.; Sugita, Y. Prognostic factors of 634 HIV-negative patients with Mycobacterium avium complex lung disease. Am. J. Respir. Crit. Care Med. 2012, 185, 575–583. [Google Scholar] [CrossRef]
- Gochi, M.; Takayanagi, N.; Kanauchi, T.; Ishiguro, T.; Yanagisawa, T.; Sugita, Y. Retrospective study of the predictors of mortality and radiographic deterioration in 782 patients with nodular/bronchiectatic Mycobacterium avium complex lung disease. BMJ. Open 2015, 5, e008058. [Google Scholar] [CrossRef]
- Kadota, T.; Matsui, H.; Hirose, T.; Suzuki, J.; Saito, M.; Akaba, T.; Kobayashi, K.; Akashi, S.; Kawashima, M.; Tamura, A.; et al. Analysis of drug treatment outcome in clarithromycin-resistant Mycobacterium avium complex lung disease. BMC Infect. Dis. 2016, 16, 31. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Miwa, S.; Shirai, M.; Kanai, M.; Fujita, K.; Ohba, H.; Iwaizumi, E.; Oshima, T.; Kojima, S.; Suda, T.; et al. Macrolide resistant Mycobacterium avium complex pulmonary disease following clarithromycin and ethambutol combination therapy. Respir. Med. 2020, 169, 106025. [Google Scholar] [CrossRef]
- Iwao, T.; Kato, G.; Ito, I.; Aramaki, E.; Kuroda, T. A survey of clarithromycin monotherapy and long-term administration of ethambutol for patients with MAC lung disease in Japan: A retrospective cohort study using the database of health insurance claims. Pharmacoepidemiol. Drug Saf. 2020, 29, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Yamaba, Y.; Ito, Y.; Suzuki, K.; Kikuchi, T.; Ogawa, K.; Fujiuchi, S.; Hasegawa, N.; Kurashima, A.; Higuchi, T.; Uchiya, K.I.; et al. Moxifloxacin resistance and genotyping of Mycobacterium avium and Mycobacterium intracellulare isolates in Japan. J. Infect. Chemother. 2019, 25, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Woods, G.L.; Williams-Bouyer, N.; Wallace, R.J., Jr.; Brown-Elliott, B.A.; Witebsky, F.G.; Conville, P.S.; Plaunt, M.; Hall, G.; Aralar, P.; Inderlied, C. Multisite reproducibility of results obtained by two broth dilution methods for susceptibility testing of Mycobacterium avium complex. J. Clin. Microbiol. 2003, 41, 627–631. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Morimoto, K.; Nakagawa, T.; Asami, T.; Morino, E.; Fujiwara, H.; Hase, I.; Tsujimoto, Y.; Izumi, K.; Hayashi, Y.; Matsuda, S.; et al. Clinico-microbiological analysis of 121 patients with pulmonary Mycobacteroides abscessus complex disease in Japan - An NTM-JRC study with RIT. Respir. Med. 2018, 145, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, K.; Furuuchi, K.; Aono, A.; Uesugi, F.; Shirai, T.; Nakamoto, K.; Shimada, T.; Mochizuki, F.; Tanaka, Y.; Iijima, H.; et al. Clinical risk factors related to treatment failure in Mycobacterium abscessus lung disease. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Aono, A.; Morimoto, K.; Chikamatsu, K.; Yamada, H.; Igarashi, Y.; Murase, Y.; Takaki, A.; Mitarai, S. Antimicrobial susceptibility testing of Mycobacteroides (Mycobacterium) abscessus complex, Mycolicibacterium (Mycobacterium) fortuitum, and Mycobacteroides (Mycobacterium) chelonae. J. Infect. Chemother 2019, 25, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Kamada, K.; Yoshida, A.; Iguchi, S.; Arai, Y.; Uzawa, Y.; Konno, S.; Shimojima, M.; Kikuchi, K. Nationwide surveillance of antimicrobial susceptibility of 509 rapidly growing mycobacteria strains isolated from clinical specimens in Japan. Sci. Rep. 2021, 11, 12208. [Google Scholar] [CrossRef] [PubMed]
- Kadota, N.; Shinohara, T.; Hino, H.; Goda, Y.; Murase, Y.; Mitarai, S.; Ogushi, F. Mycobacterium abscessus ssp. abscessus infection progressing to empyema from vertebral osteomyelitis in an immunocompetent patient without pulmonary disease: A case report. BMC Pulm. Med. 2019, 19, 100. [Google Scholar] [CrossRef]
- Seki, M.; Kamioka, Y.; Takano, K.; Imai, H.; Shoji, M.; Hariu, M.; Kabutoya, Y.; Watanabe, Y. Mycobacterium abscessus Associated Peritonitis with CAPD Successfully Treated Using a Linezolid and Tedizolid Containing Regimen Suggested Immunomodulatory Effects. Am. J. Case Rep. 2020, 21, e924642. [Google Scholar] [CrossRef] [PubMed]
- Bahuguna, A.; Rawat, D.S. An overview of new antitubercular drugs, drug candidates, and their targets. Med. Res. Rev. 2020, 40, 263–292. [Google Scholar] [CrossRef] [PubMed]
- Cynamon, M.H.; Klemens, S.P.; Sharpe, C.A.; Chase, S. Activities of several novel oxazolidinones against Mycobacterium tuberculosis in a murine model. Antimicrob. Agents Chemother. 1999, 43, 1189–1191. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.N.; Brickner, S.J.; Stover, C.K.; Zhu, T.; Ogden, A.; Tasneen, R.; Tyagi, S.; Grosset, J.H.; Nuermberger, E.L. Addition of PNU-100480 to first-line drugs shortens the time needed to cure murine tuberculosis. Am. J. Respir. Crit. Care Med. 2009, 180, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Wallis, R.S.; Dawson, R.; Friedrich, S.O.; Venter, A.; Paige, D.; Zhu, T.; Silvia, A.; Gobey, J.; Ellery, C.; Zhang, Y.; et al. Mycobactericidal activity of sutezolid (PNU-100480) in sputum (EBA) and blood (WBA) of patients with pulmonary tuberculosis. PLoS ONE 2014, 9, e94462. [Google Scholar] [CrossRef] [PubMed]
- Robertson, G.T.; Ramey, M.E.; Massoudi, L.M.; Carter, C.L.; Zimmerman, M.; Kaya, F.; Graham, B.G.; Gruppo, V.; Hastings, C.; Woolhiser, L.K.; et al. Comparative Analysis of Pharmacodynamics in the C3HeB/FeJ Mouse Tuberculosis Model for DprE1 Inhibitors TBA-7371, PBTZ169, and OPC-167832. Antimicrob. Agents Chemother. 2021, 65, e0058321. [Google Scholar] [CrossRef] [PubMed]
- Hariguchi, N.; Chen, X.; Hayashi, Y.; Kawano, Y.; Fujiwara, M.; Matsuba, M.; Shimizu, H.; Ohba, Y.; Nakamura, I.; Kitamoto, R.; et al. OPC-167832, a Novel Carbostyril Derivative with Potent Antituberculosis Activity as a DprE1 Inhibitor. Antimicrob. Agents Chemother. 2020, 64, e02020-19. [Google Scholar] [CrossRef]
- Sutherland, H.S.; Tong, A.S.T.; Choi, P.J.; Blaser, A.; Conole, D.; Franzblau, S.G.; Lotlikar, M.U.; Cooper, C.B.; Upton, A.M.; Denny, W.A.; et al. 3,5-Dialkoxypyridine analogues of bedaquiline are potent antituberculosis agents with minimal inhibition of the hERG channel. Bioorg. Med. Chem. 2019, 27, 1292–1307. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Converse, P.J.; Upton, A.M.; Mdluli, K.; Fotouhi, N.; Nuermberger, E.L. Comparative Efficacy of the Novel Diarylquinoline TBAJ-587 and Bedaquiline against a Resistant Rv0678 Mutant in a Mouse Model of Tuberculosis. Antimicrob. Agents Chemother. 2021, 65, e02418-20. [Google Scholar] [CrossRef]
- Almeida, D.; Converse, P.J.; Li, S.Y.; Upton, A.M.; Fotouhi, N.; Nuermberger, E.L. Comparative efficacy of the novel diarylquinoline TBAJ-876 and bedaquiline against a resistant Rv0678 mutant in a mouse model of tuberculosis. Antimicrob. Agents Chemother. 2021, 65, e0141221. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Jhun, B.W.; Moon, S.M.; Kim, S.Y.; Jeon, K.; Kwon, O.J.; Huh, H.J.; Lee, N.Y.; Shin, S.J.; Daley, C.L.; et al. In Vitro Activity of Bedaquiline and Delamanid against Nontuberculous Mycobacteria, Including Macrolide-Resistant Clinical Isolates. Antimicrob. Agents Chemother. 2019, 63, e00665-19. [Google Scholar] [CrossRef] [PubMed]
- Philley, J.V.; Wallace, R.J., Jr.; Benwill, J.L.; Taskar, V.; Brown-Elliott, B.A.; Thakkar, F.; Aksamit, T.R.; Griffith, D.E. Preliminary Results of Bedaquiline as Salvage Therapy for Patients with Nontuberculous Mycobacterial Lung Disease. Chest 2015, 148, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.C.; Vasireddy, R.; Vasireddy, S.; Philley, J.V.; Brown-Elliott, B.A.; Perry, B.J.; Griffith, D.E.; Benwill, J.L.; Cameron, A.D.; Wallace, R.J., Jr. Emergence of mmpT5 Variants during Bedaquiline Treatment of Mycobacterium intracellulare Lung Disease. J. Clin. Microbiol. 2017, 55, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Locher, C.P.; Jones, S.M.; Hanzelka, B.L.; Perola, E.; Shoen, C.M.; Cynamon, M.H.; Ngwane, A.H.; Wiid, I.J.; van Helden, P.D.; Betoudji, F.; et al. A novel inhibitor of gyrase B is a potent drug candidate for treatment of tuberculosis and nontuberculosis mycobacterial infections. Antimicrob. Agents Chemother. 2015, 59, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Pennings, L.J.; Ruth, M.M.; Wertheim, H.F.L.; van Ingen, J. The Benzimidazole SPR719 Shows Promising Concentration-Dependent Activity and Synergy against Nontuberculous Mycobacteria. Antimicrob. Agents Chemother. 2021, 65, e02469-20. [Google Scholar] [CrossRef] [PubMed]
- Madariaga, M.G. Omadacycline in the Treatment of Mycobacterium abscessus Infection. Clin. Infect. Dis. 2020, 71, 1124. [Google Scholar] [CrossRef]
- Bax, H.I.; de Vogel, C.P.; Mouton, J.W.; de Steenwinkel, J.E.M. Omadacycline as a promising new agent for the treatment of infections with Mycobacterium abscessus. J. Antimicrob. Chemother. 2019, 74, 2930–2933. [Google Scholar] [CrossRef] [PubMed]
- Gotfried, M.H.; Horn, K.; Garrity-Ryan, L.; Villano, S.; Tzanis, E.; Chitra, S.; Manley, A.; Tanaka, S.K.; Rodvold, K.A. Comparison of Omadacycline and Tigecycline Pharmacokinetics in the Plasma, Epithelial Lining Fluid, and Alveolar Cells of Healthy Adult Subjects. Antimicrob. Agents Chemother. 2017, 61, e01135-17. [Google Scholar] [CrossRef]
- Morrisette, T.; Alosaimy, S.; Philley, J.V.; Wadle, C.; Howard, C.; Webb, A.J.; Veve, M.P.; Barger, M.L.; Bouchard, J.; Gore, T.W.; et al. Preliminary, Real-world, Multicenter Experience with Omadacycline for Mycobacterium abscessus Infections. Open Forum Infect. Dis. 2021, 8, ofab002. [Google Scholar] [CrossRef] [PubMed]
| Regimen | ATS/CDC/ERS/IDSA | WHO Longer Regimen | WHO Shorter Regimen * | BPaL | JSTB a |
|---|---|---|---|---|---|
| Year | 2019 | 2020 | 2020 | 2020 | 2020 |
| Number of drugs | |||||
| Intensive phase | 5 | 4 | 7 | 3 | 5 |
| Consolidation phase | 4 | 3 | 4 | ||
| Treatment duration | |||||
| Intensive phase | 5–7months ** | 6 months or longer ** | 4–6months | ||
| Total | 15–21months **b | 15–17months ** | 9–12months | 6–9months | 18months ** |
| Drugs | Strongly: LVX c (MFLX d), BDQ Conditional: LZD e, CLO f, DCS, AMK g, STR, EMB, PZA, carbapenem h DMD | Group A: LVX c (MFLX d), BDQ, LZD e, Group B: CLO f, DCS Group C: EMB, DMD, PZA, carbapenem h, AMK g, ETO, PTO i, PAS | Intensive phase: BDQ, INH, EMB, PZA, MFLX d (LVX c) ETO, CLO f Consolidation phase: EMB, PZA MFLX d, CLO f | BDQ PTM LZD | Preferred: LVX c, BDQ Second to the preferred: LZD e Additional: EMB, PZA, DMD CLO f, DCS Conditional: STR, KAN j, EVM ETO, PAS carbapenem h |
| Category | Privious Definition 2006–2021 | New Definition 2021– |
|---|---|---|
| MDR | INH and RIF | INH and RIF |
| Pre-XDR | MDR + (any fluoroquinolone or at least one of three drugs * | MDR/RR + any fluoroquinolone |
| XDR | MDR + (any fluoroquinolone + at least one of three drugs * | MDR/RR + (any fluoroquinolone + at least one additional drug ** |
| Pathogen | MAC (Slowly Growing Mycobacteria) | MABS (Rapidly Growing Mycobacteria) | ||||
|---|---|---|---|---|---|---|
| Medium | CAMHB * | CAMHB | ||||
| Supplement | 5% OADC | none | ||||
| Time to judge DST results | 7 days | 14 days ** | ||||
| MIC, μg/mL | MIC, μg/mL | |||||
| S | I | R | S | I | R | |
| CLR | ≤8 | 16 | ≥32 | ≤2 | 7 | ≥8 |
| AMK (IV) | ≤16 | 32 | ≥64 | ≤16 | 32 | ≥64 |
| AMK (liposomal inhaled) | ≤64 | - | ≥128 | |||
| MFLX | ≤1 | 2 | ≥4 | ≤1 | 2 | ≥4 |
| LZD | ≤8 | 16 | ≥32 | ≤8 | 16 | ≥32 |
| IPM | ≤4 | 8–16 | ≥32 | |||
| MEPM | ≤4 | 8–16 | ≥32 | |||
| FOX a | ≤16 | 32–64 | ≥128 | |||
| CIP b | ≤1 | 2 | ≥4 | |||
| DOX c | ≤1 | 2–4 | ≥8 | |||
| SXT d | ≤2/38 | - | ≥4/76 | |||
| Priority | Drugs |
|---|---|
| Most preferred | LVX, BDQ |
| Additional * | EMB, PZA, DMD, DCS |
| Additional ** | STR, KAN, EVMETO, PAS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamada, K.; Mitarai, S. Anti-Mycobacterial Drug Resistance in Japan: How to Approach This Problem? Antibiotics 2022, 11, 19. https://doi.org/10.3390/antibiotics11010019
Kamada K, Mitarai S. Anti-Mycobacterial Drug Resistance in Japan: How to Approach This Problem? Antibiotics. 2022; 11(1):19. https://doi.org/10.3390/antibiotics11010019
Chicago/Turabian StyleKamada, Keisuke, and Satoshi Mitarai. 2022. "Anti-Mycobacterial Drug Resistance in Japan: How to Approach This Problem?" Antibiotics 11, no. 1: 19. https://doi.org/10.3390/antibiotics11010019
APA StyleKamada, K., & Mitarai, S. (2022). Anti-Mycobacterial Drug Resistance in Japan: How to Approach This Problem? Antibiotics, 11(1), 19. https://doi.org/10.3390/antibiotics11010019






