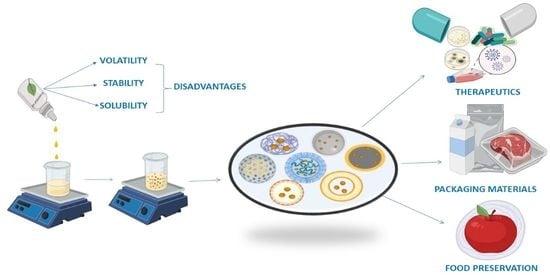
Nanoparticles—Attractive Carriers of Antimicrobial Essential Oils
Abstract
:1. Introduction
1.1. Mechanism of Action and Bacterial Spectrum
- Clove essential oil demonstrated in vitro inhibitory and bactericidal activity at a concentration of 0.304 mg/mL against S. aureus, Escherichia coli, Listeria monocytogenes, and Salmonella typhimurium [17]. The antiviral activity of eugenol, the primary component of clove essential oil, was investigated in vitro against the Herpes simplex virus (HSV)-1 and HSV-2 viruses. The replication of these viruses was inhibited with IC50 values of 25.6 µg/mL and 16.2 µg/mL against HSV-1 and HSV-2, respectively [16]. The MIC value of clove oil against L. monocytogenes was found to be 0.5 mg/mL [18].
- Lavender EO obtained from L. angustifolia Mill. has a strong antiseptic effect against antibiotic-resistant strains, e.g., Staphylococcus aureus, that are resistant to methicillin (MRSA) or vancomycin-resistant strains of Enterococcus sp. (VRE). The antimicrobial activity of Lavender EO was evaluated against L. monocytogenes (24 strains) and Salmonella enterica (10 food strains). MIC ≥ 10.0 μL/mL inhibited Salmonella; MIC of 0.3 μL/mL inhibited L. monocytogenes, revealing noticeable activity, especially on clinical strains. This activity appears to be related to EOs composition. The highest antimicrobial activities were demonstrated in the specific constituents such as linalool (38.17 and 61.98%), camphor (8.97 and 10.30%), and 1,8-cineole (6.89 and 8.11%, respectively) [19].
- Thyme EO was found to have antiviral action against Herpes simplex virus (HSV1, DNA virus) with IC50 values of 11 µg/mL [19]. Thyme EO was also tested for its ability to fight strains that cause acute bacterial pharyngitis and throat irritation. β-haemolytic Streptococci strains, such as S. pyogenes, cause this infection. T. vulgaris EO was found to be effective against S. pyogenes strains obtained from throat of patients [20]. At a concentration of 0.06%, thyme EO that was rich in γ-terpinene (68.415%) and p-thymol (24.721%) totally inhibited the growth of Fusarium graminearum Fg 06–17 [21].
- Essential Oil of Cinnamomum zeylanicum demonstrated 100% inhibition effect at 3.1 µL/mL concentration against influenza virus A1/Denver/1/57 (H1N1) with 30 min exposure. In both liquid and vapour phases, Eugenol, the main component of Cinnamomum zeylanicum EO, exhibited the most significant anti-influenza activity [22]. Cinnamon essential oil was recently used to improve zein film for food packaging, which now contains an extra 4% concentration of chitosan nanoparticles (CNP). The combined antibacterial capabilities of EO and nanoparticles not only inhibited the development of Escherichia coli (PTCC 1163) and Staphylococcus aureus (PTTC 25923), but also increased the tensile strength and decreased the elongation of the composite zein film [23].
- Tea tree EO has been used in products for oral hygiene and dermatological uses due to its antibacterial characteristics. Porphyromonas gingivalis (MIC and MBC = 0.007%) and Porphyromonas endodontalis (MIC = 0.007% and MBC = 0.5%) bacteria that cause halitosis are both inhibited by tea tree EO [24]. The antibacterial activities of tea tree essential oils (EOs) that are commercially accessible were examined. Five out of the ten EOs were active. Components identified in tea tree essential oil inhibited bacterium viability in Pseudomonas aeruginosa biofilm and caused oxidative damage in Candida glabrata [25]. Essential oil of Melaleuca alternifolia, on the other hand, displayed only minimal antifungal activity against Aspergillus niger (MIC = 625 µg/mL), which was attributed to the active components terpinen-4-ol and α-terpineol [26].
1.2. Stability and Bioavailability of Essential Oils
2. Essential Oils in Combination with Antibiotics
3. Clinical Trials and Marketed Products of EOs
Marketed Products
4. Nanotechnology in Delivery of Essential Oils
Improvement of Functional Attributes of EOs
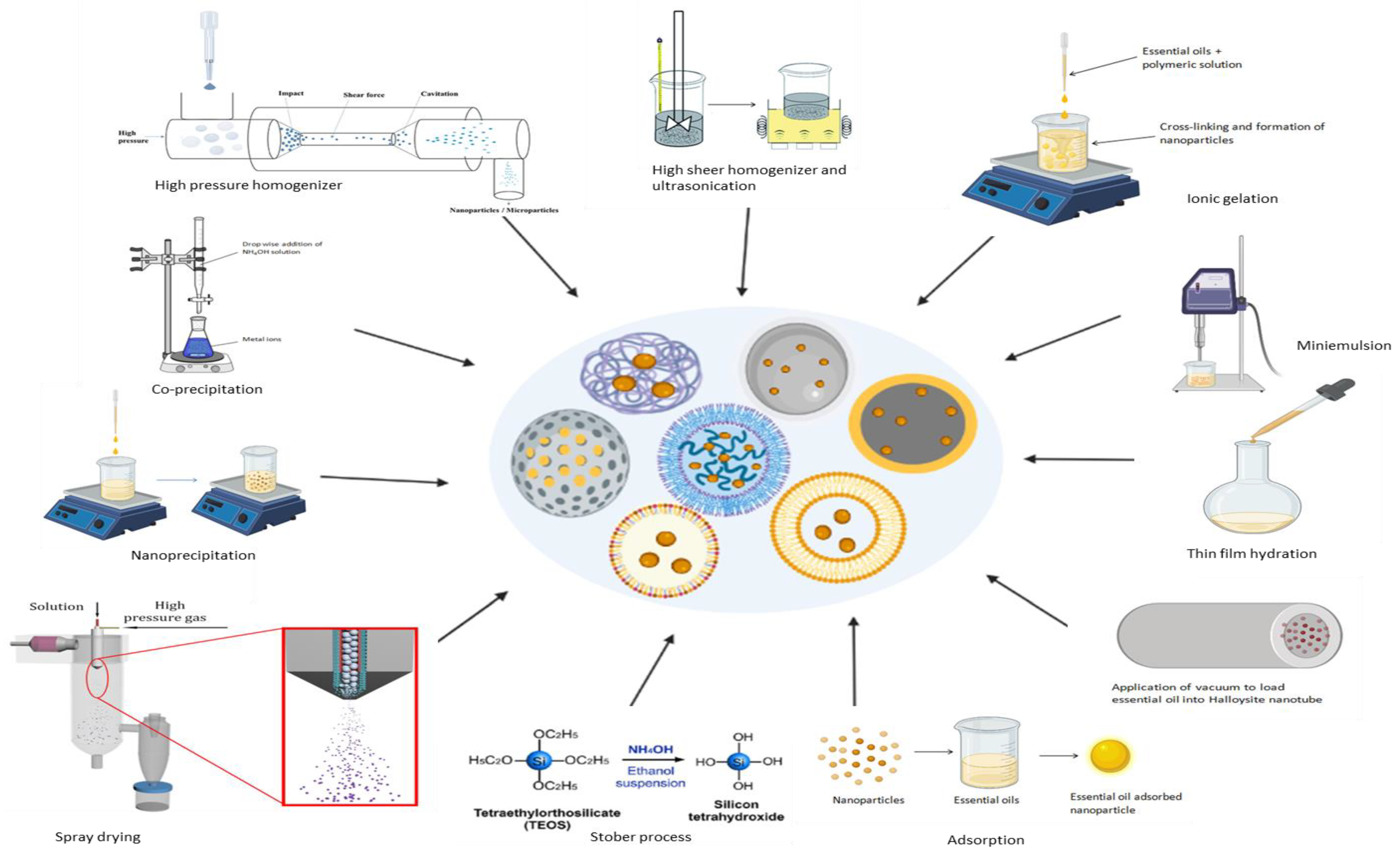
5. Synthesis of Essential Oil-Loaded Nanoparticles
5.1. Co-Precipitation Method
5.2. High-Pressure Homogenization Method
5.3. High-Speed Stirring and Ultra-Sonication Methods
5.4. Ionic Gelation Method
5.5. Miniemulsion Polymerization Method
5.6. Nanoprecipitation Method
5.7. Spray Drying Technique
5.8. Stöber Process
5.9. Thin Film Hydration, Adsorption and Vacuum Pulling Methods
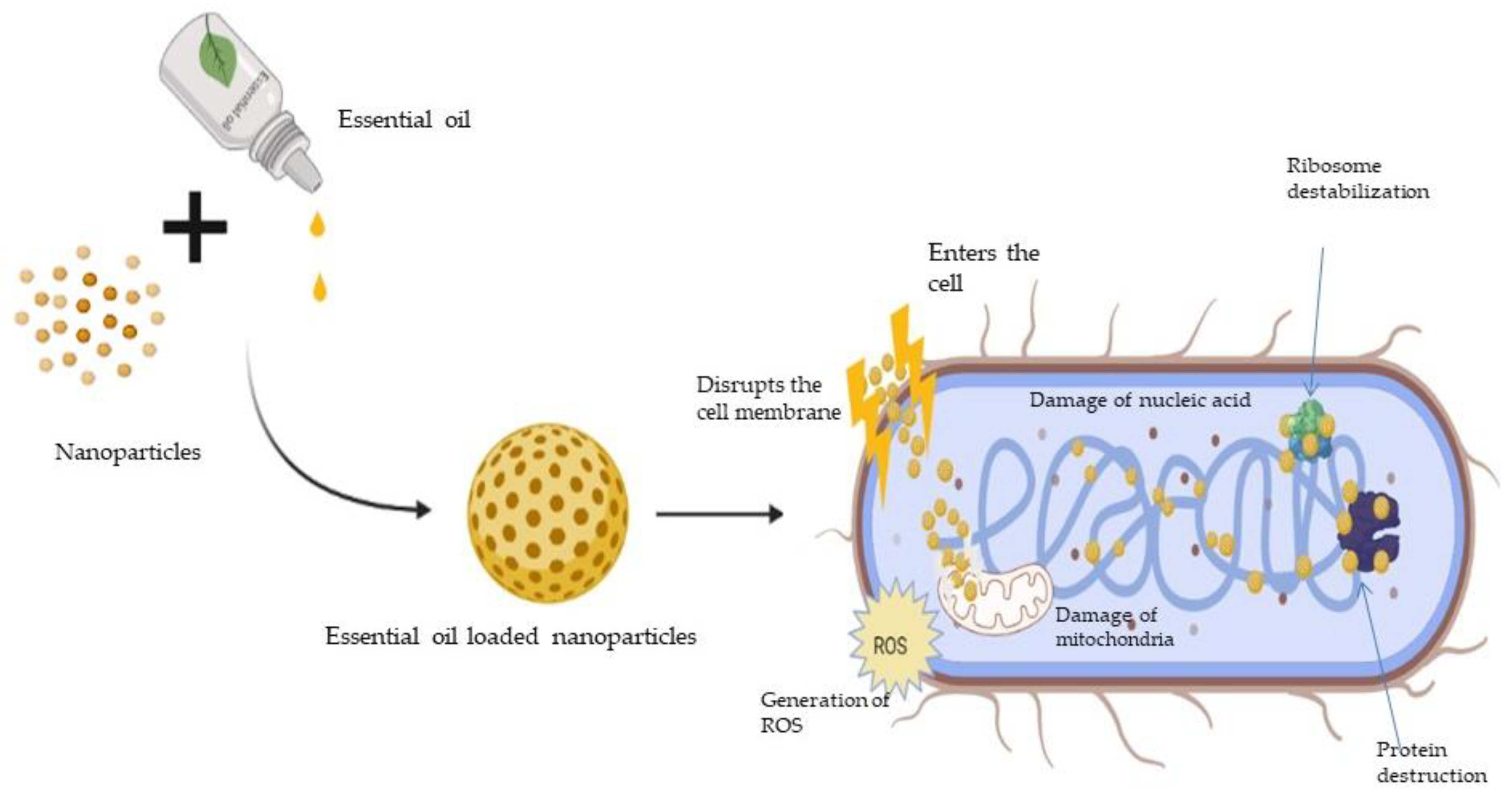
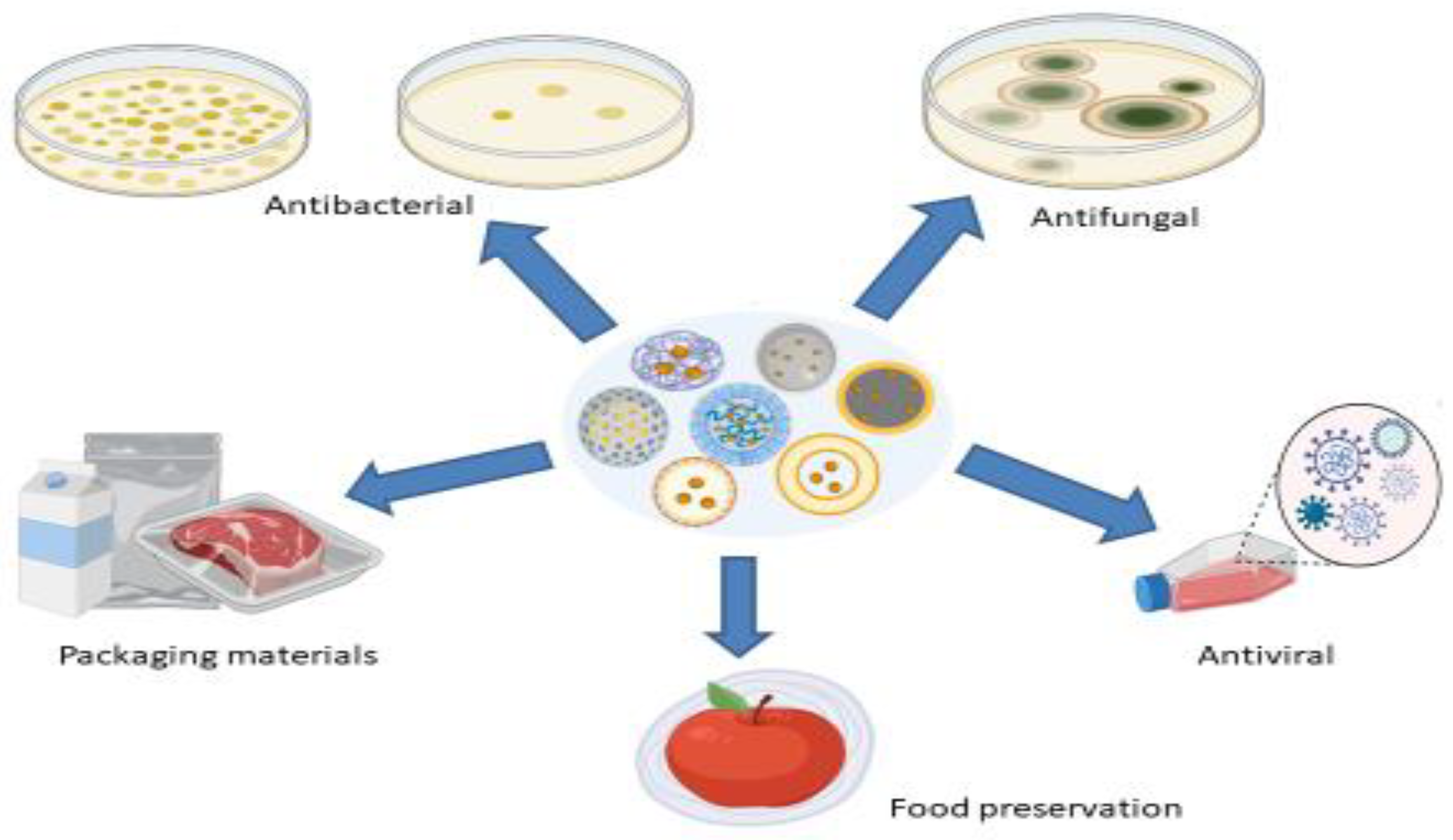
6. Nanoparticles as Carriers of EO
6.1. Inorganic Nanoparticles
6.2. Lipid Nanoparticles
6.3. Liposomes
6.4. Magnetite Nanoparticles
6.5. Metal Nanoparticles
6.6. Nanogels
6.7. Polymeric Nanoparticles
6.8. Silica Nanoparticles
6.9. Nanoemulsion-Based Nanoparticle Candies
7. Synergistic Action of EO and Nanoparticles
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Kraker, M.E.A.; Stewardson, A.J.; Harbarth, S. Will 10 million people die a year due to antimicrobial resistance by 2050? PLoS Med. 2016, 13, e1002184. [Google Scholar] [CrossRef] [Green Version]
- Miguel, M.G.; Lourenço, J.P.; Faleiro, M.L. Superparamagnetic iron oxide nanoparticles and essential oils: A new tool for biological applications. Int. J. Mol. Sci. 2020, 21, 6633. [Google Scholar] [CrossRef]
- Rai, M.; Paralikar, P.; Jogee, P.; Agarkar, G.; Ingle, A.P.; Derita, M.; Zacchino, S. Synergistic antimicrobial potential of essential oils in combination with nanoparticles: Emerging trends and future perspectives. Int. J. Pharm. 2017, 519, 67–78. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial activity of some essential oils—Present status and future perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [Green Version]
- de Groot, A.C.; Schmidt, E. Essential oils, part I: Introduction. Dermatitis 2016, 27, 39–42. [Google Scholar] [CrossRef]
- De Groot, A.C.; Schmidt, E. Essential oils, part III: Chemical composition. Dermatitis 2016, 27, 161–169. [Google Scholar] [CrossRef]
- Eslahi, H.; Fahimi, N.; Sardarian, A.R. Chemical composition of essential oils. Essent. Oils Food Process. Chem. Saf. Appl. 2017, 119–171. [Google Scholar] [CrossRef]
- Ramsey, J.T.; Shropshire, B.C.; Nagy, T.R.; Chambers, K.D.; Li, Y.; Korach, K.S. Focus: Plant-based medicine and pharmacology: Essential oils and health. Yale J. Biol. Med. 2020, 93, 291. [Google Scholar]
- Tenover, F.C. Mechanisms of antimicrobial resistance in bacteria. Am. J. Med. 2006, 119, S3–S10. [Google Scholar] [CrossRef]
- Wongkattiya, N.; Sanguansermsri, P.; Fraser, I.H.; Sanguansermsri, D. Antibacterial activity of cuminaldehyde on food-borne pathogens, the bioactive component of essential oil from Cuminum cyminum L. collected in Thailand. J. Complement. Integr. Med. 2019, 16. [Google Scholar] [CrossRef]
- Yang, L.; Zhan, C.; Huang, X.; Hong, L.; Fang, L.; Wang, W.; Su, J. Durable antibacterial cotton fabrics based on natural borneol-derived Anti-MRSA agents. Adv. Health Mater. 2020, 9, e2000186. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, F.; Ji, B.-P.; Pei, R.-S.; Xu, N. The antibacterial mechanism of carvacrol and thymol against Escherichia coli. Lett. Appl. Microbiol. 2008, 47, 174–179. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.K.; Ezzat, M.O.; Majid, A.S.A.; Majid, A.M.S.A. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef]
- Gavanji, S.; Mohammadi, E.; Larki, B.; Bakhtari, A. Antimicrobial and cytotoxic evaluation of some herbal essential oils in comparison with common antibiotics in bioassay condition. Integr. Med. Res. 2014, 3, 142–152. [Google Scholar] [CrossRef] [Green Version]
- Duarte, M.C.T.; Leme, E.E.; Delarmelina, C.; Soares, A.A.; Figueira, G.M.; Sartoratto, A. Activity of essential oils from Brazilian medicinal plants on Escherichia coli. J. Ethnopharmacol. 2007, 111, 197–201. [Google Scholar] [CrossRef]
- Reichling, J.; Schnitzler, P.; Suschke, U.; Saller, R. Essential oils of aromatic plants with antibacterial, antifungal, antiviral, and cytotoxic properties—An overview. Complement. Med. Res. 2009, 16, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Radunz, M.; da Trindade, M.L.M.; Camargo, T.M.; Radünz, A.L.; Borges, C.D.; Gandra, E.A.; Helbig, E. Antimicrobial and antioxidant activity of unencapsulated and encapsulated clove (Syzygium aromaticum, L.) essential oil. Food Chem. 2019, 276, 180–186. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Antimicrobial mechanism of clove oil on Listeria monocytogenes. Food Control 2018, 94, 140–146. [Google Scholar] [CrossRef]
- Astani, A.; Reichling, J.; Schnitzler, P. Comparative study on the antiviral activity of selected monoterpenes derived from essential oils. Phytother. Res. 2009, 24, 673–679. [Google Scholar] [CrossRef]
- Fani, M.; Kohanteb, J. In vitro antimicrobial activity of thymus vulgaris essential oil against major oral pathogens. J. Evid.-Based Integr. Med. 2017, 22, 660–666. [Google Scholar] [CrossRef] [Green Version]
- Alexa, E.; Sumalan, R.M.; Danciu, C.; Obistioiu, D.; Negrea, M.; Poiana, M.-A.; Rus, C.; Radulov, I.; Pop, G.; Dehelean, C. Synergistic antifungal, allelopatic and anti-proliferative potential of Salvia officinalis L., and Thymus vulgaris L. essential oils. Molecules 2018, 23, 185. [Google Scholar] [CrossRef] [Green Version]
- (PDF) Anti-Influenza Virus Activity of Essential Oils and Vapors. Available online: https://www.researchgate.net/publication/267035381_Anti-influenza_virus_activity_of_essential_oils_and_vapors (accessed on 5 December 2021).
- Jiang, Y.; Wang, D.; Li, F.; Li, D.; Huang, Q. Cinnamon essential oil Pickering emulsion stabilized by zein-pectin composite nanoparticles: Characterization, antimicrobial effect and advantages in storage application. Int. J. Biol. Macromol. 2020, 148, 1280–1289. [Google Scholar] [CrossRef]
- Carson, C.F.; Ashton, L.; Dry, L.; Smith, D.W.; Riley, T.V. Melaleuca alternifolia (tea tree) oil gel (6%) for the treatment of recurrent herpes labialis. J. Antimicrob. Chemother. 2001, 48, 450–451. [Google Scholar] [CrossRef]
- Powers, C.N.; Osier, J.L.; McFeeters, R.L.; Brazell, C.B.; Olsen, E.L.; Moriarity, D.M.; Satyal, P.; Setzer, W.N. Antifungal and cytotoxic activities of sixty commercially-available essential oils. Molecules 2018, 23, 1549. [Google Scholar] [CrossRef] [Green Version]
- Brun, P.; Bernabè, G.; Filippini, R.; Piovan, A. In vitro antimicrobial activities of commercially available tea tree (Melaleuca alternifolia) essential oils. Curr. Microbiol. 2019, 76, 108–116. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Stevanovic, Z.D.; Sieniawska, E.; Glowniak, K.; Obradovic, N.; Pajic-Lijakovic, I. Natural Macromolecules as carriers for essential oils: From extraction to biomedical application. Front. Bioeng. Biotechnol. 2020, 8, 563. [Google Scholar] [CrossRef]
- Singh, A.; Deepika; Chaudhari, A.K.; Das, S.; Singh, V.K.; Dwivedy, A.K.; Shivalingam, R.K.; Dubey, N.K. Assessment of preservative potential of Bunium persicum (Boiss) essential oil against fungal and aflatoxin contamination of stored masticatories and improvement in efficacy through encapsulation into chitosan nanomatrix. Environ. Sci. Pollut. Res. 2020, 27, 27635–27650. [Google Scholar] [CrossRef]
- Chahota, R.K.; Sharma, V.; Ghani, M.; Sharma, T.R.; Rana, J.C.; Sharma, S.K. Genetic and phytochemical diversity analysis in Bunium persicum populations of north-western Himalaya. Physiol. Mol. Biol. Plants 2017, 23, 429–441. [Google Scholar] [CrossRef] [Green Version]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef] [Green Version]
- Tan, L.T.H.; Lee, L.H.; Yin, W.F.; Chan, C.K.; Kadir, H.A.; Chan, K.G.; Goh, B.H. Traditional uses, phytochemistry, and bioactivities of Cananga odorata (Ylang-Ylang). Evid.-Based Complement. Altern. Med. 2015, 2015, 1–30. [Google Scholar] [CrossRef] [Green Version]
- Mbekou, M.I.K.; Dize, D.; Yimgang, V.L.; Djague, F.; Toghueo, R.M.K.; Sewald, N.; Lenta, B.N.; Boyom, F.F. Antibacterial and mode of action of extracts from endophytic fungi derived from Terminalia mantaly, Terminalia catappa, and Cananga odorata. BioMed Res. Int. 2021, 2021, 6697973. [Google Scholar] [CrossRef]
- Beatović, D.; Krstić-Milošević, D.; Trifunović, S.; Šiljegović, J.; Glamočlija, J.; Ristić, M.; Jelačić, S. Chemical composition, antioxidant and antimicrobial activities of the essential oils of twelve Ocimum basilicum L. cultivars grown in Serbia. Rec. Nat. Prod. 2015, 9, 62–75. [Google Scholar]
- Ghadimian, S.; Esmaeili, F. Chemical composition of the essential oils of Carum copticum. J. Essent. Oil Bear. Plants 2016, 19, 1834–1836. [Google Scholar] [CrossRef]
- Marchese, A.; Arciola, C.R.; Barbieri, R.; Silva, A.S.; Nabavi, S.M.; Sokeng, A.J.T.; Izadi, M.; Jafari, N.J.; Suntar, I.; Daglia, M.; et al. Update on monoterpenes as antimicrobial agents: A particular focus on p-cymene. Materials 2017, 10, 947. [Google Scholar] [CrossRef]
- Condò, C.; Anacarso, I.; Sabia, C.; Iseppi, R.; Anfelli, I.; Forti, L.; de Niederhäusern, S.; Bondi, M.; Messi, P. Antimicrobial activity of spices essential oils and its effectiveness on mature biofilms of human pathogens. Nat. Prod. Res. 2020, 34, 567–574. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, S.; Shi, W.; Zubcevik, N.; Miklossy, J.; Zhang, Y. Selective essential oils from spice or culinary herbs have high activity against stationary phase and biofilm borrelia burgdorferi. Front. Med. 2017, 4, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarra, M.; Mannucci, C.; Delbò, M.; Calapai, G. Citrus bergamia essential oil: From basic research to clinical application. Front. Pharmacol. 2015, 6, 36. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Cai, J.; Chen, H.; Zhong, Q.; Hou, Y.; Chen, W.; Chen, W. Antibacterial activity and mechanism of linalool against Pseudomonas aeruginosa. Microb. Pathog. 2020, 141, 103980. [Google Scholar] [CrossRef]
- Song, X.; Liu, T.; Wang, L.; Liu, L.; Li, X.; Wu, X. Antibacterial effects and mechanism of mandarin (Citrus reticulata L.) essential oil against Staphylococcus aureus. Molecules 2020, 25, 4956. [Google Scholar] [CrossRef]
- Sharma, S.; Habib, S.; Sahu, D.; Gupta, J. Chemical properties and therapeutic potential of citral, a monoterpene isolated from lemongrass. Med. Chem. 2020, 17, 2–12. [Google Scholar] [CrossRef]
- Shi, C.; Song, K.; Zhang, X.; Sun, Y.; Sui, Y.; Chen, Y.; Jia, Z.; Sun, H.; Sun, Z.; Xia, X. Antimicrobial activity and possible mechanism of action of citral against cronobacter sakazakii. PLoS ONE 2016, 11, e0159006. [Google Scholar] [CrossRef] [Green Version]
- Aldoghaim, F.S.; Flematti, G.R.; Hammer, K.A. Antimicrobial activity of several cineole-rich western australian eucalyptus essential oils. Microorganisms 2018, 6, 122. [Google Scholar] [CrossRef] [Green Version]
- Sebei, K.; Sakouhi, F.; Herchi, W.; Khouja, M.L.; Boukhchina, S. Chemical composition and antibacterial activities of seven Eucalyptus species essential oils leaves. Biol. Res. 2015, 48, 7. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, S.M.; Motta, J.F.G.; dos Santos, R.R.; Chávez, D.W.H.; de Melo, N.R. Functional and antimicrobial properties of cellulose acetate films incorporated with sweet fennel essential oil and plasticizers. Curr. Res. Food Sci. 2020, 3, 1–8. [Google Scholar] [CrossRef]
- Kubo, I.; Fujita, K.-I.; Nihei, K.-I. Antimicrobial activity of anethole and related compounds from aniseed. J. Sci. Food Agric. 2008, 88, 242–247. [Google Scholar] [CrossRef]
- Rozman, N.A.S.; Yenn, T.W.; Tan, W.-N.; Ring, L.C.; Yusof, F.A.B.M.; Sulaiman, B. Homalomena pineodora, a novel essential oil bearing plant and its antimicrobial activity against diabetic wound pathogens. J. Essent. Oil Bear. Plants 2018, 21, 963–971. [Google Scholar] [CrossRef]
- Verma, R.; Rahman, L.; Chanotiya, C.; Verma, R.; Chauhan, A.; Yadav, A.; Singh, A.; Yadav, A. Essential oil composition of Lavandula angustifolia Mill. cultivated in the mid hills of Uttarakhand, India. J. Serb. Chem. Soc. 2010, 75, 343–348. [Google Scholar] [CrossRef]
- de Oliveira, E.F.; DE Paula, H.C.; de Paula, R.C. Alginate/cashew gum nanoparticles for essential oil encapsulation. Colloids Surf. B Biointerfaces 2014, 113, 146–151. [Google Scholar] [CrossRef] [PubMed]
- McKay, D.L.; Blumberg, J.B. A Review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.). Phytother. Res. 2006, 20, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (Tea Tree) oil: A review of antimicrobial and other medicinal properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Feng, R.; Li, L.; Zhou, X.; Li, Z.; Jia, R.; Song, X.; Zou, Y.; Yin, L.; He, C.; et al. The Antibacterial mechanism of terpinen-4-ol against streptococcus agalactiae. Curr. Microbiol. 2018, 75, 1214–1220. [Google Scholar] [CrossRef]
- Saharkhiz, M.J.; Motamedi, M.; Zomorodian, K.; Pakshir, K.; Miri, R.; Hemyari, K. Chemical Composition, antifungal and antibiofilm activities of the essential oil of Mentha piperita L. ISRN Pharm. 2012, 2012, 718645. [Google Scholar] [CrossRef] [Green Version]
- Dawaba, A.M.; Dawaba, H.M. Application of optimization technique to develop nano-based carrier of nigella sativa essential oil: Characterization and assessment. Recent Pat. Drug Deliv. Formul. 2020, 13, 228–240. [Google Scholar] [CrossRef]
- Goel, S.; Mishra, P. Thymoquinone inhibits biofilm formation and has selective antibacterial activity due to ROS generation. Appl. Microbiol. Biotechnol. 2018, 102, 1955–1967. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, I.; Silva-Espinoza, B.; Ortega-Ramirez, L.; Leyva, J.; Siddiqui, M.W.; Valenzuela, M.R.C.; Gonzalez-Aguilar, G.; Zavala, J.F.A. Oregano essential oil as an antimicrobial and antioxidant additive in food products. Crit. Rev. Food Sci. Nutr. 2016, 56, 1717–1727. [Google Scholar] [CrossRef]
- Ellahi, H.; Sadrabad, E.K.; Hekmatimoghaddam, S.H.; Jebali, A.; Sadeghizadeh-Yazdi, J.; Rastiani, F.; Mohajeri, F.A. Antimicrobial activity and chemical composition of Pistachia Atlantica Gum Sub Sp. Kurdica. essential oil. J. Nutr. Food Secur. 2019, 4, 186–190. [Google Scholar] [CrossRef]
- Ghavam, M.; Manca, M.L.; Manconi, M. Chemical Composition and Antimicrobial Activity of Essential Oils Obtained from Leaves and Fowers of Salvia Hydrangea DC. ex Benth. Available online: https://www.nature.com/articles/s41598-020-73193-y.pdf?origin=ppub (accessed on 4 January 2022).
- Paraschos, S.; Mitakou, S.; Skaltsounis, A.-L. Chios gum mastic: A review of its biological activities. Curr. Med. Chem. 2012, 19, 2292–2302. [Google Scholar] [CrossRef]
- Gonçalves, F.A.; Neto, M.A.; Bezerra, J.N.S.; Macrae, A.; de Sousa, O.V.; Fonteles-Filho, A.A.; Vieira, R.H. Antibacterial activity of GUAVA, Psidium guajava Linnaeus, leaf extracts on diarrhea-causing enteric bacteria isolated from Seabob shrimp, Xiphopenaeus kroyeri (Heller). Rev. Inst. Med. Trop. São Paulo 2008, 50, 11–15. [Google Scholar] [CrossRef] [Green Version]
- Fathi, N.; Lotfipour, F.; Dizaj, S.M.; Hamishehkar, H.; Mohammadi, M. Antimicrobial activity of nanostructured lipid carriers loaded punica granatum seed oil against Staphylococcus epidermidis. Pharm. Nanotechnol. 2020, 8, 485–494. [Google Scholar] [CrossRef]
- Yemis, G.P.; Bach, S.; Delaquis, P. Antibacterial activity of polyphenol-rich pomegranate peel extract against Cronobacter sakazakii. Int. J. Food Prop. 2019, 22, 985–993. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Shi, C.; Wu, Q.; Zheng, Z.; Liu, P.; Li, G.; Peng, X.; Xia, X. Antimicrobial activity of punicalagin against Staphylococcus aureus and its effect on biofilm formation. Foodborne Pathog. Dis. 2017, 14, 282–287. [Google Scholar] [CrossRef]
- Ojeda-Sana, A.M.; van Baren, C.M.; Elechosa, M.A.; Juárez, M.A.; Moreno, S. New insights into antibacterial and antioxidant activities of rosemary essential oils and their main components. Food Control 2013, 31, 189–195. [Google Scholar] [CrossRef]
- Wu, K.; Lin, Y.; Chai, X.; Duan, X.; Zhao, X.; Chun, C. Mechanisms of vapor-phase antibacterial action of essential oil from Cinnamomum camphora var. linaloofera Fujita against Escherichia coli. Food Sci. Nutr. 2019, 7, 2546–2555. [Google Scholar] [CrossRef] [Green Version]
- Feyzioglu, G.C.; Tornuk, F. Development of chitosan nanoparticles loaded with summer savory (Satureja hortensis L.) essential oil for antimicrobial and antioxidant delivery applications. LWT 2016, 70, 104–110. [Google Scholar] [CrossRef]
- Kaur, K.; Kaushal, S.; Rani, R. Chemical composition, antioxidant and antifungal potential of clove (Syzygium aromaticum) essential oil, its major compound and its derivatives. J. Essent. Oil Bear. Plants 2019, 22, 1195–1217. [Google Scholar] [CrossRef]
- Aldosary, S.K.; El-Rahman, S.N.A.; Al-Jameel, S.S.; Alromihi, N.M. Antioxidant and antimicrobial activities of Thymus vulgaris essential oil contained and synthesis thymus (Vulgaris) silver nanoparticles. Braz. J. Biol. 2021, 83, e244675. [Google Scholar] [CrossRef]
- Vinciguerra, V.; Rojas, F.; Tedesco, V.; Giusiano, G.; Angiolella, L. Chemical characterization and antifungal activity of Origanum vulgare, Thymus vulgaris essential oils and carvacrol against Malassezia furfur. Nat. Prod. Res. 2019, 33, 3273–3277. [Google Scholar] [CrossRef]
- Sajed, H.; Sahebkar, A.; Iranshahi, M. Zataria multiflora Boiss. (Shirazi thyme)—An ancient condiment with modern pharmaceutical uses. J. Ethnopharmacol. 2013, 145, 686–698. [Google Scholar] [CrossRef]
- Langeveld, W.T.; Veldhuizen, E.J.A.; Burt, S.A. Synergy between essential oil components and antibiotics: A review. Crit. Rev. Microbiol. 2013, 40, 76–94. [Google Scholar] [CrossRef]
- Grădinaru, A.C.; Trifan, A.; Şpac, A.; Brebu, M.; Miron, A.; Aprotosoaie, A.C. Antibacterial activity of traditional spices against lower respiratory tract pathogens: Combinatorial effects of Trachyspermum ammi essential oil with conventional antibiotics. Lett. Appl. Microbiol. 2018, 67, 449–457. [Google Scholar] [CrossRef]
- Ben-Khalifa, R.; Gaspar, F.B.; Pereira, C.; Chekir-Ghedira, L.; Rodríguez-Rojo, S. Essential oil and hydrophilic antibiotic co-encapsulation in multiple lipid nanoparticles: Proof of concept and in vitro activity against Pseudomonas aeruginosa. Antibiotics 2021, 10, 1300. [Google Scholar] [CrossRef] [PubMed]
- Nafis, A.; Iriti, M.; Ouchari, L.; El Otmani, F.; Marraiki, N.; Elgorban, A.M.; Syed, A.; Mezrioui, N.; Hassani, L.; Custódio, L. New insight into the chemical composition, antimicrobial and synergistic effects of the moroccan endemic Thymus atlanticus (ball) roussine essential oil in combination with conventional antibiotics. Molecules 2021, 26, 5850. [Google Scholar] [CrossRef] [PubMed]
- Nafis, A.; Saad, F.E.; El Khalloufi, F.; Kasrati, A.; Abbad, A.; Mezrioui, N.; Oudra, B.; Vasconcelos, V.; Hassani, L. New insight into antimicrobial activities of Linaria ventricosa essential oil and its synergetic effect with conventional antibiotics. Arch. Microbiol. 2021, 203, 4361–4366. [Google Scholar] [CrossRef]
- Valcourt, C.; Saulnier, P.; Umerska, A.; Zanelli, M.; Montagu, A.; Rossines, E.; Joly-Guillou, M. Synergistic interactions between doxycycline and terpenic components of essential oils encapsulated within lipid nanocapsules against gram negative bacteria. Int. J. Pharm. 2016, 498, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Soulaimani, B.; Varoni, E.; Iriti, M.; Mezrioui, N.-E.; Hassani, L.; Abbad, A. Synergistic anticandidal effects of six essential oils in Combination with Fluconazole or Amphotericin B against four clinically isolated Candida Strains. Antibiotics 2021, 10, 1049. [Google Scholar] [CrossRef]
- Nidhi, P.; Rolta, R.; Kumar, V.; Dev, K.; Sourirajan, A. Synergistic potential of Citrus aurantium L. essential oil with antibiotics against Candida albicans. J. Ethnopharmacol. 2020, 262, 113135. [Google Scholar] [CrossRef]
- Nafis, A.; Kasrati, A.; Jamali, C.A.; Custódio, L.; Vitalini, S.; Iriti, M.; Hassani, L. A comparative study of the in vitro antimicrobial and synergistic effect of essential oils from Laurus nobilis L. and Prunus armeniaca L. from Morocco with antimicrobial drugs: New approach for health promoting products. Antibiotics 2020, 9, 140. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wu, H.; Jiang, D.; Yang, Y.; Tang, W.; Xu, K. The antifungal activity of essential oil from Melaleuca leucadendra (L.) L. grown in China and its synergistic effects with conventional antibiotics against Candida. Nat. Prod. Res. 2019, 33, 2545–2548. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Łopusiewicz, Ł.; Kostek, M.; Drozłowska, E.; Pruss, A.; Wojciuk, B.; Sienkiewicz, M.; Zielińska-Bliźniewska, H.; Dołęgowska, B. The antibacterial activity of lavender essential oil alone and in combination with octenidine dihydrochloride against MRSA strains. Molecules 2019, 25, 95. [Google Scholar] [CrossRef] [Green Version]
- Aelenei, P.; Rimbu, C.M.; Guguianu, E.; Dimitriu, G.; Aprotosoaie, A.C.; Brebu, M.; Horhogea, C.; Miron, A. Coriander essential oil and linalool-interactions with antibiotics against Gram-positive and Gram-negative bacteria. Lett. Appl. Microbiol. 2018, 68, 156–164. [Google Scholar] [CrossRef]
- Vasconcelos, N.G.; Queiroz, J.H.F.D.S.; Da Silva, K.E.; Vasconcelos, P.C.D.P.; Croda, J.; Simionatto, S. Synergistic effects of Cinnamomum cassia L. essential oil in combination with polymyxin B against carbapenemase-producing Klebsiella pneumoniae and Serratia marcescens. PLoS ONE 2020, 15, e0236505. [Google Scholar] [CrossRef]
- Si, H.; Hu, J.; Liu, Z.; Zeng, Z.-L. Antibacterial effect of oregano essential oil alone and in combination with antibiotics against extended-spectrum β-lactamase-producing Escherichia coli: Table 1. FEMS Immunol. Med. Microbiol. 2008, 53, 190–194. [Google Scholar] [CrossRef] [Green Version]
- El Atki, Y.; Aouam, I.; El Kamari, F.; Taroq, A.; Nayme, K.; Timinouni, M.; Lyoussi, B.; Abdellaoui, A. Antibacterial activity of cinnamon essential oils and their synergistic potential with antibiotics. J. Adv. Pharm. Technol. Res. 2019, 10, 63–67. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Łopusiewicz, Ł.; Pruss, A.; Kostek, M.; Sienkiewicz, M.; Bonikowski, R.; Wojciechowska-Koszko, I.; Dołęgowska, B. Antibacterial activity of selected essential oil compounds alone and in combination with β-lactam antibiotics against MRSA strains. Int. J. Mol. Sci. 2020, 21, 7106. [Google Scholar] [CrossRef]
- Paul, I.M.; Beiler, J.S.; King, T.S.; Clapp, E.R.; Vallati, J.; Berlin, C.M. Vapor rub, petrolatum, and no treatment for children with nocturnal cough and cold symptoms. Pediatrics 2010, 126, 1092–1099. [Google Scholar] [CrossRef] [Green Version]
- Treatment of Acute Rhino-Sinusitis with Essential Oils of Aromatic Plants-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT00610779?term=eucalyptus&cond=Infections&draw=2&rank=5 (accessed on 2 December 2021).
- Treatment of Acute Tracheitis and Laryngitis with Essential Oils of Aromatic Plants-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/study/NCT00611390?term=eucalyptus&cond=Infections&draw=2&rank=6 (accessed on 2 December 2021).
- Vennemann, M.M.; Hummel, T.; Berger, K. The association between smoking and smell and taste impairment in the general population. J. Neurol. 2008, 255, 1121–1126. [Google Scholar] [CrossRef]
- A Randomized Study to Evaluate the Efficacy of Herbal Ingredients Combined with a Carrier System (Phytonail) Compared with Amorolfine 5% Nail Lacquer (Loceryl) in the Treatment of Toenail Onychomycosis-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01929187?term=lavender+oil&cond=Infections&draw=2&rank=1 (accessed on 2 December 2021).
- Gamaleldin, M.; Nashat, S. COVID-19 Treatment Modalities. 2020. Available online: https://doi.org/10.31219/OSF.IO/U56FC (accessed on 2 December 2021).
- Use of Vagitories Based on St. John’s Wort, Tea Tree Oil and Shepherd’s Purse in the Treatment of Vaginal Inflammation-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04558697?term=chamomile+oil&cond=Infections&draw=2&rank=2 (accessed on 2 December 2021).
- Efficacy of a Plaque Disclosing Toothpaste on Home Oral Hygiene Procedures. Available online: https://clinicaltrials.gov/ct2/show/NCT03287011 (accessed on 2 December 2021).
- Irshad, M.; Subhani, M.A.; Ali, S.; Hussain, A. Biological importance of essential oils. Essent. Oils-Oils Nat. 2020. [Google Scholar] [CrossRef] [Green Version]
- Guzmán, E.; Lucia, A. Essential oils and their individual components in cosmetic products. Cosmetics 2021, 8, 114. [Google Scholar] [CrossRef]
- Lammari, N.; Louaer, O.; Meniai, A.H.; Elaissari, A. Encapsulation of essential oils via nanoprecipitation process: Overview, progress, challenges and prospects. Pharmaceutics 2020, 12, 431. [Google Scholar] [CrossRef]
- Tiwari, S.; Singh, B.K.; Dubey, N.K. Encapsulation of essential oils—A booster to enhance their bio-efficacy as botanical preservatives. J. Sci. Res. 2020, 64, 175–178. [Google Scholar] [CrossRef]
- Carpena, M.; Nuñez-Estevez, B.; Soria-Lopez, A.; Garcia-Oliveira, P.; Prieto, M.A. Essential oils and their application on active packaging systems: A review. Resources 2021, 10, 7. [Google Scholar] [CrossRef]
- Jamil, B.; Abbasi, R.; Abbasi, S.; Imran, M.; Khan, S.U.; Ihsan, A.; Javed, S.; Bokhari, H. Encapsulation of cardamom essential oil in chitosan nano-composites: In-vitro efficacy on antibiotic-resistant bacterial pathogens and cytotoxicity studies. Front. Microbiol. 2016, 7, 1580. [Google Scholar] [CrossRef]
- Mihai, A.D.; Chircov, C.; Grumezescu, A.M.; Holban, A.M. Magnetite nanoparticles and essential oils systems for advanced antibacterial therapies. Int. J. Mol. Sci. 2020, 21, 7355. [Google Scholar] [CrossRef]
- Duong, V.-A.; Nguyen, T.-T.-L.; Maeng, H.-J. Preparation of solid lipid nanoparticles and nanostructured lipid carriers for drug delivery and the effects of preparation parameters of solvent injection method. Molecules 2020, 25, 4781. [Google Scholar] [CrossRef]
- Bazzaz, B.S.F.; Khameneh, B.; Namazi, N.; Iranshahi, M.; Davoodi, D.; Golmohammadzadeh, S. Solid lipid nanoparticles carrying Eugenia caryophyllata essential oil: The novel nanoparticulate systems with broad-spectrum antimicrobial activity. Lett. Appl. Microbiol. 2018, 66, 506–513. [Google Scholar] [CrossRef]
- Giri, T.; Verma, S.; Alexander, A.; Ajazuddin, B.H.; Tripathy, M.; Tripathi, D. Crosslinked biodegradable alginate hydrogel floating beads for stomach site specific controlled delivery of metronidazole. Farmacia 2013, 61, 533–550. [Google Scholar]
- Krishnamoorthy, K.; Mahalingam, M. Selection of a suitable method for the preparation of polymeric nanoparticles: Multi-Criteria decision making approach. Adv. Pharm. Bull. 2015, 5, 57–67. [Google Scholar] [CrossRef]
- Zhang, F.; Ramachandran, G.; Mothana, R.A.; Noman, O.M.; Alobaid, W.A.; Rajivgandhi, G.; Manoharan, N. Anti-bacterial activity of chitosan loaded plant essential oil against multi drug resistant K. pneumoniae. Saudi J. Biol. Sci. 2020, 27, 3449–3455. [Google Scholar] [CrossRef]
- Amato, D.; Amato, D.; Mavrodi, O.V.; Braasch, D.A.; Walley, S.E.; Douglas, J.R.; Mavrodi, D.V.; Patton, D.L. Destruction of opportunistic pathogens via polymer nanoparticle-mediated release of plant-based antimicrobial payloads. Adv. Health Mater. 2016, 5, 1094–1103. [Google Scholar] [CrossRef] [Green Version]
- Froiio, F.; Ginot, L.; Paolino, D.; Lebaz, N.; Bentaher, A.; Fessi, H.; Elaissari, A. Essential oils-loaded polymer particles: Preparation, characterization and antimicrobial property. Polymers 2019, 11, 1017. [Google Scholar] [CrossRef] [Green Version]
- Abreu, F.O.M.S.; de Oliveira, E.F.; Paula, H.C.B.; de Paula, R.C.M. Chitosan/cashew gum nanogels for essential oil encapsulation. Carbohydr. Polym. 2012, 89, 1277–1282. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Vörös-Horváth, B.; Bencsik, T.; Micalizzi, G.; Mondello, L.; Horváth, G.; Kőszegi, T.; Széchenyi, A. Antimicrobial activity of different artemisia essential oil formulations. Molecules 2020, 25, 2390. [Google Scholar] [CrossRef]
- Quick Guide: Chitosan Nanoparticles Preparation with Ionic Gelation Method. Available online: https://chitolytic.com/chitosan-nanoparticles-ionic-gelation/ (accessed on 7 November 2021).
- Cruz, I.F.; Freire, C.; Araújo, J.P.; Pereira, C.; Pereira, A.M. Multifunctional ferrite nanoparticles: From current trends toward the future. In Magnetic Nanostructured Materials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 59–116. [Google Scholar] [CrossRef]
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous silica nanoparticles: A comprehensive review on synthesis and recent advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef] [Green Version]
- Badea, M.L.; Iconaru, S.L.; Groza, A.; Chifiriuc, M.C.; Beuran, M.; Predoi, D. Peppermint Essential Oil-Doped Hydroxyapatite Nanoparticles with Antimicrobial Properties. Molecules 2019, 24, 2169. [Google Scholar] [CrossRef] [Green Version]
- Alizadeh-Sani, M.; Mohammadian, E.; McClements, D.J. Eco-friendly active packaging consisting of nanostructured biopolymer matrix reinforced with TiO2 and essential oil: Application for preservation of refrigerated meat. Food Chem. 2020, 322, 126782. [Google Scholar] [CrossRef]
- Mizielińska, M.; Nawrotek, P.; Stachurska, X.; Ordon, M.; Bartkowiak, A. Packaging covered with antiviral and antibacterial coatings based on ZnO nanoparticles supplemented with geraniol and carvacrol. Int. J. Mol. Sci. 2021, 22, 1717. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Buton, N.; Badea, M.L.; Marutescu, L. Antimicrobial Activity of New Materials Based on Lavender and Basil Essential Oils and Hydroxyapatite. Nanomaterials 2018, 8, 291. [Google Scholar] [CrossRef] [Green Version]
- Babapour, H.; Jalali, H.; Nafchi, A.M. The synergistic effects of zinc oxide nanoparticles and fennel essential oil on physicochemical, mechanical, and antibacterial properties of potato starch films. Food Sci. Nutr. 2021, 9, 3893–3905. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- de Souza, M.E.; Clerici, D.J.; Verdi, C.M.; Fleck, G.; Quatrin, P.M.; Spat, L.E.; Bonez, P.C.; dos Santos, C.F.; Antoniazzi, R.P.; Zanatta, F.B.; et al. Antimicrobial activity of Melaleuca alternifolia nanoparticles in polymicrobial biofilm in situ. Microb. Pathog. 2017, 113, 432–437. [Google Scholar] [CrossRef]
- Piran, P.; Kafil, H.S.; Ghanbarzadeh, S.; Safdari, R.; Hamishehkar, H. Formulation of menthol-loaded nanostructured lipid carriers to enhance its antimicrobial activity for food preservation. Adv. Pharm. Bull. 2017, 7, 261–268. [Google Scholar] [CrossRef]
- Comin, V.M.; Lopes, L.Q.; Quatrin, P.M.; de Souza, M.E.; Bonez, P.C.; Pintos, F.G.; Raffin, R.; Vaucher, R.D.A.; Martinez, D.S.T.; Santos, R.C.V. Influence of Melaleuca alternifolia oil nanoparticles on aspects of Pseudomonas aeruginosa biofilm. Microb. Pathog. 2016, 93, 120–125. [Google Scholar] [CrossRef]
- Saporito, F.; Sandri, G.; Bonferoni, M.C.; Rossi, S.; Boselli, C.; Icaro Cornaglia, A.; Mannucci, B.; Grisoli, P.; Vigani, B.; Ferrari, F. Essential oil-loaded lipid nanoparticles for wound healing. Int. J. Nanomed. 2017, 11, 175–186. [Google Scholar] [CrossRef] [Green Version]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.; Sood, A.K.; Hua, S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [Green Version]
- Abdulbaqi, I.M.; Darwis, Y.; Khan, N.A.K.; Assi, R.A.; Khan, A.A. Ethosomal nanocarriers: The impact of constituents and formulation techniques on ethosomal properties, in vivo studies, and clinical trials. Int. J. Nanomed. 2016, 11, 2279–2304. [Google Scholar] [CrossRef] [Green Version]
- Zahid, S.R.; Upmanyu, N.; Dangi, S.; Ray, S.K.; Jain, P.; Parkhe, G. Ethosome: A novel vesicular carrier for transdermal drug delivery. J. Drug Deliv. Ther. 2018, 8, 318–326. [Google Scholar] [CrossRef]
- Aguilar-Pérez, K.; Medina, D.; Narayanan, J.; Parra-Saldívar, R.; Iqbal, H. Synthesis and nano-sized characterization of bioactive oregano essential oil molecule-loaded small unilamellar nanoliposomes with antifungal potentialities. Molecules 2021, 26, 2880. [Google Scholar] [CrossRef] [PubMed]
- Sinico, C.; De Logu, A.; Lai, F.; Valenti, D.; Manconi, M.; Loy, G.; Bonsignore, L.; Fadda, A.M. Liposomal incorporation of Artemisia arborescens L. essential oil and in vitro antiviral activity. Eur. J. Pharm. Biopharm. 2005, 59, 161–168. [Google Scholar] [CrossRef]
- Wu, Z.; Zhou, W.; Pang, C.; Deng, W.; Xu, C.; Wang, X. Multifunctional chitosan-based coating with liposomes containing laurel essential oils and nanosilver for pork preservation. Food Chem. 2019, 295, 16–25. [Google Scholar] [CrossRef]
- Jin, P.; Yao, R.; Qin, D.; Chen, Q.; Du, Q. Enhancement in Antibacterial activities of eugenol-entrapped ethosome nanoparticles via strengthening its permeability and sustained release. J. Agric. Food Chem. 2019, 67, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Anghel, I.; Grumezescu, A.M. Hybrid nanostructured coating for increased resistance of prosthetic devices to staphylococcal colonization. Nanoscale Res. Lett. 2013, 8, 6. [Google Scholar] [CrossRef] [Green Version]
- Rădulescu, M.; Andronescu, E.; Holban, A.M.; Vasile, B.S.; Iordache, F.; Mogoantă, L.; Mogoșanu, G.D.; Grumezescu, A.M.; Georgescu, M.; Chifiriuc, M.C. Antimicrobial nanostructured bioactive coating based on Fe3O4 and patchouli oil for wound dressing. Metals 2016, 6, 103. [Google Scholar] [CrossRef] [Green Version]
- Bilcu, M.; Grumezescu, A.M.; Oprea, A.E.; Popescu, R.C.; Mogoșanu, G.D.; Hristu, R.; Stanciu, G.A.; Mihailescu, D.F.; Lazar, V.; Bezirtzoglou, E.; et al. Efficiency of Vanilla, Patchouli and Ylang Ylang essential oils stabilized by iron oxide@C14 nanostructures against bacterial adherence and biofilms formed by Staphylococcus aureus and Klebsiella pneumoniae clinical strains. Molecules 2014, 19, 17943–17956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anghel, A.G.; Grumezescu, A.M.; Chirea, M.; Grumezescu, V.; Socol, G.; Iordache, F.; Oprea, A.E.; Anghel, I.; Holban, A.M. MAPLE Fabricated Fe3O4@cinnamomum verum antimicrobial surfaces for improved gastrostomy tubes. Molecules 2014, 19, 8981–8994. [Google Scholar] [CrossRef] [Green Version]
- Negut, I.; Grumezescu, V.; Ficai, A.; Grumezescu, A.M.; Holban, A.M.; Popescu, R.C.; Savu, D.; Vasile, B.S.; Socol, G. MAPLE deposition of Nigella sativa functionalized Fe3O4 nanoparticles for antimicrobial coatings. Appl. Surf. Sci. 2018, 455, 513–521. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Ramakrishna, S.; Esmaeili, H.; Bahrani, S.; Koosha, M.; Babapoor, A. Green synthesis of supermagnetic Fe3O4–MgO nanoparticles via Nutmeg essential oil toward superior anti-bacterial and anti-fungal performance. J. Drug Deliv. Sci. Technol. 2019, 54, 101352. [Google Scholar] [CrossRef]
- Grumezescu, V.; Holban, A.M.; Iordache, F.; Socol, G.; Mogoşanu, G.D.; Grumezescu, A.M.; Ficai, A.; Vasile, B.Ş.; Truşcă, R.; Chifiriuc, M.C.; et al. MAPLE fabricated magnetite@eugenol and (3-hidroxybutyric acid-co-3-hidroxyvaleric acid)–polyvinyl alcohol microspheres coated surfaces with anti-microbial properties. Appl. Surf. Sci. 2014, 306, 16–22. [Google Scholar] [CrossRef]
- Anghel, I.; Holban, A.M.; Grumezescu, A.M.; Andronescu, E.; Ficai, A.; Anghel, A.G.; Maganu, M.; Lazǎr, V.; Chifiriuc, M.C. Modified wound dressing with phyto-nanostructured coating to prevent staphylococcal and pseudomonal biofilm development. Nanoscale Res. Lett. 2012, 7, 690. [Google Scholar] [CrossRef] [Green Version]
- Anghel, I.; Holban, A.M.; Andronescu, E.; Grumezescu, A.M.; Chifiriuc, M.C.; Mihai Grumezescu, A. Efficient surface functionalization of wound dressings by a phytoactive nanocoating refractory to Candida albicansbiofilm development. Biointerphases 2013, 8, 12. [Google Scholar] [CrossRef] [Green Version]
- Palza, H. Antimicrobial polymers with metal nanoparticles. Int. J. Mol. Sci. 2015, 16, 2099–2116. [Google Scholar] [CrossRef] [Green Version]
- Maduray, K.; Parboosing, R. Metal nanoparticles: A promising treatment for viral and arboviral infections. Biol. Trace Elem. Res. 2021, 199, 3159–3176. [Google Scholar] [CrossRef] [PubMed]
- Jabir, M.S.; Taha, A.A.; Sahib, U.I. Linalool loaded on glutathione-modified gold nanoparticles: A drug delivery system for a successful antimicrobial therapy. Artif. Cells Nanomed. Biotechnol. 2018, 46, 345–355. [Google Scholar] [CrossRef]
- Manju, S.; Malaikozhundan, B.; Vijayakumar, S.; Shanthi, S.; Jaishabanu, A.; Ekambaram, P.; Vaseeharan, B. Antibacterial, antibiofilm and cytotoxic effects of Nigella sativa essential oil coated gold nanoparticles. Microb. Pathog. 2016, 91, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Sana, S.S.; Li, H.; Zhang, Z.; Sharma, M.; Usmani, Z.; Hou, T.; Netala, V.R.; Wang, X.; Gupta, V.K. Recent advances in essential oils-based metal nanoparticles: A review on recent developments and biopharmaceutical applications. J. Mol. Liq. 2021, 333, 115951. [Google Scholar] [CrossRef]
- Vilas, V.; Philip, D.; Mathew, J. Catalytically and biologically active silver nanoparticles synthesized using essential oil. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 132, 743–750. [Google Scholar] [CrossRef]
- Vilas, V.; Philip, D.; Mathew, J. Essential oil mediated synthesis of silver nanocrystals for environmental, anti-microbial and antioxidant applications. Mater. Sci. Eng. C 2016, 61, 429–436. [Google Scholar] [CrossRef]
- Muniyappan, N.; Nagarajan, N. Green synthesis of gold nanoparticles using Curcuma pseudomontana essential oil, its biological activity and cytotoxicity against human ductal breast carcinoma cells T47D. J. Environ. Chem. Eng. 2014, 2, 2037–2044. [Google Scholar] [CrossRef]
- Kousalová, J.; Etrych, T. Polymeric nanogels as drug delivery systems. Physiol. Res. 2018, 67, S305–S317. [Google Scholar] [CrossRef]
- Mohsenabadi, N.; Rajaei, A.; Tabatabaei, M.; Mohsenifar, A. Physical and antimicrobial properties of starch-carboxy methyl cellulose film containing rosemary essential oils encapsulated in chitosan nanogel. Int. J. Biol. Macromol. 2018, 112, 148–155. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, S.Y.B.; Almeida, R.R.; Pinto, N.A.R.; de Mayrinck, C.; Vieira, S.S.; Haddad, J.F.; Leitão, A.A.; Guimarães, L.G.D.L. Encapsulation of essential oils using cinnamic acid grafted chitosan nanogel: Preparation, characterization and antifungal activity. Int. J. Biol. Macromol. 2020, 166, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Almeida, K.B.; Araujo, J.L.; Cavalcanti, J.F.; Romanos, M.T.V.; Mourão, S.C.; Amaral, A.C.F.; Falcão, D.Q. In vitro release and anti-herpetic activity of Cymbopogon citratus volatile oil-loaded nanogel. Rev. Bras. Farm. 2018, 28, 495–502. [Google Scholar] [CrossRef]
- Rashidipour, M.; Ashrafi, B.; Nikbakht, M.R.; Veiskarami, S.; Taherikalani, M.; Soroush, S. Encapsulation of Satureja khuzistanica jamzad essential oil in chitosan nanoparticles with enhanced antibacterial and anticancer activities. Prep. Biochem. Biotechnol. 2021, 51, 971–978. [Google Scholar] [CrossRef]
- Mohammadi, A.; Hashemi, M.; Hosseini, S.M. Nanoencapsulation of Zataria multiflora essential oil preparation and characterization with enhanced antifungal activity for controlling Botrytis cinerea, the causal agent of gray mould disease. Innov. Food Sci. Emerg. Technol. 2015, 28, 73–80. [Google Scholar] [CrossRef]
- Hasheminejad, N.; Khodaiyan, F. The effect of clove essential oil loaded chitosan nanoparticles on the shelf life and quality of pomegranate arils. Food Chem. 2020, 309, 125520. [Google Scholar] [CrossRef] [PubMed]
- Hasheminejad, N.; Khodaiyan, F.; Safari, M. Improving the antifungal activity of clove essential oil encapsulated by chitosan nanoparticles. Food Chem. 2019, 275, 113–122. [Google Scholar] [CrossRef]
- da Silva, N.P.; Pereira, E.D.C.R.L.; Duarte, L.M.; Freitas, J.C.D.O.; de Almeida, C.G.; da Silva, T.P.; Melo, R.C.; Apolônio, A.C.M.; de Oliveira, M.A.L.; Brandão, H.D.M.; et al. Improved anti-Cutibacterium acnes activity of tea tree oil-loaded chitosan-poly(ε-caprolactone) core-shell nanocapsules. Colloids Surf. B Biointerfaces 2020, 196, 111371. [Google Scholar] [CrossRef]
- Rozman, N.A.S.; Tong, W.Y.; Leong, C.R.; Anuar, M.R.; Karim, S.; Ong, S.K.; Yusof, F.A.M.; Tan, W.-N.; Sulaiman, B.; Ooi, M.L.; et al. Homalomena pineodora essential oil nanoparticle inhibits diabetic wound pathogens. Sci. Rep. 2020, 10, 3307–3311. [Google Scholar] [CrossRef]
- Esmaeili, A.; Asgari, A. In vitro release and biological activities of Carum copticum essential oil (CEO) loaded chitosan nanoparticles. Int. J. Biol. Macromol. 2015, 81, 283–290. [Google Scholar] [CrossRef]
- Granata, G.; Stracquadanio, S.; Leonardi, M.; Napoli, E.; Malandrino, G.; Cafiso, V.; Stefani, S.; Geraci, C. Oregano and thyme essential oils encapsulated in chitosan nanoparticles as effective antimicrobial agents against foodborne pathogens. Molecules 2021, 26, 4055. [Google Scholar] [CrossRef]
- Song, X.; Wang, L.; Liu, T.; Liu, Y.; Wu, X.; Liu, L. Mandarin (Citrus reticulata L.) essential oil incorporated into chitosan nanoparticles: Characterization, anti-biofilm properties and application in pork preservation. Int. J. Biol. Macromol. 2021, 185, 620–628. [Google Scholar] [CrossRef]
- Zhang, Y.; Niu, Y.; Luo, Y.; Ge, M.; Yang, T.; Yu, L.; Wang, Q. Fabrication, characterization and antimicrobial activities of thymol-loaded zein nanoparticles stabilized by sodium caseinate–chitosan hydrochloride double layers. Food Chem. 2014, 142, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Li, K.-K.; Yin, S.-W.; Yang, X.-Q.; Tang, C.-H.; Wei, Z.-H. Fabrication and Characterization of novel antimicrobial films derived from thymol-loaded zein–sodium caseinate (SC) nanoparticles. J. Agric. Food Chem. 2012, 60, 11592–11600. [Google Scholar] [CrossRef]
- Vrouvaki, I.; Koutra, E.; Kornaros, M.; Avgoustakis, K.; Lamari, F.N.; Hatziantoniou, S. Polymeric nanoparticles of Pistacia lentiscus var. chia essential oil for cutaneous applications. Pharmaceutics 2020, 12, 353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iannitelli, A.; Grande, R.; Di Stefano, A.; Di Giulio, M.; Sozio, P.; Bessa, L.J.; Laserra, S.; Paolini, C.; Protasi, F.; Cellini, L. Potential antibacterial activity of carvacrol-loaded poly(DL-lactide-co-glycolide) (PLGA) nanoparticles against microbial biofilm. Int. J. Mol. Sci. 2011, 12, 5039–5051. [Google Scholar] [CrossRef]
- Qiu, C.; Chang, R.; Yang, J.; Ge, S.; Xiong, L.; Zhao, M.; Li, M.; Sun, Q. Preparation and characterization of essential oil-loaded starch nanoparticles formed by short glucan chains. Food Chem. 2017, 221, 1426–1433. [Google Scholar] [CrossRef]
- Andriotis, E.G.; Papi, R.M.; Paraskevopoulou, A.; Achilias, D.S. Synthesis of D-limonene loaded polymeric nanoparticles with enhanced antimicrobial properties for potential application in food packaging. Nanomaterials 2021, 11, 191. [Google Scholar] [CrossRef]
- Wang, Y.; Xue, Y.; Bi, Q.; Qin, D.; Du, Q.; Jin, P. Enhanced antibacterial activity of eugenol-entrapped casein nanoparticles amended with lysozyme against gram-positive pathogens. Food Chem. 2021, 360, 130036. [Google Scholar] [CrossRef]
- Liakos, I.L.; Abdellatif, M.H.; Innocenti, C.; Scarpellini, A.; Carzino, R.; Brunetti, V.; Marras, S.; Brescia, R.; Drago, F.; Pompa, P.P. Antimicrobial lemongrass essential oil—Copper ferrite cellulose acetate nanocapsules. Molecules 2016, 21, 520. [Google Scholar] [CrossRef] [Green Version]
- Liakos, I.L.; D’Autilia, F.; Garzoni, A.; Bonferoni, C.; Scarpellini, A.; Brunetti, V.; Carzino, R.; Bianchini, P.; Pompa, P.P.; Athanassiou, A. All natural cellulose acetate—Lemongrass essential oil antimicrobial nanocapsules. Int. J. Pharm. 2016, 510, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Liakos, I.L.; Iordache, F.; Carzino, R.; Scarpellini, A.; Oneto, M.; Bianchini, P.; Grumezescu, A.M.; Holban, A.M. Cellulose acetate-essential oil nanocapsules with antimicrobial activity for biomedical applications. Colloids Surf. B Biointerfaces 2018, 172, 471–479. [Google Scholar] [CrossRef]
- Upadhyay, N.; Singh, V.K.; Dwivedy, A.K.; Chaudhari, A.K.; Dubey, N.K. Assessment of nanoencapsulated Cananga odorata essential oil in chitosan nanopolymer as a green approach to boost the antifungal, antioxidant and in situ efficacy. Int. J. Biol. Macromol. 2021, 171, 480–490. [Google Scholar] [CrossRef]
- Flores, F.C.; De Lima, J.A.; Ribeiro, R.F.; Alves, S.H.; Rolim, C.M.B.; Beck, R.C.R.; Da Silva, C.B. Antifungal activity of nanocapsule suspensions containing tea tree oil on the growth of trichophyton rubrum. Mycopathologia 2013, 175, 281–286. [Google Scholar] [CrossRef]
- Antonioli, G.; Fontanella, G.; Echeverrigaray, S.; Delamare, A.P.L.; Pauletti, G.F.; Barcellos, T. Poly(lactic acid) nanocapsules containing lemongrass essential oil for postharvest decay control: In vitro and in vivo evaluation against phytopathogenic fungi. Food Chem. 2020, 326, 126997. [Google Scholar] [CrossRef]
- Karam, T.K.; Ortega, S.; Nakamura, T.U.; Auzély-Velty, R.; Nakamura, C.V. Development of chitosan nanocapsules containing essential oil of Matricaria chamomilla L. for the treatment of cutaneous leishmaniasis. Int. J. Biol. Macromol. 2020, 162, 199–208. [Google Scholar] [CrossRef]
- Jang, S.-H.; Jang, S.-R.; Lee, G.-M.; Ryu, J.-H.; Park, S.-I.; Park, N.-H. Halloysite nanocapsules containing thyme essential oil: Preparation, characterization, and application in packaging materials. J. Food Sci. 2017, 82, 2113–2120. [Google Scholar] [CrossRef] [PubMed]
- López-Meneses, A.; Plascencia-Jatomea, M.; Lizardi-Mendoza, J.; Fernández-Quiroz, D.; Rodríguez-Félix, F.; Mouriño-Pérez, R.R.; Cortez-Rocha, M. Schinus molle L. essential oil-loaded chitosan nanoparticles: Preparation, characterization, antifungal and anti-aflatoxigenic properties. LWT 2018, 96, 597–603. [Google Scholar] [CrossRef]
- Yilmaz, M.T.; Yilmaz, A.; Akman, P.K.; Bozkurt, F.; Dertli, E.; Basahel, A.; Al-Sasi, B.; Taylan, O.; Sagdic, O. Electrospraying method for fabrication of essential oil loaded-chitosan nanoparticle delivery systems characterized by molecular, thermal, morphological and antifungal properties. Innov. Food Sci. Emerg. Technol. 2019, 52, 166–178. [Google Scholar] [CrossRef]
- Ways, T.M.M.; Ng, K.W.; Lau, W.M.; Khutoryanskiy, V.V. Silica nanoparticles in transmucosal drug delivery. Pharmaceutics 2020, 12, 751. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Rico, M.; Pérez-Esteve, É.; Bernardos, A.; Sancenón, F.; Martínez-Máñez, R.; Marcos, M.D.; Barat, J.M. Enhanced antimicrobial activity of essential oil components immobilized on silica particles. Food Chem. 2017, 233, 228–236. [Google Scholar] [CrossRef]
- Das, S.; Horváth, B.; Šafranko, S.; Jokić, S.; Széchenyi, A.; Kőszegi, T. Antimicrobial activity of chamomile essential oil: Effect of different formulations. Molecules 2019, 24, 4321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sattary, M.; Amini, J.; Hallaj, R. Antifungal activity of the lemongrass and clove oil encapsulated in mesoporous silica nanoparticles against wheat’s take-all disease. Pestic. Biochem. Physiol. 2020, 170, 104696. [Google Scholar] [CrossRef]
- Jin, L.; Teng, J.; Hu, L.; Lan, X.; Xu, Y.; Sheng, J.; Song, Y.; Wang, M. Pepper fragrant essential oil (PFEO) and functionalized MCM-41 nanoparticles: Formation, characterization, and bactericidal activity. J. Sci. Food Agric. 2019, 99, 5168–5175. [Google Scholar] [CrossRef]
- Ellahi, H.; Sadrabad, E.K.; Hekmatimoghaddam, S.; Jebali, A.; Sarmast, E.; Mohajeri, F.A. Application of essential oil of Pistacia atlantica Gum, polypropylene and silica nanoparticles as a new milk packaging. Food Sci. Nutr. 2020, 8, 4037–4043. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Liu, Y.; Qin, Y.; Chen, H.; Zhou, L. Release of clove essential oil loaded by mesoporous nano-silica in polylactic acid-based food packaging on postharvest preservation of white button mushroom. Int. J. Food Sci. Technol. 2021, 57, 457–465. [Google Scholar] [CrossRef]
- Karimi, N.; Jabbari, V.; Nazemi, A.; Ganbarov, K.; Karimi, N.; Tanomand, A.; Karimi, S.; Abbasi, A.; Yousefi, B.; Khodadadi, E.; et al. Thymol, cardamom and Lactobacillus plantarum nanoparticles as a functional candy with high protection against Streptococcus mutans and tooth decay. Microb. Pathog. 2020, 148, 104481. [Google Scholar] [CrossRef]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef] [PubMed]
- Jalill, R.D.A. Green synthesis of titanium dioxide nanoparticles with volatile oil of Eugenia caryophyllata for enhanced antimicrobial activities. IET Nanobiotechnol. 2018, 12, 678–687. [Google Scholar] [CrossRef]
- Scandorieiro, S.; De Camargo, L.C.; Lancheros, C.A.C.; Yamada-Ogatta, S.F.; Nakamura, C.V.; de Oliveira, A.G.; Andrade, C.G.T.J.; Durán, N.; Nakazato, G.; Kobayashi, R.K.T. Synergistic and Additive effect of oregano essential oil and biological silver nanoparticles against multidrug-resistant bacterial strains. Front. Microbiol. 2016, 7, 760. [Google Scholar] [CrossRef]
- Biasi-Garbin, R.P.; Otaguiri, E.S.; Morey, A.T.; Da Silva, M.F.; Morguette, A.E.B.; Lancheros, C.; Kian, D.; Perugini, M.R.E.; Nakazato, G.; Durán, N.; et al. Effect of Eugenol against Streptococcus agalactiae and Synergistic Interaction with Biologically Produced Silver Nanoparticles. Evid.-Based Complement. Altern. Med. 2015, 2015, 861497. [Google Scholar] [CrossRef] [Green Version]
- Navani, N.K.; Ghosh, I.N.; Patil, S.D.; Sharma, T.K.; Srivastava, S.K.; Pathania, R. Synergistic action of cinnamaldehyde with silver nanoparticles against spore-forming bacteria: A case for judicious use of silver nanoparticles for antibacterial applications. Int. J. Nanomed. 2013, 8, 4721–4731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheikholeslami, S.; Mousavi, S.E.; Ashtiani, H.R.A.; Doust, S.R.H.; Rezayat, S.M. Antibacterial activity of silver nanoparticles and their combination with zataria multiflora essential oil and methanol extract. Jundishapur J. Microbiol. 2016, 9, 36070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Aziz, N.K.A.; Ammar, A.M.; El-Naenaeey, E.-S.Y.M.; El Damaty, H.M.; Elazazy, A.A.; Hefny, A.A.; Shaker, A.; Eldesoukey, I.E. Antimicrobial and antibiofilm potentials of cinnamon oil and silver nanoparticles against Streptococcus agalactiae isolated from bovine mastitis: New avenues for countering resistance. BMC Veter. Res. 2021, 17, 136. [Google Scholar] [CrossRef] [PubMed]
- Cinteza, L.O.; Scomoroscenco, C.; Voicu, S.N.; Nistor, C.L.; Nitu, S.G.; Trica, B.; Jecu, M.-L.; Petcu, C. Chitosan-stabilized Ag nanoparticles with superior biocompatibility and their synergistic antibacterial effect in mixtures with essential oils. Nanomaterials 2018, 8, 826. [Google Scholar] [CrossRef] [Green Version]
| Biological Source of Essential Oils | Part | Antimicrobial Activities | Major Chemical Components | Mechanism of Action | References |
|---|---|---|---|---|---|
| Bunium persicum | Seeds | L. monocytogenes, Listeria grayi andAspergillusflavus | γ-Terpinene, 1-phellandrene, γ-terpene, cuminaldehyde | Cell membrane disruption and cytolytic leakage Swelling and reduction in membrane function | [10,29,30,31] |
| Cananga odorata | Flower | Hepatitis B virus (HBV), Bacillus. subtilis, E. coli, S. typhi, Shigella shiga, Streptococcus-β-haemolyticus and A. flavus | Linalool, β-caryophyllene | Disruption of cell membrane integrity Induces apoptosis via nuclear condensation and fragmentation pathways including disruption of mitochondrial membrane potential | [13,32,33,34] |
| Carum copticum | Seeds | S. aureus, Staphylococcus epidermidis, Bacillus cereus, E. coli, S. typhimurium, Proteus vulgaris | Thymol, γ-Terpinene, ρ-cymene | Depolarization of the cytoplasmic membrane and disruption of cell membrane integrity and decrease intracellular ATP levels | [12,35,36] |
| Cinnamomum zeylanicum | Bark | Borrelia burgdorferi, E. coli., S. aureus, and P. aeruginosa | Carvacrol | Depolarization of the cytoplasmic membrane and disruption of cell membrane integrity | [12,37,38] |
| Citrus bergamia | Peel | Campylobacter jejuni, E. coli, L. monocytogenes, B. cereus, and S. aureus | Linalool, Citral, Linalyl acetate | Disruption of cell membrane integrity Induction of changes in ATP concentration, cell membrane hyperpolarization, and reduction in cytoplasmic pH | [39,40] |
| Citrus reticulata | Peel | S. aureus, E. coli, Penicillium italicum and Penicillium. digitatum | Limonene and γ-Terpinene | Cell membrane disruption and cytolytic leakage | [41] |
| Cymbopogon citratus | Leaves | HSV-1, HSV-2, S. aureus, E. coli and Gaeumannomyces graminis | Citral | Induction of changes in ATP concentration, cell membrane hyperpolarization, and reduction in cytoplasmic pH | [42,43] |
| Eugenia caryophyllata | Flower buds | B. cereus, S. typhimurium and E. coli | Eugenol, β-caryophyllene | Cell membrane disruption and cytolytic leakage Induces apoptosis via nuclear condensation and fragmentation pathways including disruption of mitochondrial membrane potential | [13,31,44] |
| Eucalyptus globulus | Leaves | S. aureus and S. pyogenes | 1,8-cineol α-pinene | Disruption of cell membrane integrity and cytolytic leakage | [45] |
| Foeniculum vulgare | Seeds and Leaves | S. aureus, E. coli, and A. flavus | Anethole | Disruption of cell membrane integrity | [46,47] |
| Homalomena pineodora | Leaves | B. cereus, B. subtilis, S. aureus, MRSA, E. coli, Proteus mirabilis, Yersinia sp., K. pneumoniae, Shigella boydii, S. typhimurium, Acinetobacter anitratus, P. aeruginosa, Candida albicans and Candida utilis | 2-octylcyclopentanone | Cell membrane disruption and cytolytic leakage | [48] |
| Lavandula angustifolia Sevastopolis | Whole plant | MRSA, S. aureus and E. coli | Linalool, Borneol, Camphor | Disruption of cell membrane integrity and cytolytic leakage | [11,49] |
| Lippia sidoides | Leaves | Stegomyia aegypti larvae | Thymol | Depolarization of the cytoplasmic membrane and disruption of cell membrane integrity | [12,50] |
| Matricaria chamomilla | Fresh or dried flower heads | Leishmania amazonensis,E. coli, P. aeruginosa, B. subtilis, S. aureus, S. pyogenes, Schizosaccharomyces pombe, C. albicans and Candida tropicalis | α-Bisabolol | Cell membrane disruption and cytolytic leakage | [51] |
| Melaleuca alternifolia | Leaves | S. aureus, E. coli, L. monocytogenes, C. albicans, P. aeruginosa and A. niger | Terpinen-4-ol | Cell membrane disruption and cytolytic leakage | [52,53] |
| Mentha piperita | Leaves | C. albicans, C. tropicalis, Pichia anomala andSaccharomycescerevisiae | Menthol, Menthone | Depolarization of the cytoplasmic membrane and disruption of cell membrane integrity | [54] |
| Nigella sativa | Seeds | S. aureus and Vibrio harveyii | Thymoquinone | Apoptosis by production of reactive oxygen species | [55,56] |
| Ocimum basilicum | Whole plant | C. albicans, S. aureus | Linalool | Disruption of cell membrane integrity and cytolytic leakage | [34,40] |
| Origanum vulgare | Leaves | Trichophyton tonsurans, Trichophyton violaceum, Trichophyton floccosum, T mentagrophytes | Carvacrol, Thymol | Depolarization of the cytoplasmic membrane and disruption of cell membrane integrity | [12,57] |
| Pistacia atlantica | Gum | S. aureus, S. enterica, E. coli and L. monocytogenes | α-Thujene, α-Pinene, Camphorene, Sabinene, β-Pinene, ∆3-Carene, Limonene | Disruption of cell membrane integrity and cytolytic leakage | [58,59] |
| Pistacia lentiscus | Resin | E. coli and B. subtilis | α-Pinene, β-Pinene, β-myrcene, Linalool, trans-Caryophyllene and Camphene | Disruption of cell membrane integrity and cytolytic leakage | [59,60] |
| Psidium guajava | Leaves | S. aureus, Salmonella spp. and E. coli | β- caryophyllene | Induction of apoptosis via nuclear condensation and fragmentation pathways including disruption of mitochondrial membrane | [13,61] |
| Punica granatum | Seeds | S. epidermidis | Punicalagin, punicalin | Cell membrane disruption and cytolytic leakage | [62,63,64] |
| Rosmarinus officinalis | Leaves | C. albicans, C. tropicalis | 1,8-Cineole, camphor | Disruption of cell membrane integrity and cytolytic leakage | [65,66] |
| Satureja hortensis | Leaves | S. aureus, Corynebacterium glutamicum, P. aeruginosa and E. coli, and C. albicans | Carvacrol, Thymol | Depolarization of the cytoplasmic membrane and disruption of cell membrane integrity | [12,67] |
| Syzygium aromaticum | Floral bud | E. coli, S. aureus, S. typhi, P. aeruginosa, B. cereus, L. monocytogenes | Eugenol, eugenyl acetate | Cell membrane disruption and cytolytic leakage | [31,68] |
| Thymus vulgaris | Leaves | M. furfur, C. albican, C. tropicalis, Candida glabrata, Candida kefyr and Candida guillermondii, S. aureus, S. pyogenes and E. coli | Thymol, p-cymene, Carvacrol | Depolarization of the cytoplasmic membrane and disruption of cell membrane integrity | [12,69,70] |
| Zataria multiflora | Aerial parts | S. aureus, MRSA, S. epidermidis and P. aeruginosa | Carvacrol, Thymol, p-cymene | Depolarization of the cytoplasmic membrane and disruption of cell membrane integrity | [12,71] |
| Antibiotics | Essential Oils/Essential Oil Constituents | * FICI | Organisms | Interaction | Reference |
|---|---|---|---|---|---|
| Amoxicillin, Ciprofloxacin | Ajowan oil Thymol | 0.36–1 | P. aeruginosa, S. aureus and S. pneumoniae | Synergism—EO/thymol with amoxicillin against MRSA; EO with ciprofloxacin against P. aeruginosa, S. aureus and S. pneumoniae; Thymol with ciprofloxacin against P. aeruginosa and S. pneumoniae | [73] |
| Cefepime | Rosemary oil | - | P. aeruginosa | Synergism | [74] |
| Ciprofloxacin Fluconazole | Thymus atlanticus | 0.25–0.50 | Bacillus subtilis, Micrococcus luteus, Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, K. pneumoniae and Candida parapsilosis, Candida albicans, Candida glabrata, Candida krusei | Synergism | [75] |
| Ciprofloxacin Fluconazole | Linaria ventricosa | 0.26 to 0.50 | E. coli, C. albicans and C. glabrata | Synergism | [76] |
| Doxycycline | Carvacrol, eugenol and cinnamaldehyde | 0.7–1.3 | Acinetobacter baumannii K. pneumoniae E. coli P. aeruginosa | Additive or indifferent inhibitory activity Synergistic bactericidal activity | [77] |
| Fluconazole Amphotericin B | T. satureioides T. pallidus A. leucotrichus T. leptobotrys O. compactum A. herba alba | 0.25–0.31 | C. albicans C. glabrata C. krusei C. parapsilosis | Synergism | [78] |
| Fluconazole Amphotericin B | Citrus aurantium | 0.36 and 0.24 | Candida albicans | Synergism | [79] |
| Fluconazole, Ciprofloxacin Vancomycin | Laurus nobilis Prunus armeniaca | 0.258–0.75 | M. luteus,S. aureus, B. subtilis, E. coli, P. aeruginosa, K. pneumoniae andC. parapsilosis,Candida albicans, Candida glabrata, Candida krusei | Synergism | [80] |
| Fluconazole, Econazole, Ketoconazole Itraconazole | Melaleuca leucadendra | 0.35–0.46 | C. albicans | Synergism | [81] |
| Octenidine dihydrochloride | Lavender | 0.11–0.26 | MRSA | Synergism | [82] |
| Oxacillin, Amoxicillin, Gentamicin, Ciprofloxacin, Tetracycline, Erythromycin, Clindamycin | coriander oil | 0.25–1 | MRSA S. epidermidis P. aeruginosa E. coli | Synergism—coriander oil with amoxicillin, gentamicin, oxacillin and tetracycline against MRSA; coriander oil with gentamicin against P. aeruginosa; coriander oil with erythromycin and tetracycline against E. coli Additive—coriander oil with amoxicillin and clindamycin against MRSA; coriander oil with gentamicin and ciprofloxacin against E. coli | [83] |
| Polymyxin B | Cinnamomum cassia | 0.006 | carbapenemase-producing Klebsiella pneumoniae and Serratia marcescens | Synergism | [84] |
| Sarafloxacin, Levofloxacin, Polymycin, Lincomycin, Amoxicillin, Ceftiofur, Ceftriaxone, Maquindox, Florfenicol, Doxycycline, Kanamycin | Oregano | 0.375–1.5 | E. coli | Synergism—oregano oil with Sarafloxacin, Levofloxacin, Maquindox, Florfenicol, Doxycycline Additive—oregano oil with Polymycin, Lincomycin, Amoxicillin, Ceftiofur, Ceftriaxone Independent—oregano oil with Kanamycin | [85] |
| Streptomycin Ampicillin Chloramphenicol | Cinnamomum cassia | 0.38–0.125 | E. coli, S. aureus, and P. aeruginosa | Synergism—EO with chloramphenicol against E. coli and S. aureus Additive—EO with Streptomycin and Ampicillin against E. coli, S. aureus and P. aeruginosa | [86] |
| β-lactam antibiotics (methicillin, penicillin G) | 1,8-cineole, eugenol, carvacrol, linalool, linalyl acetate, trans-anethole, thymol, menthone, menthol, β-caryophyllene | 0.2–5.0 | MSRA | Synergism—linalyl acetate with methicillin and 1,8-cineole with penicillin G Additive—linalyl acetate with penicillin G Antagonism—methicillin with thymol and methicillin with menthone | [87] |
| Sr. No. | Study Title | Condition | Interventions | Study Design | Phase | Location | Status | Outcome Measurement | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Effect of a Medicated Topical Therapy, Petrolatum, and No Treatment on Nocturnal Cough | Respiratory tract Diseases | Other: Ointment containing camphor, eucalyptus oil, and menthol One time use Other: Petroleum jelly One time use | Study Type: | Interventional (Clinical Trial) | - | United States, Pennsylvania | Complete | Subjective assessment of cough and congestion symptoms (Time Frame: 24 h) | [88] |
| Actual Enrollment: | 143 participants | |||||||||
| Allocation: | Randomized | |||||||||
| Intervention Model: | Parallel Assignment | |||||||||
| Masking: | Double (Participant, Investigator) | |||||||||
| Primary Purpose: | Treatment | |||||||||
| 2. | Treatment of Acute Rhino-Sinusitis with Essential Oils of Aromatic Plants | Rhino-Sinusitis | Dietary Supplement: mixture of aromatic essential oils. 1% of mixture containing aromatic essential oils of Eucalyptus citriodora, Eucalyptus globulus, Mentha piperita, Origanum syriacum, and Rosmarinus Officinalis, spraying to the nose. Dietary Supplement: placebo 0.1% of Lemon VIP (Florasynth, Israel), spraying to the nose. | Study Type: | Interventional (Clinical Trial) | I and II | Israel | Complete | To demonstrate a relief in the nasal obstruction within the first 20 min after first administration of treatment with the spray. (Time Frame: 20 min) To demonstrate a reduction in a defined symptoms sum score based on symptoms and signs comparing baseline therapy from the beginning to the end of 3 days treatment. (Time Frame: 3 days) | [89] |
| Actual Enrollment: | 14 participants | |||||||||
| Allocation: | Randomized | |||||||||
| Intervention Model: | Parallel Assignment | |||||||||
| Masking: | Double (Participant, Investigator) | |||||||||
| Primary Purpose: | Treatment | |||||||||
| 3. | Treatment of Acute Tracheitis and Laryngitis With Essential Oils of Aromatic Plants | Viral Laryngitis Viral Tracheitis | Dietary Supplement: mixture of aromatic essential oils. 3% of mixture containing aromatic essential oils of Eucalyptus citriodora, Eucalyptus globulus, Mentha piperita, Origanum syriacum, and Rosmarinus Officinalis, spraying to the larynx. Dietary Supplement: placebo 0.1% of Lemon VIP (Florasynth, Israel), spraying to the larynx | Study Type: | Interventional (Clinical Trial) | I and II | Israel | complete | To demonstrate a cough or hoarseness relief within the first 20 min after first administration of treatment with the spray. (Time Frame: 20 min) To demonstrate a reduction in a defined symptoms sum score based on symptoms and signs comparing baseline therapy from the beginning to the end of 3 days treatment. (Time Frame: 3 days) | [90] |
| Actual Enrollment: | 29 participants | |||||||||
| Allocation: | Randomized | |||||||||
| Intervention Model: | Parallel Assignment | |||||||||
| Masking: | Double (Participant, Investigator) | |||||||||
| Primary Purpose: | Treatment | |||||||||
| 4. | Anosmia Rehabilitation in Patients Post Coronavirus Disease (COVID 19) | Olfactory Disorder | Other: Olfactory retraining Olfactory retraining Olfactory training is performed by exposing patients twice daily to essential oils with four specific odors, present in glass jars with soaked cotton pads: phenyl ethyl alcohol, rose; eucalyptol, eucalyptus; citronellal, lemon; eugenol, cloves. Drug: corticosteroid nasal irrigation Other: smell household Items Other: Nasal Irrigation | Study Type: | Interventional (Clinical Trial) | IV | Canada, Ontario | With-drawn | Change from Baseline Snap and Sniff Threshold Test and Smell Identification Test (SIT) at 3 months (Time Frame: 3 and 6 months) Score from the Snap and Sniff Olfactory Test results and Smell Identification test results. | [91] |
| Actual Enrollment: | 0 participants | |||||||||
| Allocation: | Randomized | |||||||||
| Intervention Model: | Parallel Assignment | |||||||||
| Masking: | None (Open Label) | |||||||||
| Primary Purpose: | Treatment | |||||||||
| 5. | A Randomized Study to Evaluate the Efficacy of Herbal Ingredients Combined With a Carrier System (Phytonail) Compared With Amorolfine 5% Nail Lacquer (Loceryl) in the Treatment of Toenail Onychomycosis | Onychomycosis | Drug: Phytonail Other Name: herbal ingredients combined with a carrier system (Phytonail) Drug: Loceryl Other Name: amorolfine 5% nail lacquer (Loceryl) | Study Type: | Interventional (Clinical Trial) | - | Taiwan | Unknown | Mycological cure (Time Frame: At week 16) | [92] |
| Estimated Enrollment: | 72 participants | |||||||||
| Allocation: | Randomized | |||||||||
| Intervention Model: | Parallel Assignment | |||||||||
| Masking: | None (Open Label) | |||||||||
| Primary Purpose: | Treatment | |||||||||
| 6. | Omega-3, Nigella Sativa, Indian Costus, Quinine, Anise Seed, Deglycyrrhizinated Licorice, Artemisinin, Febrifugine on Immunity of Patients With (COVID-19) | Covid19 Immunodeficiency | Drug: Omega 3/Nigella Sativa Oil Drug: Omega 3/Nigella Sativa Oil/Indian Costus Drug: Omega 3/Nigella Sativa Oil/Quinine pills Drug: Omega 3/Nigella Sativa Oil/Anise seed capsule Drug: Omega 3/Nigella Sativa Oil/Deglycyrrhizinated Licorice Drug: Active Comparator | Study Type: | Interventional (Clinical Trial) | II and III | Saudi Arabia | Recruiting | Clinical improvement (Time Frame: 30 Days) Time to Clinical recovery Recovery rate from positive to negative swaps (Time Frame: 14 Days) | [93] |
| Estimated Enrollment: | 200 participants | |||||||||
| Allocation: | Randomized | |||||||||
| Intervention Model: | Sequential Assignment | |||||||||
| Masking: | Double (Participant, Care Provider) | |||||||||
| Primary Purpose: | Treatment | |||||||||
| 7. | Use of Vagitories based on St. John’s Wort, Tea Tree Oil and Shepherd’s Purse in the Treatment of Vaginal Inflammation | Non-Specific Vaginitis | Drug: Shepherd’s Purse extractum oleosum vagitories Drug: Tea tree vagitories Drug: Hyperici extractum oleosum vagitories Drug: Vagitories—Probiotic | Study Type: | Interventional (Clinical Trial) | IV | Bosnia and Herzegovina | Complete | Change in objective symptoms of non-specific vaginitis, assessed by gynecological examination (Time Frame: 1 day after treatment completion) | [94] |
| Actual Enrollment: | 210 participants | |||||||||
| Allocation: | Randomized | |||||||||
| Intervention Model: | Parallel Assignment | |||||||||
| Masking: | None (Open Label) | |||||||||
| Primary Purpose: | Treatment | |||||||||
| 8. | Efficacy of a Plaque Disclosing Toothpaste on Home Oral Hygiene Procedures | Chronic Gingivitis, Plaque Induced | Other: Colgate toothpaste fluoridated Other: Shoplaq toothpaste Active Ingredient -Sodium Monofluorophosphate 1000 PPM Ingredients -Precipitated Calcium Carbonate, Sorbitol, Glycerin, Precipitated Silica, Sodium Carboxy Methyl Cellulose, Sodium Benzoate, DM Water, Colour CI-45410, Holy Basil Oil, Neem Oil, Citrus Oil, Thymol Oi, Clove Oil, Piper Betel Leaf Oil, Tea Tree Oil, Eucalyptus Oil, Peppermint Oil, Spearmint Oil. Dye containing tooth paste for disclosing plaque and efficient brushing for better oral health. | Study Type: | Interventional (Clinical Trial) | - | Malaysia | Unknown | Plaque removal efficacy of a disclosing toothpaste (Time Frame: from Baseline to 1 year) | [95] |
| Estimated Enrollment: | 50 participants | |||||||||
| Allocation: | Randomized | |||||||||
| Intervention Model: | Parallel Assignment | |||||||||
| Intervention Model Description: | interventional preventive trial | |||||||||
| Masking: | Double (Care Provider, Outcomes Assessor) | |||||||||
| Masking Description: | Toothpaste tubes will be masked so the care provider would not know which tube he/she allocating to the participants as well the outcome assessor would be masked from both groups (test and control) so data is assessed unbiased. | |||||||||
| Primary Purpose: | Prevention | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nair, A.; Mallya, R.; Suvarna, V.; Khan, T.A.; Momin, M.; Omri, A. Nanoparticles—Attractive Carriers of Antimicrobial Essential Oils. Antibiotics 2022, 11, 108. https://doi.org/10.3390/antibiotics11010108
Nair A, Mallya R, Suvarna V, Khan TA, Momin M, Omri A. Nanoparticles—Attractive Carriers of Antimicrobial Essential Oils. Antibiotics. 2022; 11(1):108. https://doi.org/10.3390/antibiotics11010108
Chicago/Turabian StyleNair, Arya, Rashmi Mallya, Vasanti Suvarna, Tabassum Asif Khan, Munira Momin, and Abdelwahab Omri. 2022. "Nanoparticles—Attractive Carriers of Antimicrobial Essential Oils" Antibiotics 11, no. 1: 108. https://doi.org/10.3390/antibiotics11010108
APA StyleNair, A., Mallya, R., Suvarna, V., Khan, T. A., Momin, M., & Omri, A. (2022). Nanoparticles—Attractive Carriers of Antimicrobial Essential Oils. Antibiotics, 11(1), 108. https://doi.org/10.3390/antibiotics11010108