Comparison of Pharmaceutical Characteristics between Brand-Name Meropenem and Its Generics
Abstract
1. Introduction
2. Results
2.1. Particle Morphology Characterization under SEM
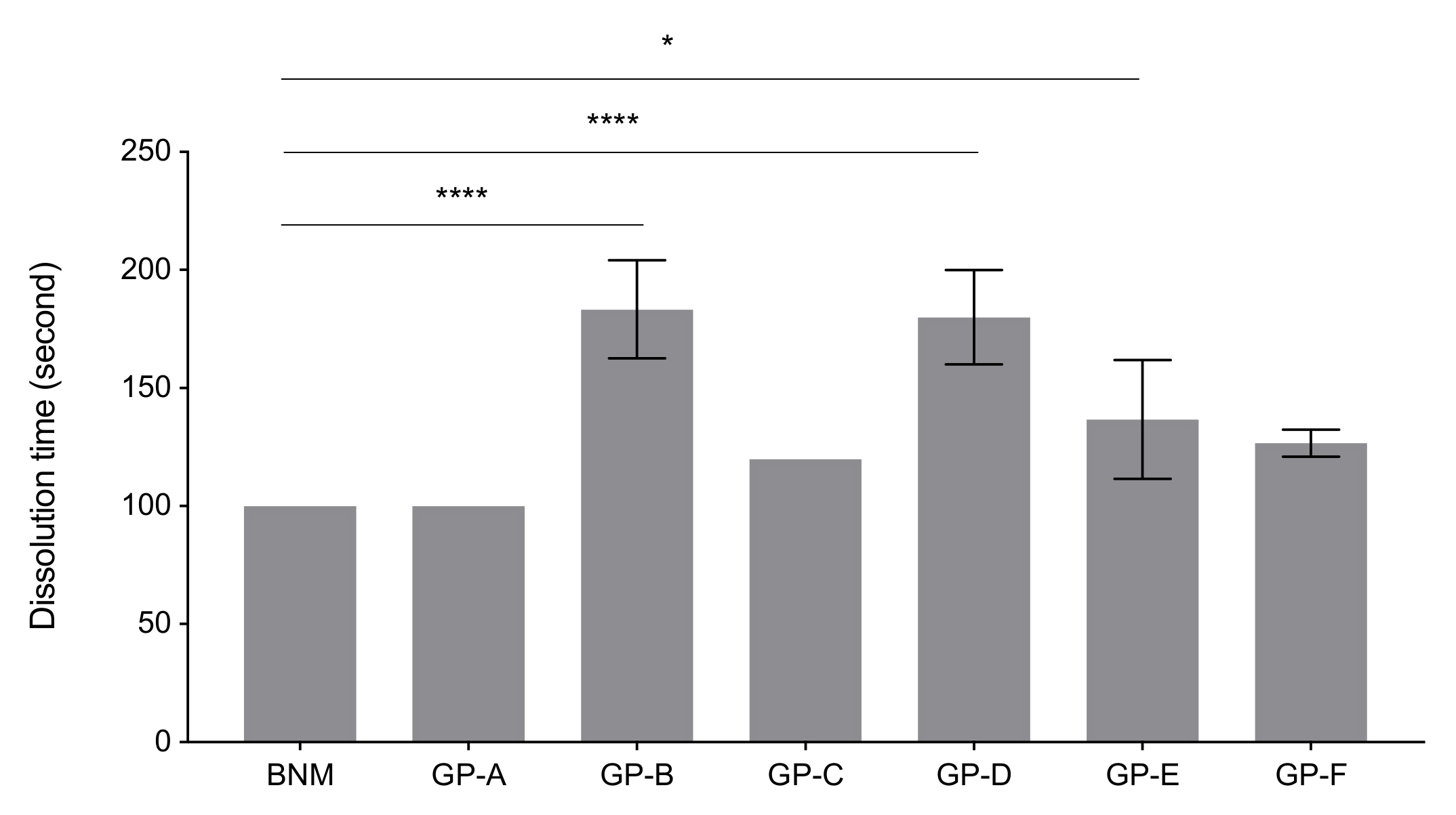
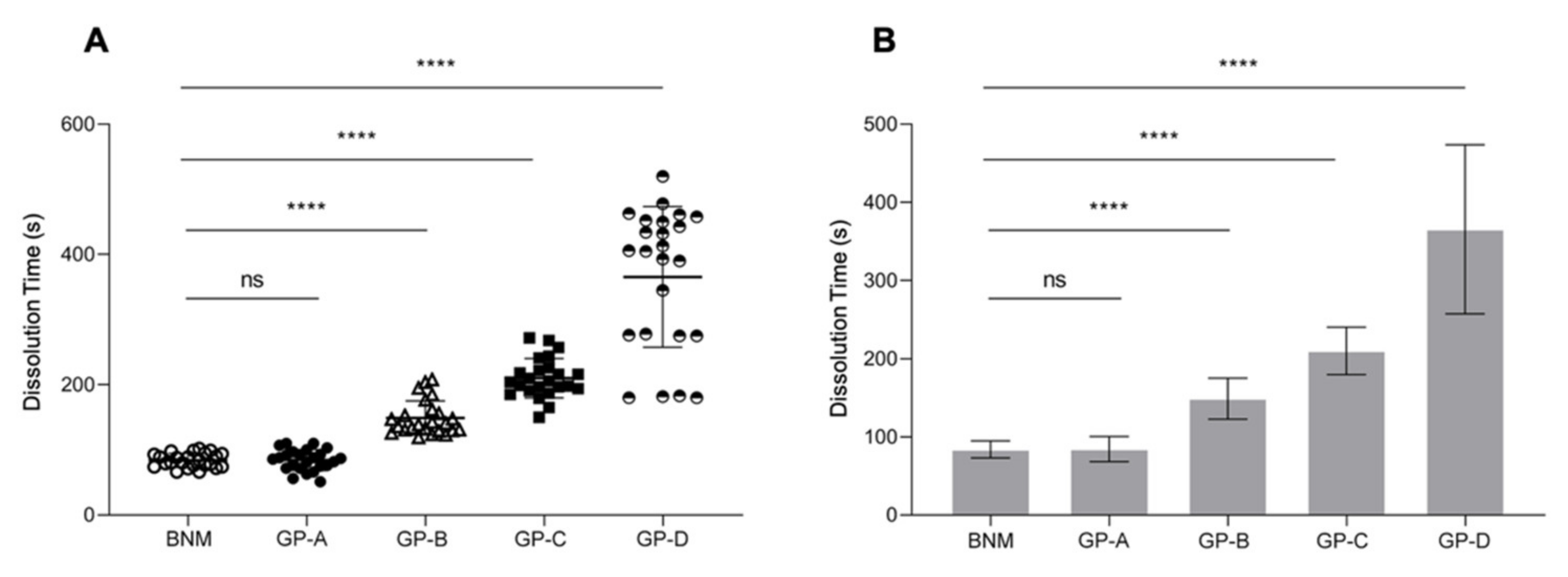
2.2. Dissolution Time Measurements
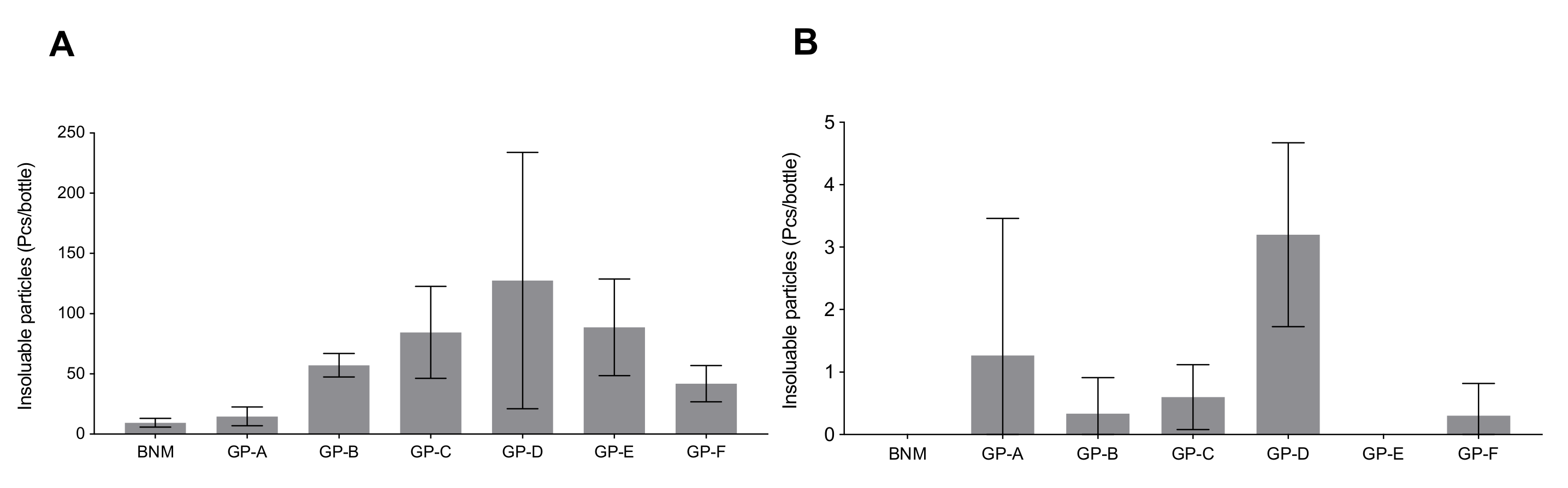
2.3. Insoluble Particles Quantification
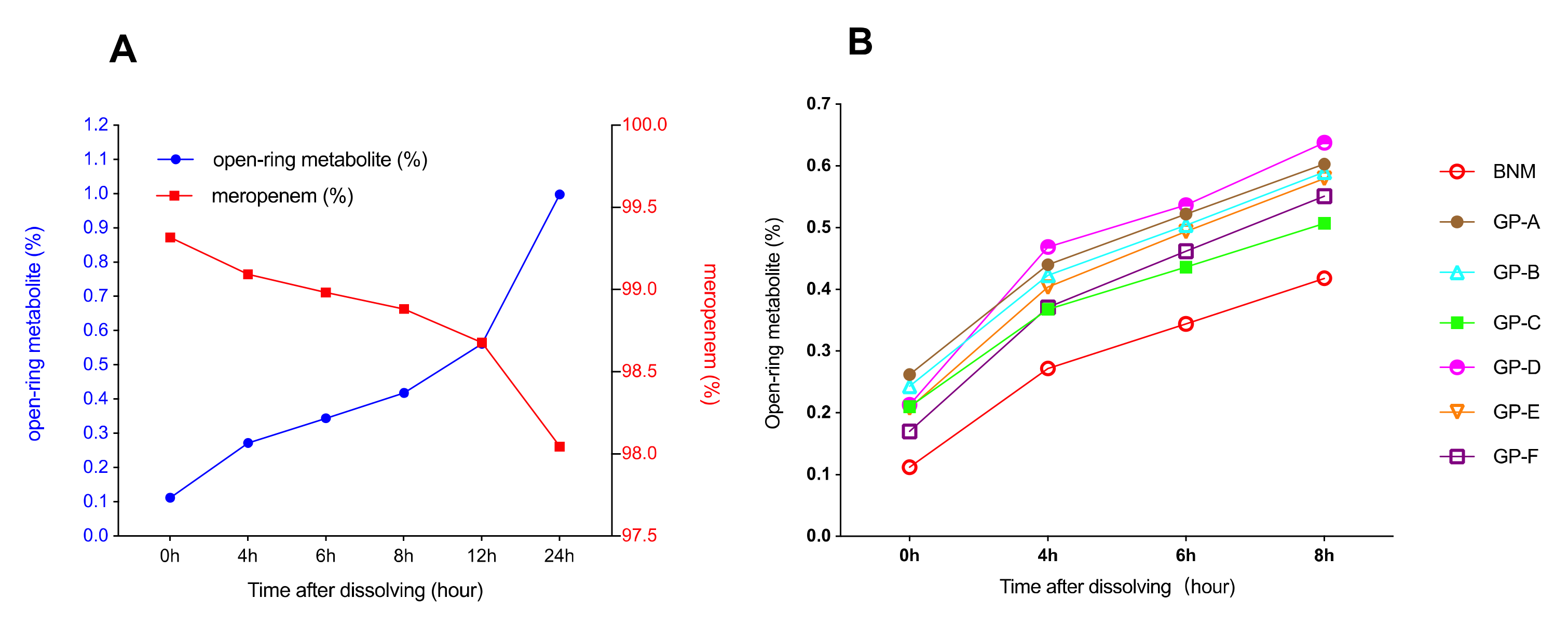
2.4. Solution Stability
2.5. Residual Solvent Detection
2.6. Antimicrobial Susceptibility Test
3. Discussion
4. Materials and Methods
4.1. Study Design and Subjects
4.2. Particle Morphology Characterization under SEM
4.3. Dissolution Time Measurements
4.4. Insoluble Particles Quantification
4.5. Solution Stability
4.6. Residual Solvent Detection
4.7. Antimicrobial Susceptibility Test
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Collier, R. Drug patents: The evergreening problem. Can. Med. Assoc. J. 2013, 185, E385–E386. [Google Scholar] [CrossRef] [PubMed]
- Manzoli, L.; Flacco, M.E.; Boccia, S.; Andrea, D.E.; Panic, N.; Marzuillo, C.; Siliquini, R.; Ricciardi, W.; Villari, P.; Ioannidis, J.P.A. Generic versus brand-name drugs used in cardiovascular diseases. Eur. J. Epidemiol. 2016, 31, 351–368. [Google Scholar] [CrossRef]
- Morgan, S.G.; Leopold, C.; Wagner, A.K. Drivers of expenditure on primary care prescription drugs in 10 high-income countries with universal health coverage. Can. Med. Assoc. J. 2017, 189, E794–E799. [Google Scholar] [CrossRef]
- Segal, J.B.; Onasanya, O.; Daubresse, M.; Lee, C.-Y.; Moechtar, M.; Pu, X.; Dutcher, S.K.; Romanelli, R.J. Determinants of Generic Drug Substitution in the United States. Ther. Innov. Regul. Sci. 2019, 54, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.L.; El-Sayed, M.H.; Kao, J.-H.; Lazarus, J.V.; Lemoine, M.; Lok, A.S.; Zoulim, F. Progress towards elimination goals for viral hepatitis. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 533–542. [Google Scholar] [CrossRef]
- Pathak, H. Capsule Commentary on Romanelli et al., Predictors of Statin Compliance after Switching from Branded to Generic Agents Among Managed-Care Beneficiaries. J. Gen. Intern. Med. 2014, 29, 1391. [Google Scholar] [CrossRef][Green Version]
- Gauzit, R.; Lakdhari, M. Generic antibiotic drugs: Is effectiveness guaranteed? Méd. Mal. Infect. 2012, 42, 141–148. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, W.; Ren, J. Use of carbapenems in urban hospitals in China. Shanghai Yiyao 2009, 30, 370–372. [Google Scholar]
- Wouters, O.J.; Kanavos, P.G.; McKee, M. Comparing Generic Drug Markets in Europe and the United States: Prices, Volumes, and Spending. Milbank Q. 2017, 95, 554–601. [Google Scholar] [CrossRef]
- Spelsberg, A.; Prugger, C.; Doshi, P.; Ostrowski, K.; Witte, T.; Hüsgen, D.; Keil, U. Contribution of industry funded post-marketing studies to drug safety: Survey of notifications submitted to regulatory agencies. BMJ 2017, 356, j337. [Google Scholar] [CrossRef] [PubMed]
- Delattre, I.K.; Briquet, C.; Wallemacq, P.; Tulkens, P.M.; Van Bambeke, F. Comparative in vitro antimicrobial potency, stability, colouration and dissolution time of generics versus innovator of meropenem in Europe. Int. J. Antimicrob. Agents 2019, 55, 105825. [Google Scholar] [CrossRef]
- Cojutti, P.; Sartor, A.; Righi, E.; Scarparo, C.; Bassetti, M.; Pea, F. Population Pharmacokinetics of High-Dose Continuous-Infusion Meropenem and Considerations for Use in the Treatment of Infections Due to KPC-Producing Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2017, 61, e00794-17. [Google Scholar] [CrossRef] [PubMed]
- Griffith, D.C.; Sabet, M.; Tarazi, Z.; Lomovskaya, O.; Dudley, M.N. Pharmacokinetics/Pharmacodynamics of Vaborbactam, a Novel Beta-Lactamase Inhibitor, in Combination with Meropenem. Antimicrob. Agents CH 2019, 63, e01659-18. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, S. Meropenem/Vaborbactam: A Review in Complicated Urinary Tract Infections. DRUGS 2018, 78, 1259–1270. [Google Scholar] [CrossRef]
- Pascale, R.; Giannella, M.; Bartoletti, M.; Viale, P.; Pea, F. Use of meropenem in treating carbapenem-resistant Enterobacteriaceae infections. Expert Rev. Anti Infe. 2019, 17, 819–827. [Google Scholar] [CrossRef]
- Patel, T.S.; Pogue, J.M.; Mills, J.P.; Kaye, K.S. Meropenem–vaborbactam: A new weapon in the war against infections due to resistant Gram-negative bacteria. Futur. Microbiol. 2018, 13, 971–983. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, S.C.J.; Rybak, M.J. Meropenem and Vaborbactam: Stepping up the Battle against Carbapenem-resistant Enterobacteriaceae. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2018, 38, 444–461. [Google Scholar] [CrossRef]
- Shields, R.K.; McCreary, E.K.; Marini, R.V.; Kline, E.G.; Jones, E.C.; Hao, B.; Chen, L.; Kreiswirth, B.N.; Doi, Y.; Clancy, C.J.; et al. Early Experience with Meropenem-Vaborbactam for Treatment of Carbapenem-resistant Enterobacteriaceae Infections. Clin. Infect. Dis. 2020, 71, 667–671. [Google Scholar] [CrossRef]
- Fujimura, S.; Watanabe, A. Generic antibiotics in Japan. J. Infect. Chemother. 2012, 18, 421–427. [Google Scholar] [CrossRef]
- Agudelo, M.; Rodriguez, C.A.; Pelaez, C.A.; Vesga, O. Even Apparently Insignificant Chemical Deviations among Bioequivalent Generic Antibiotics Can Lead to Therapeutic Nonequivalence: The Case of Meropenem. Antimicrob. Agents CH 2014, 58, 1005–1018. [Google Scholar] [CrossRef]
- Ordóñez, K.; Feinstein, M.M.; Reyes, S.; Hernández-Gómez, C.; Pallares, C.; Villegas, M.V. Clinical and economic impact of generic versus brand name meropenem use in an intensive care unit in Colombia. Braz. J. Infect. Dis. 2019, 23, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Jana, B.; Dhar, K.; Goel, G.; Bhattacharya, S.; Chandy, M. Relative Potency of Different Generic Brands of Meropenem, Colistin and Fosfomycin: Implications for Antimicrobial Therapy and Antimicrobial Formulary. Indian J. Med. Microbiol. 2019, 37, 95–98. [Google Scholar] [CrossRef]
- Barbosa, F.D.S.; Pezzi, L.C.; Tsao, M.; Macedo, S.M.D.; de Oliveira, T.F.; Schapoval, E.E.; Mendez, A.S. Stability in clinical use and stress testing of meropenem antibiotic by direct infusion ESI-Q-TOF: Quantitative method and identification of degradation products. J. Pharm. Biomed. Anal. 2020, 179, 112973. [Google Scholar] [CrossRef] [PubMed]
- Elragehy, N.A.; Abdel-Moety, E.M.; Hassan, N.Y.; Rezk, M.R. Stability-indicating determination of meropenem in presence of its degradation product. Talanta 2008, 77, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.A.; Tripathi, R.; Mishra, B. Floating Elementary Osmotic Pump Tablet (FEOPT) for Controlled Delivery of Diethylcarbamazine Citrate: A Water-Soluble Drug. AAPS PharmSciTech 2011, 12, 1312–1323. [Google Scholar] [CrossRef]
- Yung, M.M.N.; Fougères, P.; Leung, Y.H.; Liu, F.; Djurišić, A.B.; Giesy, J.P.; Leung, K.M.Y. Physicochemical characteristics and toxicity of surface-modified zinc oxide nanoparticles to freshwater and marine microalgae. Sci. Rep. UK 2017, 7, 15909. [Google Scholar] [CrossRef]
- Gigliobianco, M.R.; Casadidio, C.; Censi, R.; Di Martino, P. Nanocrystals of Poorly Soluble Drugs: Drug Bioavailability and Physicochemical Stability. Pharmaceutics 2018, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- Commission, C.P. Chinese Pharmacopoeia; China Medical Science Press: Beijing, China, 2020. [Google Scholar]
- Guideline ICH. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. Available online: https://database.ich.org/sites/default/files/ICH_Q3C-R8_Guideline_Step4_2021_0422_1.pdf (accessed on 8 May 2021).
- Berube, B.J.; Rangel, S.M.; Hauser, A.R. Pseudomonas aeruginosa: Breaking down barriers. Curr. Genet. 2016, 62, 109–113. [Google Scholar] [CrossRef]
- Ibrahim, D.; Jabbour, J.-F.; Kanj, S.S. Current choices of antibiotic treatment for Pseudomonas aeruginosa infections. Curr. Opin. Infect. Dis. 2020, 33, 464–473. [Google Scholar] [CrossRef]
- Zahra, M.J.; Hamed, H.; Mohammad, R.Y.; Nosratollah, Z.; Akbarzadeh, A.; Morteza, M. Evaluation and study of antimicrobial activity of nanoliposomal meropenem against Pseudomonas aeruginosa isolates. Artif. Cells Nanomed. Biotechnol. 2017, 45, 975–980. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, C.; Wang, N.; Shih, S. In vitro antibacterial activity of rifampicin in combination with imipenem, meropenem and doripenem against multidrug-resistant clinical isolates of Pseudomonas aeruginosa. BMC Infect. Dis. 2016, 16, 444. [Google Scholar] [CrossRef] [PubMed]
- Feld, G.K.; Tiongson, J.; Oshodi, G. Particle formation and risk of embolization during transseptal catheterization: Comparison of standard transseptal needles and a new radiofrequency transseptal needle. J. Interv. Card. Electrophysiol. 2011, 30, 31–36. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ruimy, R.; Angebault, C.; Djossou, F.; Dupont, C.; Epelboin, L.; Jarraud, S.; Lefevre, L.A.; Bes, M.; Lixandru, B.E.; Bertine, M.; et al. Are Host Genetics the Predominant Determinant of Persistent NasalStaphylococcus aureus Carriage in Humans? J. Infect. Dis. 2010, 202, 924–934. [Google Scholar] [CrossRef]
- Bharat, A.; Cunningham, S.A.; Scott Budinger, G.R.; Kreisel, D.; DeWet, C.J.; Gelman, A.E.; Waites, K.; Crabb, D.; Xiao, L.; Bhorade, S.; et al. Disseminated Ureaplasma infection as a cause of fatal hyperammonemia in humans. Sci. Transl. Med. 2015, 7, 284re3. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing. In Proceedings of the 22nd Informational Supplement CLSI M100-S22; CLSI: Wayne, PA, USA, 2012. [Google Scholar]


| MIC (µg/mL) | Concordance Rate (%) of the MIC | |||||||
|---|---|---|---|---|---|---|---|---|
| MIC Range | MIC50 | MIC80 | MIC88 | ×1 | ×2 | ×4 | ×8 | |
| BNM | <0.6–32 | 0.5 | 2 | 4 | n/a | n/a | n/a | n/a |
| GP-A | 0.125–32 | 0.5 | 4 | 4 | 89.5 | 10.5 | 0 | 0 |
| GP-B | 0.125–32 | 0.5 | 2 | 8 | 85 | 14 | 1 | 0 |
| GP-C | 0.125–32 | 0.5 | 2 | 4 | 87.5 | 12 | 0.5 | 0 |
| GP-D | 0.125–32 | 0.5 | 2 | 8 | 88 | 11.5 | 0.5 | 0 |
| GP-E | 0.125–32 | 0.5 | 2 | 4 | 88.5 | 11 | 0.5 | 0 |
| GP-F | 0.125–32 | 0.5 | 2 | 8 | 86.5 | 12 | 1.5 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, P.; Fujimura, S.; Du, Y.; Zhang, B.; Yang, L.; Kawamura, M.; Zhang, Z.; Zhai, S. Comparison of Pharmaceutical Characteristics between Brand-Name Meropenem and Its Generics. Antibiotics 2021, 10, 1096. https://doi.org/10.3390/antibiotics10091096
Yang P, Fujimura S, Du Y, Zhang B, Yang L, Kawamura M, Zhang Z, Zhai S. Comparison of Pharmaceutical Characteristics between Brand-Name Meropenem and Its Generics. Antibiotics. 2021; 10(9):1096. https://doi.org/10.3390/antibiotics10091096
Chicago/Turabian StyleYang, Ping, Shigeru Fujimura, Yawei Du, Bei Zhang, Li Yang, Masato Kawamura, Zhenhua Zhang, and Suodi Zhai. 2021. "Comparison of Pharmaceutical Characteristics between Brand-Name Meropenem and Its Generics" Antibiotics 10, no. 9: 1096. https://doi.org/10.3390/antibiotics10091096
APA StyleYang, P., Fujimura, S., Du, Y., Zhang, B., Yang, L., Kawamura, M., Zhang, Z., & Zhai, S. (2021). Comparison of Pharmaceutical Characteristics between Brand-Name Meropenem and Its Generics. Antibiotics, 10(9), 1096. https://doi.org/10.3390/antibiotics10091096






