Phages for Africa: The Potential Benefit and Challenges of Phage Therapy for the Livestock Sector in Sub-Saharan Africa
Abstract
1. Introduction
2. Antibiotic Resistance in Livestock Farming
3. Alternatives to Antibiotics Used in Livestock Farming
3.1. Attributes of Phage-Based Products That Could Be Compelling for Livestock Farming
3.1.1. Single-Dose Potential
3.1.2. Inexpensive Drugs of Infectious Diseases
3.1.3. Short Product Development Time Frames
3.1.4. Decreased Probability of Resistance Development
4. Current Phage Research in Africa
5. Hurdles of Phage Research and Regulatory Aspects of Phage Development/Products in SSA with a Focus on Kenya
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Enahoro, D.; Mason-D’Croz, D.; Mul, M.; Rich, K.M.; Robinson, T.P.; Thornton, P.; Staal, S.S. Supporting sustainable expansion of livestock production in South Asia and Sub-Saharan Africa: Scenario analysis of investment options. Glob. Food Secur. 2019, 20, 114–121. [Google Scholar] [CrossRef]
- Agriculture Organization of the United Nations; Animal Production, Health Division, Agriculture Organization of the United Nations. Emergency Prevention System for Transboundary Animal, Plant Pests. In Improved Animal Health for Poverty Reduction and Sustainable Livelihoods; Food & Agriculture Organization: Rome, Italy, 2002. [Google Scholar]
- Halliday, J.E.; Allan, K.J.; Ekwem, D.; Cleaveland, S.; Kazwala, R.R.; Crump, J.A. Endemic zoonoses in the tropics: A public health problem hiding in plain sight. Vet. Rec. 2015, 176, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Andrew Selaledi, L.; Mohammed Hassan, Z.; Manyelo, T.G.; Mabelebele, M. The Current Status of the Alternative Use to Antibiotics in Poultry Production: An African Perspective. Antibiotics 2020, 9, 594. [Google Scholar] [CrossRef]
- World Health Organization. Joint FAO/OIE/WHO Expert Workshop on Non-Human Antimicrobial Usage and Antimicrobial Resistance: Scientific Assessment: Geneva, 1–5 December 2003; World Health Organization: Geneva, Switzerland, 2004.
- Iskandar, K.; Molinier, L.; Hallit, S.; Sartelli, M.; Hardcastle, T.C.; Haque, M.; Lugova, H.; Dhingra, S.; Sharma, P.; Islam, S. Surveillance of antimicrobial resistance in low-and middle-income countries: A scattered picture. Antimicrob. Resist. Infect. Control 2021, 10, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Appiah, B. US Pharmacopeia fighting counterfeit medicines in Africa. Can. Med. Assoc. J. 2013, 185, E666. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martín, P.V.; Muñoz, M.A.; Pigolotti, S. Bet-hedging strategies in expanding populations. PLoS Comput. Biol. 2019, 15, e1006529. [Google Scholar]
- Sultan, I.; Rahman, S.; Jan, A.T.; Siddiqui, M.T.; Mondal, A.H.; Haq, Q.M.R. Antibiotics, resistome and resistance mechanisms: A bacterial perspective. Front. Microbiol. 2018, 9, 2066. [Google Scholar] [CrossRef]
- Wangai, F.K.; Masika, M.M.; Lule, G.N.; Karari, E.M.; Maritim, M.C.; Jaoko, W.G.; Museve, B.; Kuria, A. Bridging antimicrobial resistance knowledge gaps: The East African perspective on a global problem. PLoS ONE 2019, 14, e0212131. [Google Scholar] [CrossRef]
- World Health Organization. WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. 2017. Available online: https://www.who.int/ru/news-room/detail/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed. (accessed on 1 April 2021).
- van den Bogaard, A.E.; Stobberingh, E.E. Epidemiology of resistance to antibiotics: Links between animals and humans. Int. J. Antimicrob. Agents 2000, 14, 327–335. [Google Scholar] [CrossRef]
- Iovine, N.M. Resistance mechanisms in Campylobacter jejuni. Virulence 2013, 4, 230–240. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Glennon, E.E.; Chen, D.; Gilbert, M.; Robinson, T.P.; Grenfell, B.T.; Levin, S.A.; Bonhoeffer, S.; Laxminarayan, R. Reducing antimicrobial use in food animals. Science 2017, 357, 1350–1352. [Google Scholar] [CrossRef]
- Monistero, V.; Barberio, A.; Biscarini, F.; Cremonesi, P.; Castiglioni, B.; Graber, H.; Bottini, E.; Ceballos-Marquez, A.; Kroemker, V.; Petzer, I. Different distribution of antimicrobial resistance genes and virulence profiles of Staphylococcus aureus strains isolated from clinical mastitis in six countries. J. Dairy Sci. 2020, 103, 3431–3446. [Google Scholar] [CrossRef] [PubMed]
- Theobald, S.; Etter, E.M.C.; Gerber, D.; Abolnik, C. Antimicrobial resistance trends in Escherichia coli in South African poultry: 2009–2015. Foodborne Pathog. Dis. 2019, 16, 652–660. [Google Scholar] [CrossRef]
- Fri, J.; Njom, H.A.; Ateba, C.N.; Ndip, R.N. Antibiotic resistance and virulence gene characteristics of methicillin-resistant Staphylococcus aureus (MRSA) isolated from healthy Edible Marine Fish. Int. J. Microbiol. 2020, 2020, 9803903. [Google Scholar] [CrossRef] [PubMed]
- Pillay, S.; Amoako, D.G.; Abia, A.L.; Somboro, A.M.; Shobo, C.O.; Perrett, K.; Bester, L.A.; Essack, S.Y. Characterisation of Campylobacter spp. isolated from poultry in KwaZulu-Natal, South Africa. Antibiotics 2020, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Langata, L.M.; Maingi, J.M.; Musonye, H.A.; Kiiru, J.; Nyamache, A.K. Antimicrobial resistance genes in Salmonella and Escherichia coli isolates from chicken droppings in Nairobi, Kenya. BMC Res. Notes 2019, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.O.; Raji, M.A.; Mamman, P.H.; Raufu, I.A.; Aremu, A.; Akorede, G.J.; Kwanashie, C.N. Salmonellosis: Serotypes, prevalence and multi-drug resistant profiles of Salmonella enterica in selected poultry farms, Kwara State, North Central Nigeria. Onderstepoort J. Vet. Res. 2019, 86, 1–8. [Google Scholar] [CrossRef]
- Adinortey, C.A.; Aheto, D.W.; Boateng, A.A.; Agbeko, R. Multiple Antibiotic Resistance-Coliform Bacteria in Some Selected Fish Farms of the Central Region of Ghana. Scientifica 2020, 2020, 6641461. [Google Scholar] [CrossRef]
- Montso, P.K.; Mlambo, V.; Ateba, C.N. The first isolation and molecular characterization of Shiga Toxin-producing virulent multi-drug resistant atypical enteropathogenic Escherichia coli O177 serogroup from South African Cattle. Front. Cell. Infect. Microbiol. 2019, 9, 333. [Google Scholar] [CrossRef]
- Tshitshi, L.; Manganyi, M.C.; Montso, P.K.; Mbewe, M.; Ateba, C.N. Extended Spectrum Beta-Lactamase-Resistant Determinants among Carbapenem-Resistant Enterobacteriaceae from Beef Cattle in the North West Province, South Africa: A Critical Assessment of Their Possible Public Health Implications. Antibiotics 2020, 9, 820. [Google Scholar] [CrossRef]
- Hartnack, S.; Odoch, T.; Kratzer, G.; Furrer, R.; Wasteson, Y.; L’Abée-Lund, T.M.; Skjerve, E. Additive Bayesian networks for antimicrobial resistance and potential risk factors in non-typhoidal Salmonella isolates from layer hens in Uganda. BMC Vet. Res. 2019, 15, 212. [Google Scholar] [CrossRef] [PubMed]
- Messele, Y.E.; Abdi, R.D.; Tegegne, D.T.; Bora, S.K.; Babura, M.D.; Emeru, B.A.; Werid, G.M. Analysis of milk-derived isolates of E. coli indicating drug resistance in central Ethiopia. Trop. Anim. Health Prod. 2019, 51, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Kazibwe, G.; Katami, P.; Alinaitwe, R.; Alafi, S.; Nanteza, A.; Nakavuma, J.L. Bacteriophage activity against and characterisation of avian pathogenic Escherichia coli isolated from colibacillosis cases in Uganda. PLoS ONE 2020, 15, e0239107. [Google Scholar] [CrossRef] [PubMed]
- Igbinosa, I.H. Prevalence and detection of antibiotic-resistant determinant in Salmonella isolated from food-producing animals. Trop. Anim. Health Prod. 2015, 47, 37–43. [Google Scholar] [CrossRef]
- Ngbede, E.O.; Raji, M.A.; Kwanashie, C.N.; Kwaga, J.K.P. Antimicrobial resistance and virulence profile of enterococci isolated from poultry and cattle sources in Nigeria. Trop. Anim. Health Prod. 2017, 49, 451–458. [Google Scholar] [CrossRef]
- Vounba, P.; Rhouma, M.; Arsenault, J.; Alambédji, R.B.; Fravalo, P.; Fairbrother, J.M. Prevalence of colistin resistance and mcr-1/mcr-2 genes in extended-spectrum β-lactamase/AmpC-producing Escherichia coli isolated from chickens in Canada, Senegal and Vietnam. J. Glob. Antimicrob. Resist. 2019, 19, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Igbinosa, I.H.; Igbinosa, E.O.; Okoh, A.I. Detection of antibiotic resistance, virulence gene determinants and biofilm formation in Aeromonas species isolated from cattle. Environ. Sci. Pollut. Res. 2015, 22, 17596–17605. [Google Scholar] [CrossRef]
- Guetiya Wadoum, R.; Zambou, N.; Anyangwe, F.; Njimou, J.; Coman, M.; Verdenelli, M.; Cecchini, C.; Silvi, S.; Orpianesi, C.; Cresci, A. Abusive use of antibiotics in poultry farming in Cameroon and the public health implications. Br. Poult. Sci. 2016, 57, 483–493. [Google Scholar] [CrossRef]
- Iweriebor, B.C.; Obi, L.C.; Okoh, A.I. Macrolide, glycopeptide resistance and virulence genes in Enterococcus species isolates from dairy cattle. J. Med. Microbiol. 2016, 65, 641–648. [Google Scholar] [CrossRef]
- Mainda, G.; Bessell, P.R.; Muma, J.B.; McAteer, S.P.; Chase-Topping, M.E.; Gibbons, J.; Stevens, M.P.; Gally, D.L.; Barend, M. Prevalence and patterns of antimicrobial resistance among Escherichia coli isolated from Zambian dairy cattle across different production systems. Sci. Rep. 2015, 5, 12439. [Google Scholar] [CrossRef]
- Adegoke, A.A.; Okoh, A.I. Species diversity and antibiotic resistance properties of Staphylococcus of farm animal origin in Nkonkobe Municipality, South Africa. Folia Microbiol. 2014, 59, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Chenia, H.Y.; Jacobs, A. Antimicrobial resistance, heavy metal resistance and integron content in bacteria isolated from a South African tilapia aquaculture system. Dis. Aquat. Org. 2017, 126, 199–209. [Google Scholar] [CrossRef]
- Raufu, I.A.; Fashae, K.; Ameh, J.A.; Ambali, A.; Ogunsola, F.T.; Coker, A.O.; Hendriksen, R.S. Persistence of fluoroquinolone-resistant Salmonella enterica serovar Kentucky from poultry and poultry sources in Nigeria. J. Infect. Dev. Ctries. 2014, 8, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Kemal, J.; Sibhat, B.; Menkir, S.; Beyene, D. Prevalence, assessment, and antimicrobial resistance patterns of Salmonella from raw chicken eggs in Haramaya, Ethiopia. J. Infect. Dev. Ctries. 2016, 10, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, T.; Karimuribo, E.; Mdegela, R. Prevalence of bovine subclinical mastitis and antibiotic susceptibility patterns of major mastitis pathogens isolated in Unguja island of Zanzibar, Tanzania. Trop. Anim. Health Prod. 2018, 50, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Katakweba, A.; Møller, K.; Muumba, J.; Muhairwa, A.; Damborg, P.; Rosenkrantz, J.; Minga, U.; Mtambo, M.; Olsen, J. Antimicrobial resistance in faecal samples from buffalo, wildebeest and zebra grazing together with and without cattle in Tanzania. J. Appl. Microbiol. 2015, 118, 966–975. [Google Scholar] [CrossRef]
- Rugumisa, B.T.; Call, D.R.; Mwanyika, G.O.; Mrutu, R.I.; Luanda, C.M.; Lyimo, B.M.; Subbiah, M.; Buza, J.J. Prevalence of antibiotic-resistant fecal Escherichia coli isolates from penned broiler and scavenging local chickens in arusha, Tanzania. J. Food Prot. 2016, 79, 1424–1429. [Google Scholar] [CrossRef]
- Massot, M.; Couffignal, C.; Clermont, O.; D’Humières, C.; Chatel, J.; Plault, N.; Andremont, A.; Caron, A.; Mentré, F.; Denamur, E. Day-to-day dynamics of commensal Escherichia coli in Zimbabwean cows evidence temporal fluctuations within a host-specific population structure. Appl. Environ. Microbiol. 2017, 83, e00659-17. [Google Scholar] [CrossRef]
- Abrahmsén, M.; Persson, Y.; Kanyima, B.M.; Båge, R. Prevalence of subclinical mastitis in dairy farms in urban and peri-urban areas of Kampala, Uganda. Trop. Anim. Health Prod. 2014, 46, 99–105. [Google Scholar] [CrossRef]
- Olowe, O.A.; Adewumi, O.; Odewale, G.; Ojurongbe, O.; Adefioye, O.J. Phenotypic and molecular characterisation of extended-spectrum beta-lactamase producing Escherichia coli obtained from animal fecal samples in Ado Ekiti, Nigeria. J. Environ. Public Health 2015, 2015, 497980. [Google Scholar] [CrossRef]
- Nworie, A.; Onyema, A.S.; Okekpa, S.I.; Elom, M.O.; Umoh, N.O.; Usanga, V.U.; Ibiam, G.A.; Ukwah, B.N.; Nwadi, L.C.; Ezeruigbo, C. A novel methicillin-resistant Staphylococcus aureus t11469 and a poultry endemic strain t002 (ST5) are present in chicken in Ebonyi State, Nigeria. BioMed Res. Int. 2017, 2017, 2936461. [Google Scholar] [CrossRef]
- Saidi, B.; Mafirakureva, P.; Mbanga, J. Antimicrobial resistance of Escherichia coli isolated from chickens with colibacillosis in and around Harare, Zimbabwe. Avian Dis. 2013, 57, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Gitau, G.K.; Bundi, R.M.; Mulei, C.M.; Vanleeuwen, J. Mastitogenic bacteria isolated from dairy cows in Kenya and their antimicrobial sensitivity. J. S. Afr. Vet. Assoc. 2014, 85, 1–8. [Google Scholar] [CrossRef]
- van den Honert, M.S.; Gouws, P.A.; Hoffman, L.C. Escherichia coli Antibiotic Resistance Patterns from Co-Grazing and Non-Co-Grazing Livestock and Wildlife Species from Two Farms in the Western Cape, South Africa. Antibiotics 2021, 10, 618. [Google Scholar] [CrossRef] [PubMed]
- Perreten, V.; Strauss, C.; Collaud, A.; Gerber, D. Colistin resistance gene mcr-1 in avian-pathogenic Escherichia coli in South Africa. Antimicrob. Agents Chemother. 2016, 60, 4414–4415. [Google Scholar] [CrossRef] [PubMed]
- Haftu, R.; Taddele, H.; Gugsa, G.; Kalayou, S. Prevalence, bacterial causes, and antimicrobial susceptibility profile of mastitis isolates from cows in large-scale dairy farms of Northern Ethiopia. Trop. Anim. Health Prod. 2012, 44, 1765–1771. [Google Scholar] [CrossRef]
- Alders, R.; Pym, R. Village poultry: Still important to millions, eight thousand years after domestication. World’s Poult. Sci. J. 2009, 65, 181–190. [Google Scholar] [CrossRef]
- Mapiye, C.; Mwale, M.; Mupangwa, J.; Chimonyo, M.; Foti, R.; Mutenje, M. A research review of village chicken production constraints and opportunities in Zimbabwe. Asian-Australas. J. Anim. Sci. 2008, 21, 1680–1688. [Google Scholar] [CrossRef]
- Vaarst, M.; Steenfeldt, S.; Horsted, K. Sustainable development perspectives of poultry production. World’s Poult. Sci. J. 2015, 71, 609–620. [Google Scholar] [CrossRef]
- Caly, D.L.; D’Inca, R.; Auclair, E.; Drider, D. Alternatives to antibiotics to prevent necrotic enteritis in broiler chickens: A microbiologist’s perspective. Front. Microbiol. 2015, 6, 1336. [Google Scholar] [CrossRef]
- Cardinal, K.M.; Kipper, M.; Andretta, I.; Ribeiro, A.M.L. Withdrawal of antibiotic growth promoters from broiler diets: Performance indexes and economic impact. Poult. Sci. 2019, 98, 6659–6667. [Google Scholar] [CrossRef]
- AlSheikh, H.M.A.; Sultan, I.; Kumar, V.; Rather, I.A.; Al-Sheikh, H.; Tasleem Jan, A.; Haq, Q.M.R. Plant-based phytochemicals as possible alternative to antibiotics in combating bacterial drug resistance. Antibiotics 2020, 9, 480. [Google Scholar] [CrossRef]
- Aiyegoro, O.; Okoh, A. Use of bioactive plant products in combination with standard antibiotics: Implications in antimicrobial chemotherapy. J. Med. Plants Res. 2009, 3, 1147–1152. [Google Scholar]
- Tian, M.; Xu, X.; Liu, F.; Fan, X.; Pan, S. Untargeted metabolomics reveals predominant alterations in primary metabolites of broccoli sprouts in response to pre-harvest selenium treatment. Food Res. Int. 2018, 111, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Castelani, L.; Arcaro, J.; Braga, J.; Bosso, A.; Moura, Q.; Esposito, F.; Sauter, I.; Cortez, M.; Lincopan, N. Activity of nisin, lipid bilayer fragments and cationic nisin-lipid nanoparticles against multidrug-resistant Staphylococcus spp. isolated from bovine mastitis. J. Dairy Sci. 2019, 102, 678–683. [Google Scholar] [CrossRef]
- Dal Pozzo, M.; Santurio, D.; Rossatto, L.; Vargas, A.; Alves, S.; Loreto, E.; Viegas, J. Activity of essential oils from spices against Staphylococcus spp. isolated from bovine mastitis. Arq. Bras. Med. Vet. Zootec. 2011, 63, 1229–1232. [Google Scholar] [CrossRef]
- Budri, P.E.; Silva, N.C.; Bonsaglia, E.C.; Júnior, A.F.; Júnior, J.A.; Doyama, J.T.; Gonçalves, J.L.; Santos, M.; Fitzgerald-Hughes, D.; Rall, V.L. Effect of essential oils of Syzygium aromaticum and Cinnamomum zeylanicum and their major components on biofilm production in Staphylococcus aureus strains isolated from milk of cows with mastitis. J. Dairy Sci. 2015, 98, 5899–5904. [Google Scholar] [CrossRef] [PubMed]
- Cerioli, M.F.; Moliva, M.V.; Cariddi, L.N.; Reinoso, E.B. Effect of the essential oil of minthostachys verticillata (Griseb.) epling and limonene on biofilm production in pathogens causing bovine mastitis. Front. Vet. Sci. 2018, 5, 146. [Google Scholar] [CrossRef]
- Golkar, Z.; Bagasra, O.; Pace, D.G. Bacteriophage therapy: A potential solution for the antibiotic resistance crisis. J. Infect. Dev. Ctries. 2014, 8, 129–136. [Google Scholar] [CrossRef]
- Chibani-Chennoufi, S.; Bruttin, A.; Dillmann, M.-L.; Brüssow, H. Phage-host interaction: An ecological perspective. J. Bacteriol. 2004, 186, 3677–3686. [Google Scholar] [CrossRef]
- de Melo, A.G.; Levesque, S.; Moineau, S. Phages as friends and enemies in food processing. Curr. Opin. Biotechnol. 2018, 49, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Viertel, T.M.; Ritter, K.; Horz, H.-P. Viruses versus bacteria—Novel approaches to phage therapy as a tool against multidrug-resistant pathogens. J. Antimicrob. Chemother. 2014, 69, 2326–2336. [Google Scholar] [CrossRef]
- Clokie, M.R.; Millard, A.D.; Letarov, A.V.; Heaphy, S. Phages in nature. Bacteriophage 2011, 1, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Huff, G.; Huff, W.; Rath, N.; Donoghue, A. Critical evaluation of bacteriophage to prevent and treat colibacillosis in poultry. J. Ark. Acad. Sci. 2009, 63, 93–98. [Google Scholar]
- Żbikowska, K.; Michalczuk, M.; Dolka, B. The use of bacteriophages in the poultry industry. Animals 2020, 10, 872. [Google Scholar] [CrossRef]
- Gopalaiah, H. Bacteriophage as Antimicrobial Agents: A Milestone. J. Indian Acad. Oral Med. Radiol. 2013, 25, 40. [Google Scholar] [CrossRef]
- Fortier, L.-C.; Sekulovic, O. Importance of prophages to evolution and virulence of bacterial pathogens. Virulence 2013, 4, 354–365. [Google Scholar] [CrossRef]
- Monteiro, R.; Pires, D.P.; Costa, A.R.; Azeredo, J. Phage therapy: Going temperate? Trends Microbiol. 2019, 27, 368–378. [Google Scholar] [CrossRef]
- Harper, D.R. Criteria for selecting suitable infectious diseases for phage therapy. Viruses 2018, 10, 177. [Google Scholar] [CrossRef]
- Petrovic Fabijan, A.; Khalid, A.; Maddocks, S.; Ho, J.; Gilbey, T.; Sandaradura, I.; Lin, R.C.; Ben Zakour, N.; Venturini, C.; Bowring, B. Phage therapy for severe bacterial infections: A narrative review. Med. J. Aust. 2020, 212, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Nagel, T.E.; Chan, B.K.; De Vos, D.; El-Shibiny, A.; Kang’ethe, E.K.; Makumi, A.; Pirnay, J.-P. The developing world urgently needs phages to combat pathogenic bacteria. Front. Microbiol. 2016, 7, 882. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, O.; Gabriel-Ajobiewe, R.; Taiwo, M.; Kayode, S. Phage therapy: A potential alternative in the treatment of multi-drug resistant bacterial infections. J. Microbiol. Exp. 2017, 5, 00173. [Google Scholar]
- Islam, M.S.; Zhou, Y.; Liang, L.; Nime, I.; Liu, K.; Yan, T.; Wang, X.; Li, J. Application of a Phage Cocktail for Control of Salmonella in Foods and Reducing Biofilms. Viruses 2019, 11, 841. [Google Scholar] [CrossRef]
- Abdelsattar, A.S.; Abdelrahman, F.; Dawoud, A.; Connerton, I.F.; El-Shibiny, A. Encapsulation of E. coli phage ZCEC5 in chitosan-alginate beads as a delivery system in phage therapy. AMB Express 2019, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Janež, N.; Kokošin, A.; Zaletel, E.; Vranac, T.; Kovač, J.; Vučković, D.; Smole Možina, S.; Curin Šerbec, V.; Zhang, Q.; Accetto, T.; et al. Identification and characterisation of new Campylobacter group III phages of animal origin. FEMS Microbiol. Lett. 2014, 359, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, D.; Rodríguez-Rubio, L.; Fernández, L.; Martínez, B.; Rodríguez, A.; García, P. Applicability of commercial phage-based products against Listeria monocytogenes for improvement of food safety in Spanish dry-cured ham and food contact surfaces. Food Control 2017, 73, 1474–1482. [Google Scholar] [CrossRef]
- Atterbury, R.J.; Van Bergen, M.A.; Ortiz, F.; Lovell, M.A.; Harris, J.A.; De Boer, A.; Wagenaar, J.A.; Allen, V.M.; Barrow, P.A. Bacteriophage therapy to reduce salmonella colonization of broiler chickens. Appl. Environ. Microbiol. 2007, 73, 4543–4549. [Google Scholar] [CrossRef]
- Carvalho, C.M.; Gannon, B.W.; Halfhide, D.E.; Santos, S.B.; Hayes, C.M.; Roe, J.M.; Azeredo, J. The in vivo efficacy of two administration routes of a phage cocktail to reduce numbers of Campylobacter coli and Campylobacter jejuni in chickens. BMC Microbiol. 2010, 10, 232. [Google Scholar] [CrossRef]
- Clavijo, V.; Baquero, D.; Hernandez, S.; Farfan, J.C.; Arias, J.; Arévalo, A.; Donado-Godoy, P.; Vives-Flores, M. Phage cocktail SalmoFREE® reduces Salmonella on a commercial broiler farm. Poult. Sci. 2019, 98, 5054–5063. [Google Scholar] [CrossRef]
- Kawacka, I.; Olejnik-Schmidt, A.; Schmidt, M.; Sip, A. Effectiveness of Phage-Based Inhibition of Listeria monocytogenes in Food Products and Food Processing Environments. Microorganisms 2020, 8, 1764. [Google Scholar] [CrossRef]
- Zhang, X.; Niu, Y.D.; Nan, Y.; Stanford, K.; Holley, R.; McAllister, T.; Narváez-Bravo, C. SalmoFresh™ effectiveness in controlling Salmonella on romaine lettuce, mung bean sprouts and seeds. Int. J. Food Microbiol. 2019, 305, 108250. [Google Scholar] [CrossRef] [PubMed]
- Vikram, A.; Woolston, J.; Sulakvelidze, A. Phage Biocontrol Applications in Food Production and Processing. Curr. Issues Mol. Biol. 2021, 40, 267–302. [Google Scholar] [CrossRef] [PubMed]
- Maciejewska, B.; Olszak, T.; Drulis-Kawa, Z. Applications of bacteriophages versus phage enzymes to combat and cure bacterial infections: An ambitious and also a realistic application? Appl. Microbiol. Biotechnol. 2018, 102, 2563–2581. [Google Scholar] [CrossRef] [PubMed]
- Luong, T.; Salabarria, A.-C.; Roach, D.R. Phage therapy in the resistance era: Where do we stand and where are we going? Clin. Ther. 2020, 42, 1659–1680. [Google Scholar] [CrossRef]
- Kortright, K.E.; Chan, B.K.; Koff, J.L.; Turner, P.E. Phage therapy: A renewed approach to combat antibiotic-resistant bacteria. Cell Host Microbe 2019, 25, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.H.; Islam, G.S.; Wu, Y.; Sabour, P.M.; Chambers, J.R.; Wang, Q.; Wu, S.X.; Griffiths, M.W. Temporal distribution of encapsulated bacteriophages during passage through the chick gastrointestinal tract. Poult. Sci. 2016, 95, 2911–2920. [Google Scholar] [CrossRef]
- Malik, D.J.; Sokolov, I.J.; Vinner, G.K.; Mancuso, F.; Cinquerrui, S.; Vladisavljevic, G.T.; Clokie, M.R.J.; Garton, N.J.; Stapley, A.G.F.; Kirpichnikova, A. Formulation, stabilisation and encapsulation of bacteriophage for phage therapy. Adv. Colloid Interface Sci. 2017, 249, 100–133. [Google Scholar] [CrossRef]
- Naghizadeh, M.; Karimi Torshizi, M.A.; Rahimi, S.; Engberg, R.M.; Sørensen Dalgaard, T. Effect of serum anti-phage activity on colibacillosis control by repeated phage therapy in broilers. Vet. Microbiol. 2019, 234, 61–71. [Google Scholar] [CrossRef]
- Stanford, K.; McAllister, T.A.; Niu, Y.D.; Stephens, T.P.; Mazzocco, A.; Waddell, T.E.; Johnson, R.P. Oral delivery systems for encapsulated bacteriophages targeted at Escherichia coli O157:H7 in feedlot cattle. J. Food Prot. 2010, 73, 1304–1312. [Google Scholar] [CrossRef]
- Vaz, C.S.L.; Voss-Rech, D.; Alves, L.; Coldebella, A.; Brentano, L.; Trevisol, I.M. Effect of time of therapy with wild-type lytic bacteriophages on the reduction of Salmonella Enteritidis in broiler chickens. Vet. Microbiol. 2020, 240, 108527. [Google Scholar] [CrossRef]
- Imklin, N.; Nasanit, R. Characterization of Salmonella bacteriophages and their potential use in dishwashing materials. J. Appl. Microbiol. 2020, 129, 266–277. [Google Scholar] [CrossRef]
- Iannetti, L.; Neri, D.; Santarelli, G.A.; Cotturone, G.; Vulpiani, M.P.; Salini, R.; Antoci, S.; Di Serafino, G.; Di Giannatale, E.; Pomilio, F. Animal welfare and microbiological safety of poultry meat: Impact of different at-farm animal welfare levels on at-slaughterhouse Campylobacter and Salmonella contamination. Food Control 2020, 109, 106921. [Google Scholar] [CrossRef]
- Plackett, B. Why big pharma has abandoned antibiotics. Nature 2020, 586, S50–S52. [Google Scholar] [CrossRef]
- Parfitt, T. Georgia: An unlikely stronghold for bacteriophage therapy. Lancet 2005, 365, 2166–2167. [Google Scholar] [CrossRef]
- Torres-Acosta, M.A.; Clavijo, V.; Vaglio, C.; González-Barrios, A.F.; Vives-Flórez, M.J.; Rito-Palomares, M. Economic evaluation of the development of a phage therapy product for the control of Salmonella in poultry. Biotechnol. Prog. 2019, 35, e2852. [Google Scholar] [CrossRef]
- Hughes, D.; Karlén, A. Discovery and preclinical development of new antibiotics. Upsala J. Med. Sci. 2014, 119, 162–169. [Google Scholar] [CrossRef]
- Hyman, P. Phages for Phage Therapy: Isolation, Characterization, and Host Range Breadth. Pharmaceuticals 2019, 12, 35. [Google Scholar] [CrossRef]
- Cross, T.; Schoff, C.; Chudoff, D.; Graves, L.; Broomell, H.; Terry, K.; Farina, J.; Correa, A.; Shade, D.; Dunbar, D. An optimized enrichment technique for the isolation of Arthrobacter bacteriophage species from soil sample isolates. JoVE (J. Vis. Exp.) 2015, e52781. [Google Scholar] [CrossRef]
- Patey, O.; McCallin, S.; Mazure, H.; Liddle, M.; Smithyman, A.; Dublanchet, A. Clinical indications and compassionate use of phage therapy: Personal experience and literature review with a focus on osteoarticular infections. Viruses 2019, 11, 18. [Google Scholar] [CrossRef]
- Li, P.; Zhang, X.; Xie, X.; Tu, Z.; Gu, J.; Zhang, A. Characterization and whole-genome sequencing of broad-host-range Salmonella-specific bacteriophages for bio-control. Microb. Pathog. 2020, 143, 104119. [Google Scholar] [CrossRef]
- Ross, A.; Ward, S.; Hyman, P. More Is Better: Selecting for Broad Host Range Bacteriophages. Front. Microbiol. 2016, 7, 1352. [Google Scholar] [CrossRef]
- de Jonge, P.A.; Nobrega, F.L.; Brouns, S.J.J.; Dutilh, B.E. Molecular and Evolutionary Determinants of Bacteriophage Host Range. Trends Microbiol. 2019, 27, 51–63. [Google Scholar] [CrossRef]
- Li, M.; Lin, H.; Jing, Y.; Wang, J. Broad-host-range Salmonella bacteriophage STP4-a and its potential application evaluation in poultry industry. Poult. Sci. 2020, 99, 3643–3654. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Liu, Y.; Peng, L.; Cai, X.; Shen, L.; Duan, M.; Ning, Y.; Liu, S.; Li, C.; Liu, Y. Characterization of the narrow-spectrum bacteriophage LSE7621 towards Salmonella Enteritidis and its biocontrol potential on lettuce and tofu. LWT 2020, 118, 108791. [Google Scholar] [CrossRef]
- Alves, D.; Cerqueira, M.A.; Pastrana, L.M.; Sillankorva, S. Entrapment of a phage cocktail and cinnamaldehyde on sodium alginate emulsion-based films to fight food contamination by Escherichia coli and Salmonella Enteritidis. Food Res. Int. 2020, 128, 108791. [Google Scholar] [CrossRef] [PubMed]
- Zaczek-Moczydłowska, M.A.; Young, G.K.; Trudgett, J.; Plahe, C.; Fleming, C.C.; Campbell, K.; Hanlon, R.O. Phage cocktail containing Podoviridae and Myoviridae bacteriophages inhibits the growth of Pectobacterium spp. under in vitro and in vivo conditions. PLoS ONE 2020, 15, e0230842. [Google Scholar] [CrossRef] [PubMed]
- Naghizadeh, M.; Karimi Torshizi, M.A.; Rahimi, S.; Dalgaard, T.S. Synergistic effect of phage therapy using a cocktail rather than a single phage in the control of severe colibacillosis in quails. Poult. Sci. 2019, 98, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Chadha, P.; Katare, O.P.; Chhibber, S. In vivo efficacy of single phage versus phage cocktail in resolving burn wound infection in BALB/c mice. Microb. Pathog. 2016, 99, 68–77. [Google Scholar] [CrossRef]
- Al-Shayeb, B.; Sachdeva, R.; Chen, L.-X.; Ward, F.; Munk, P.; Devoto, A.; Castelle, C.J.; Olm, M.R.; Bouma-Gregson, K.; Amano, Y. Clades of huge phages from across Earth’s ecosystems. Nature 2020, 578, 425–431. [Google Scholar] [CrossRef]
- Bumunang, E.W.; McAllister, T.A.; Stanford, K.; Anany, H.; Niu, Y.D.; Ateba, C.N. Characterization of Non-O157 STEC infecting bacteriophages isolated from cattle faeces in North-West South Africa. Microorganisms 2019, 7, 615. [Google Scholar] [CrossRef]
- Kering, K.K.; Zhang, X.; Nyaruaba, R.; Yu, J.; Wei, H. Application of adaptive evolution to improve the stability of bacteriophages during storage. Viruses 2020, 12, 423. [Google Scholar] [CrossRef]
- Akhwale, J.K.; Rohde, M.; Rohde, C.; Bunk, B.; Spröer, C.; Boga, H.I.; Klenk, H.-P.; Wittmann, J. Isolation, characterization and analysis of bacteriophages from the haloalkaline lake Elmenteita, Kenya. PLoS ONE 2019, 14, e0215734. [Google Scholar] [CrossRef] [PubMed]
- Akhwale, J.K.; Rohde, M.; Rohde, C.; Bunk, B.; Spröer, C.; Klenk, H.-P.; Boga, H.I.; Wittmann, J. Comparative genomic analysis of eight novel haloalkaliphilic bacteriophages from Lake Elmenteita, Kenya. PLoS ONE 2019, 14, e0212102. [Google Scholar] [CrossRef] [PubMed]
- van Zyl, L.J.; Abrahams, Y.; Stander, E.A.; Kirby-McCollough, B.; Jourdain, R.; Clavaud, C.; Breton, L.; Trindade, M. Novel phages of healthy skin metaviromes from South Africa. Sci. Rep. 2018, 8, 12265. [Google Scholar] [CrossRef] [PubMed]
- Yahya, M.; Hmaied, F.; Jebri, S.; Jofre, J.; Hamdi, M. Bacteriophages as indicators of human and animal faecal contamination in raw and treated wastewaters from Tunisia. J. Appl. Microbiol. 2015, 118, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Maje, M.D.; Kaptchouang Tchatchouang, C.D.; Manganyi, M.C.; Fri, J.; Ateba, C.N. Characterisation of vibrio species from surface and drinking water sources and assessment of biocontrol potentials of their bacteriophages. Int. J. Microbiol. 2020, 2020, 8863370. [Google Scholar] [CrossRef]
- Hassim, A.; Lekota, K.E.; Van Dyk, D.S.; Dekker, E.H.; Van Heerden, H. A Unique Isolation of a Lytic Bacteriophage Infected Bacillus anthracis Isolate from Pafuri, South Africa. Microorganisms 2020, 8, 932. [Google Scholar] [CrossRef]
- Bumunang, E.W.; Ateba, C.N.; Stanford, K.; Niu, Y.D.; Wang, Y.; McAllister, T.A. Activity of bacteriophage and complex tannins against biofilm-forming shiga toxin-producing Escherichia coli from Canada and South Africa. Antibiotics 2020, 9, 257. [Google Scholar] [CrossRef]
- Van Zyl, L.J.; Nemavhulani, S.; Cass, J.; Cowan, D.A.; Trindade, M. Three novel bacteriophages isolated from the East African Rift Valley soda lakes. Virol. J. 2016, 13, 204. [Google Scholar] [CrossRef]
- Montso, P.K.; Mlambo, V.; Ateba, C.N. Characterization of lytic bacteriophages infecting multidrug-resistant shiga toxigenic atypical Escherichia coli O177 strains isolated from cattle feces. Front. Public Health 2019, 7, 355. [Google Scholar] [CrossRef]
- Damelin, L.H.; Paximadis, M.; Mavri-Damelin, D.; Birkhead, M.; Lewis, D.A.; Tiemessen, C.T. Identification of predominant culturable vaginal Lactobacillus species and associated bacteriophages from women with and without vaginal discharge syndrome in South Africa. J. Med. Microbiol. 2011, 60, 180–183. [Google Scholar] [CrossRef][Green Version]
- Kakabadze, E.; Makalatia, K.; Grdzelishvili, N.; Bakuradze, N.; Goderdzishvili, M.; Kusradze, I.; Phoba, M.-F.; Lunguya, O.; Lood, C.; Lavigne, R. Selection of potential therapeutic bacteriophages that lyse a CTX-M-15 extended spectrum β-lactamase producing Salmonella enterica serovar typhi strain from the democratic republic of the Congo. Viruses 2018, 10, 172. [Google Scholar] [CrossRef]
- Akindolire, M.A.; Aremu, B.R.; Ateba, C.N. Complete genome sequence of Escherichia coli O157: H7 phage PhiG17. Microbiol. Resour. Announc. 2019, 8, e01296-18. [Google Scholar] [CrossRef]
- Essoh, C.; Vernadet, J.-P.; Vergnaud, G.; Coulibaly, A.; Kakou-N’Douba, A.; Assavo, S.-P.G.; Ouassa, T.; Pourcel, C. Characterization of sixteen Achromobacter xylosoxidans phages from Abidjan, Côte d’Ivoire, isolated on a single clinical strain. Arch. Virol. 2020, 165, 725–730. [Google Scholar] [CrossRef]
- Mahmoud, M.; Askora, A.; Barakat, A.B.; Rabie, O.E.-F.; Hassan, S.E. Isolation and characterization of polyvalent bacteriophages infecting multi drug resistant Salmonella serovars isolated from broilers in Egypt. Int. J. Food Microbiol. 2018, 266, 8–13. [Google Scholar] [CrossRef]
- Brown, B.P.; Chopera, D.; Havyarimana, E.; Wendoh, J.; Jaumdally, S.; Nyangahu, D.D.; Gray, C.M.; Martin, D.P.; Varsani, A.; Jaspan, H.B. crAssphage genomes identified in fecal samples of an adult and infants with evidence of positive genomic selective pressure within tail protein genes. Virus Res. 2021, 292, 198219. [Google Scholar] [CrossRef]
- Maina, A.N.; Mwaura, F.B.; Oyugi, J.; Goulding, D.; Toribio, A.L.; Kariuki, S. Characterization of Vibrio cholerae bacteriophages isolated from the environmental waters of the Lake Victoria region of Kenya. Curr. Microbiol. 2014, 68, 64–70. [Google Scholar] [CrossRef]
- Montso, P.K.; Mlambo, V.; Ateba, C.N. Efficacy of novel phages for control of multi-drug resistant Escherichia coli O177 on artificially contaminated beef and their potential to disrupt biofilm formation. Food Microbiol. 2021, 94, 103647. [Google Scholar] [CrossRef] [PubMed]
- Essoh, C.; Latino, L.; Midoux, C.; Blouin, Y.; Loukou, G.; Nguetta, S.-P.A.; Lathro, S.; Cablanmian, A.; Kouassi, A.K.; Vergnaud, G. Investigation of a large collection of Pseudomonas aeruginosa bacteriophages collected from a single environmental source in Abidjan, Côte d’Ivoire. PLoS ONE 2015, 10, e0130548. [Google Scholar] [CrossRef] [PubMed]
- Marie, V.; Lin, J. Viruses in the environment–presence and diversity of bacteriophage and enteric virus populations in the Umhlangane River, Durban, South Africa. J. Water Health 2017, 15, 966–981. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ndongmo Teytsa, H.; Tsanou, B.; Bowong, S.; Lubuma, J.M. Bifurcation analysis of a phage-bacteria interaction model with prophage induction. Math. Med. Biol. J. IMA 2021, 38, 28–58. [Google Scholar] [CrossRef] [PubMed]
- Bettarel, Y.; Combe, M.; Adingra, A.; Ndiaye, A.; Bouvier, T.; Panfili, J.; Durand, J.-D. Hordes of Phages in the Gut of the Tilapia Sarotherodon melanotheron. Sci. Rep. 2018, 8, 11311. [Google Scholar] [CrossRef] [PubMed]
- Mtimka, S.; Pillay, P.; Rashamuse, K.; Gildenhuys, S.; Tsekoa, T.L. Functional screening of a soil metagenome for DNA endonucleases by acquired resistance to bacteriophage infection. Mol. Biol. Rep. 2020, 47, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Ezemokwe, C.G.; Agwom, F.M.; Okoliegbe, I.; Okonkwo, F.O.; Ngene, A.C.; Gimba, N.; Morenikeji, O.R.; Egwuenu, A.; Okonkwo, C.H.; Aguiyi, J.C. Complete Genome Sequence of Pseudomonas Phage Zikora. Microbiol. Resour. Announc. 2021, 10, e00489-21. [Google Scholar] [CrossRef]
- Cinek, O.; Mazankova, K.; Kramna, L.; Odeh, R.; Alassaf, A.; Ibekwe, M.U.; Ahmadov, G.; Mekki, H.; Abdullah, M.A.; Elmahi, B.M. Quantitative CrAssphage real--time PCR assay derived from data of multiple geographically distant populations. J. Med. Virol. 2018, 90, 767–771. [Google Scholar] [CrossRef]
- Rodwell, E.V.; Wenner, N.; Pulford, C.V.; Cai, Y.; Bowers-Barnard, A.; Beckett, A.; Rigby, J.; Picton, D.M.; Blower, T.R.; Feasey, N.A. Isolation and characterisation of bacteriophages with activity against invasive non-typhoidal Salmonella causing bloodstream infection in Malawi. Viruses 2021, 13, 478. [Google Scholar] [CrossRef]
- El Didamony, G.; Askora, A.; Shehata, A.A. Isolation and characterization of T7-like lytic bacteriophages infecting multidrug resistant Pseudomonas aeruginosa isolated from Egypt. Curr. Microbiol. 2015, 70, 786–791. [Google Scholar] [CrossRef]
- El--Shibiny, A.; El--Sahhar, S.; Adel, M. Phage applications for improving food safety and infection control in Egypt. J. Appl. Microbiol. 2017, 123, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.A.; Elhalag, K.M.; Addy, H.S.; Nasr-Eldin, M.A.; Hussien, A.S.; Huang, Q. Sequencing, genome analysis and host range of a novel Ralstonia phage, RsoP1EGY, isolated in Egypt. Arch. Virol. 2018, 163, 2271–2274. [Google Scholar] [CrossRef]
- Adriaenssens, E.M.; Van Zyl, L.; De Maayer, P.; Rubagotti, E.; Rybicki, E.; Tuffin, M.; Cowan, D.A. Metagenomic analysis of the viral community in N amib D esert hypoliths. Environ. Microbiol. 2015, 17, 480–495. [Google Scholar] [CrossRef]
- Ganz, H.H.; Law, C.; Schmuki, M.; Eichenseher, F.; Calendar, R.; Loessner, M.J.; Getz, W.M.; Korlach, J.; Beyer, W.; Klumpp, J. Novel giant siphovirus from Bacillus anthracis features unusual genome characteristics. PLoS ONE 2014, 9, e85972. [Google Scholar] [CrossRef] [PubMed]
- Elhalag, K.; Nasr--Eldin, M.; Hussien, A.; Ahmad, A. Potential use of soilborne lytic Podoviridae phage as a biocontrol agent against Ralstonia solanacearum. J. Basic Microbiol. 2018, 58, 658–669. [Google Scholar] [CrossRef] [PubMed]
- Zablocki, O.; Van Zyl, L.J.; Kirby, B.; Trindade, M. Diversity of dsDNA viruses in a South African hot spring assessed by metagenomics and microscopy. Viruses 2017, 9, 348. [Google Scholar] [CrossRef]
- Sharaf, A.; Mercati, F.; Elmaghraby, I.; Elbaz, R.; Marei, E. Functional and comparative genome analysis of novel virulent actinophages belonging to Streptomyces flavovirens. BMC Microbiol. 2017, 17, 51. [Google Scholar] [CrossRef]
- Ochieng’Oduor, J.M.; Onkoba, N.; Maloba, F.; Arodi, W.O.; Nyachieo, A. Efficacy of lytic Staphylococcus aureus bacteriophage against multidrug-resistant Staphylococcus aureus in mice. J. Infect. Dev. Ctries. 2016, 10, 1208–1213. [Google Scholar]
- D’arc, M.; Furtado, C.; Siqueira, J.D.; Seuánez, H.N.; Ayouba, A.; Peeters, M.; Soares, M.A. Assessment of the gorilla gut virome in association with natural simian immunodeficiency virus infection. Retrovirology 2018, 15, 19. [Google Scholar] [CrossRef]
- Pope, W.H.; Jacobs-Sera, D.; Russell, D.A.; Rubin, D.H.; Kajee, A.; Msibi, Z.N.; Larsen, M.H.; Jacobs, W.R., Jr.; Lawrence, J.G.; Hendrix, R.W. Genomics and proteomics of mycobacteriophage patience, an accidental tourist in the Mycobacterium neighborhood. mBio 2014, 5, e02145-14. [Google Scholar] [CrossRef]
- Zablocki, O.; van Zyl, L.; Adriaenssens, E.M.; Rubagotti, E.; Tuffin, M.; Cary, S.C.; Cowan, D. High-level diversity of tailed phages, eukaryote-associated viruses, and virophage-like elements in the metaviromes of antarctic soils. Appl. Environ. Microbiol. 2014, 80, 6888–6897. [Google Scholar] [CrossRef]
- Safwat Mohamed, D.; Farouk Ahmed, E.; Mohamed Mahmoud, A.; Abd El-Baky, R.M.; John, J. Isolation and evaluation of cocktail phages for the control of multidrug-resistant Escherichia coli serotype O104: H4 and E. coli O157: H7 isolates causing diarrhea. FEMS Microbiol. Lett. 2018, 365, fnx275. [Google Scholar] [CrossRef]
- Montso, P.K.; Mnisi, C.M.; Ateba, C.N.; Mlambo, V. An Assessment of the Viability of Lytic Phages and Their Potency against Multidrug Resistant Escherichia coli O177 Strains under Simulated Rumen Fermentation Conditions. Antibiotics 2021, 10, 265. [Google Scholar] [CrossRef]
- Jebri, S.; Jofre, J.; Barkallah, I.; Saidi, M.; Hmaied, F. Presence and fate of coliphages and enteric viruses in three wastewater treatment plants effluents and activated sludge from Tunisia. Environ. Sci. Pollut. Res. 2012, 19, 2195–2201. [Google Scholar] [CrossRef] [PubMed]
- Fancello, L.; Trape, S.; Robert, C.; Boyer, M.; Popgeorgiev, N.; Raoult, D.; Desnues, C. Viruses in the desert: A metagenomic survey of viral communities in four perennial ponds of the Mauritanian Sahara. ISME J. 2013, 7, 359–369. [Google Scholar] [CrossRef]
- Eckstein, S.; Stender, J.; Mzoughi, S.; Vogele, K.; Kühn, J.; Friese, D.; Bugert, C.; Handrick, S.; Ferjani, M.; Wölfel, R. Isolation and characterization of lytic phage TUN1 specific for Klebsiella pneumoniae K64 clinical isolates from Tunisia. BMC Microbiol. 2021, 21, 186. [Google Scholar] [CrossRef] [PubMed]
- Peduzzi, P.; Gruber, M.; Gruber, M.; Schagerl, M. The virus’s tooth: Cyanophages affect an African flamingo population in a bottom-up cascade. ISME J. 2014, 8, 1346–1351. [Google Scholar] [CrossRef] [PubMed]
- Carrigy, N.; Liang, L.; Wang, H.; Kariuki, S.; Nagel, T.; Connerton, I.; Vehring, R. Mechanistic modeling expedites the development of spray dried biologics. In Proceedings of the IDS 2018, 21st International Drying Symposium Proceedings, Editorial Universitat Politècnica de València, Valencia, Spain, 11–14 September 2018. [Google Scholar]
- Carrigy, N.B.; Liang, L.; Wang, H.; Kariuki, S.; Nagel, T.E.; Connerton, I.F.; Vehring, R. Spray-dried anti-Campylobacter bacteriophage CP30A powder suitable for global distribution without cold chain infrastructure. Int. J. Pharm. 2019, 569, 118601. [Google Scholar] [CrossRef]
- Carrigy, N.B.; Liang, L.; Wang, H.; Kariuki, S.; Nagel, T.E.; Connerton, I.F.; Vehring, R. Trileucine and pullulan improve anti-campylobacter bacteriophage stability in engineered spray-dried microparticles. Ann. Biomed. Eng. 2019, 48, 1169–1180. [Google Scholar] [CrossRef]
- Liang, L.; Carrigy, N.B.; Kariuki, S.; Muturi, P.; Onsare, R.; Nagel, T.; Vehring, R.; Connerton, P.L.; Connerton, I.F. Development of a lyophilization process for campylobacter bacteriophage storage and transport. Microorganisms 2020, 8, 282. [Google Scholar] [CrossRef]
- Moelling, K.; Broecker, F.; Willy, C. A wake-up call: We need phage therapy now. Viruses 2018, 10, 688. [Google Scholar] [CrossRef] [PubMed]
- Kassa, T. Bacteriophages Against Pathogenic Bacteria and Possibilities for Future Application in Africa. Infect. Drug Resist. 2021, 14, 17. [Google Scholar] [CrossRef]
- García, R.; Latz, S.; Romero, J.; Higuera, G.; García, K.; Bastías, R. Bacteriophage production models: An overview. Front. Microbiol. 2019, 10, 1187. [Google Scholar] [CrossRef]
- Cooper, C.J.; Khan Mirzaei, M.; Nilsson, A.S. Adapting Drug Approval Pathways for Bacteriophage-Based Therapeutics. Front. Microbiol. 2016, 7, 1209. [Google Scholar] [CrossRef]
- Fauconnier, A. Phage Therapy Regulation: From Night to Dawn. Viruses 2019, 11, 352. [Google Scholar] [CrossRef] [PubMed]
- Naureen, Z.; Malacarne, D.; Anpilogov, K.; Dautaj, A.; Camilleri, G.; Cecchin, S.; Bressan, S.; Casadei, A.; Albion, E.; Sorrentino, E.; et al. Comparison between American and European legislation in the therapeutical and alimentary bacteriophage usage. Acta Bio-Med. Atenei Parm. 2020, 91, e2020023. [Google Scholar] [CrossRef]
- Kwiatek, M.; Parasion, S.; Nakonieczna, A. Therapeutic bacteriophages as a rescue treatment for drug--resistant infections—An in vivo studies overview. J. Appl. Microbiol. 2020, 128, 985–1002. [Google Scholar] [CrossRef] [PubMed]
- Kenya, Veterinary Medicines Directorate. In Guidelines on Submission of Documentations for Marketing Authorization for Veterinary Medicines; Draft, Doc No. VMD/GUID/O1; Veterinary Medicines Directorate: Nairobi, Kenya; 108p.
- Huys, I.; Pirnay, J.P.; Lavigne, R.; Jennes, S.; De Vos, D.; Casteels, M.; Verbeken, G. Paving a regulatory pathway for phage therapy. Europe should muster the resources to financially, technically and legally support the introduction of phage therapy. EMBO Rep. 2013, 14, 951–954. [Google Scholar] [CrossRef]
- Loponte, R.; Pagnini, U.; Iovane, G.; Pisanelli, G. Phage Therapy in Veterinary Medicine. Antibiotics 2021, 10, 421. [Google Scholar] [CrossRef]
- Verbeken, G.; Huys, I.; De Vos, D.; De Coninck, A.; Roseeuw, D.; Kets, E.; Vanderkelen, A.; Draye, J.P.; Rose, T.; Jennes, S.; et al. Access to bacteriophage therapy: Discouraging experiences from the human cell and tissue legal framework. FEMS Microbiol. Lett. 2016, 363, fnv241. [Google Scholar] [CrossRef]
- Verbeken, G.; Pirnay, J.P.; Lavigne, R.; Jennes, S.; De Vos, D.; Casteels, M.; Huys, I. Call for a dedicated European legal framework for bacteriophage therapy. Arch. Immunol. Ther. Exp. 2014, 62, 117–129. [Google Scholar] [CrossRef]
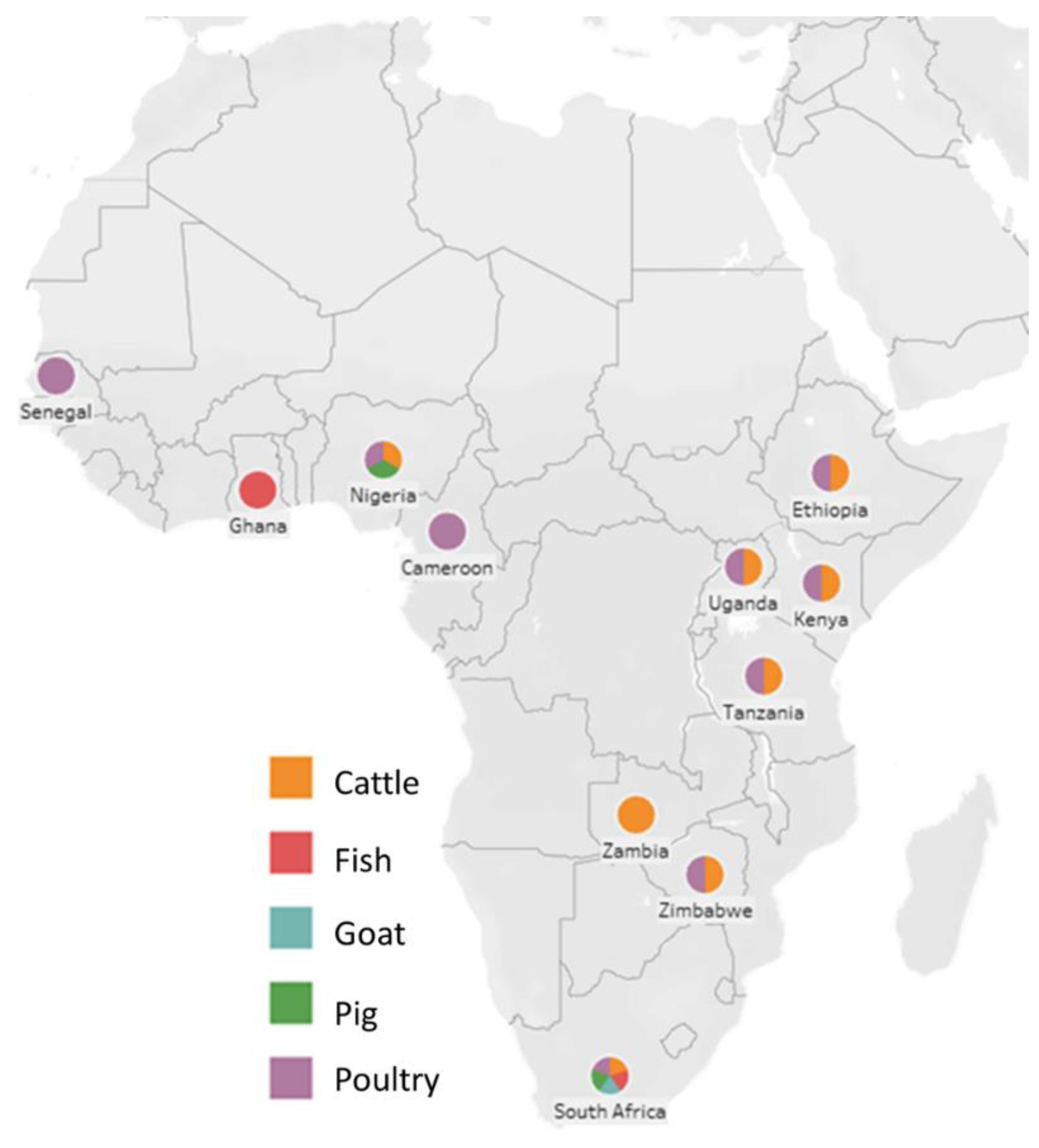
| Country | Animal | Sample | Organism | Antibiotic Resistance Data | Reference |
|---|---|---|---|---|---|
| South Africa | Cattle | Milk | S. aureus | SPN, ERY | [15] |
| South Africa | Poultry | Fecal Samples | E. coli | CST, FLO, TRS, SPE, FOS, AMX | [16] |
| South Africa | Fish | Bacterial isolates | S. aureus | RIF(82%), CLI(82%), ERY(67%), AMP(67%), TET(27%), VAN (30%) | [17] |
| South Africa | Poultry | Fecal Samples | C. jejuni | ERY (79%), CLI (75%), AMP(54%), NAL(48%), CTR(48%), CIP(33%), GEN (15%), TET(16%) | [18] |
| South Africa | Poultry | Fecal Samples | C. coli | ERY (60%), CLI (56%),AMP (36%), NAL(26%), CTR(28%), CIP(15%), GEN (8%), TET(7%) | [18] |
| Kenya | Poultry | Fecal Samples | Salmonella | STR (6%), AMP (50%), TRS (28%), TET (11%) | [19] |
| Kenya | Poultry | Fecal Samples | E. coli | STR (9%), CHL (2%), NAL(2%), AMO (54%), TRS (26%), TET (12%) | [19] |
| Nigeria | Poultry | Feces, feed, water | S. Enterica | AMP(100%), CHL(100%), CTV(100%), CIP(100%), GEN (100%), CTA(100%), NEO(100%), NAL(100%), CPDS (100%),STR (100%), TET (100%) | [20] |
| Ghana | Fish | Water and cultured fish species | Coliform Bacteria | AMP(98.4%),CUR(88.9%), TET(66.7%), CTA(52.4%), TRS (56.0%), GEN (6.4%) | [21] |
| South Africa | Cattle | Fecal Samples | E. coli | ERY(63.84%),AMP(21.54%), TET(13.37%), STR(17.01%), KAN (2.42%), CHL(1.97%),NOR (1.40%) | [22] |
| South Africa | Cattle | Fecal Samples | Enterobacteriaceae | CAA: IMI (42%), ERT (35%), DOR (30%), MER (28%) | [23] |
| Uganda | Poultry | fecal samples | Salmonella | CIP(46.5%), SULFA(24.4%), TET(15.1%), TRI(7.0%), TRS(7.0%), CHL(4.6%), AMP(4.6%) | [24] |
| Ethiopia | Cattle | Milk | E. coli | AMP (68.7%), TRS (50%), STR (25%) | [25] |
| Uganda | Poultry | post-mortem samples | E. coli | PEN G(100%), TRS(87.5%), TET(83.9%), AMP(80.4%), AMX(69.6%), STR(67.9%), NAL(60.7%), CHL (35.7%),GEN (10.7%) | [26] |
| South Africa | Cattle | Fecal Samples | Salmonella | PEN(79%), CTA(28%), NAL(7%), CLT(24%),GEN (1%), CHL(20%), TET(62%), ERY (42%), MIN (46%), VAN (100%), OXA(100%), OFL(9%), AMP(82%), TRS(62%), STR(40%) | [27] |
| South Africa | Goats | Fecal samples | Salmonella | PEN(88%), CTA(54%), NAL(6%), CLT(37%), GEN (24%), CHL(29%), TET(32%), ERY(57%), MIN (15%), VAN(100%), OXA(100%), AMP(25%), TRS (71%), STR (29%) | [27] |
| Nigeria | Cattle, Poultry | Rectal and cloacol Swabs | Enterococcus | TET (61.0%), ERY (61.0%), QUD (4.4%), CHL (8.0%) | [28] |
| Senegal | Poultry | Fecal samples | E. coli | CST(2.2%) | [29] |
| South Africa | Cattle | Fecal samples | Aeromonas | AMX (100%, 92%), CHL (7%; 2%), PEN (100%; 95%), PLB (50%; 32%) | [30] |
| Cameroon | Poultry | muscle, liver, heart, kidney and gizzards | Various bacteria | TET (63%), KAN(45%), AMC(63%), AMP(54%), TRS (36%), ERY(81%),CTF (45%),CHL (36%), ENR (45%), GEN (54%) VAN (63%) | [31] |
| South Africa | Cattle | Fecal Samples | Enterococcus | VAN (100%), CLO (100%), AMI(74%), CLT (88%), STR (94%), PEN G (91%), CLI (97%), NEO (91%), ERY (99%), IMI (0.6%), AMC (8%), CIP (12%) | [32] |
| Zambia | Cattle | Fecal Samples | E. coli | CPO, CIP, AMP, TRS, TET, GEN | [33] |
| South Africa | Pigs/piglets, Cattle, Goats, Poultry | nasal, mouth wash, and ear swabs | Staphylococcus | PEN G (75%),MER (2.3%),VAN (12%),CTA (13%),CTV (40%), OXA(38%), MIN (16%),TET (83%),ERY (12%),CLI (16%),NAL (100%),CIP (3%),OFL (5%),LEV (2%) | [34] |
| South Africa | Fish | Water | Gram-negative bacteria | ERY (100%), AMP (85%), TRI (78%) | [35] |
| Nigeria | Poultry | Fecal Samples | Salmonella | AMP, AMC, CIP, GEN, NAL, NEO; SPE, STR, SME, TET, TRI | [36] |
| Ethiopia | Poultry | Eggs | Salmonella | CLI (100%), ERY (63%), AMP (38%), AMX (38%), TET (25%) | [37] |
| Tanzania | Cattle | Milk | Staphylococcus aureus and other bacteria | AMX, CPX, GEN, KAN, NEO, TET | [38] |
| Tanzania | Cattle | Fecal Samples | E. coli | AMP (40%), TET (20%), CTA (10%),TRS (15%) | [39] |
| Tanzania | Poultry | fecal Samples | E. coli | AMP, AMX, CHL, CIP, STR, SME, TET, TRI | [40] |
| Zimbabwe | Cattle | Fecal samples | E. coli | TET, PEN, TRS | [41] |
| Uganda | Cattle | Milk | Streptococci spp. and Staphylococci spp. | TET (100%) | [42] |
| Nigeria | Cattle and Pigs | Fecal samples | E. coli | PEN (96%),AMX (88%), AMP (89%), AUG (96%), CTV (58%),CTA (92%), CIX (39%), CUR (83%), CPO(58%), TET (88%), ERY (82%), STR (79%), GEN (49%), CIP(5%), OFL (5%), CLO (84%), TRS (90%), CHL (92%) | [43] |
| Nigeria | Poultry | Cloacae and nasal samples | Staphylococcus aureus | AUG(0.8%), CXI (6.1%), CUR (5.3%), CHL (12.1%),DOX (7.7%), ERY (19.4%), GEN (5.3%), LEV (0.8%), TET (45.7%),TRS (40.9%) | [44] |
| Zimbabwe | Poultry | Fecal samples | E. coli | TET (100%), BCN (100%), CLO (100%) AMP (94.1%) | [45] |
| Kenya | Cattle | Milk | Staphylococcus aureus and Streptococcus agalactiae and other bacteria | TRS (76%), AMP (57%) | [46] |
| South Africa | Cattle | Fecal samples | E. coli | AMP, SFZ, TET, STR | [47] |
| South Africa | Poultry | Isolates | E. coli | CST (13.5%) | [48] |
| Ethiopia | Cattle | Milk | Staphylococcus species and coliforms | AMP, ERY, NAL, CLI, TRS, CHL | [49] |
| South Africa | Cattle | Milk | Bacteria | PEN (47.8), OXA (1.1%), CLT (1.1%), STR (16.7%), NEO (5.6%), TET (11.1%), TRS (1.1%), ENR (1.1%), TLS (2.2%) | [49] |
| Country | Source of Sample | Host | Phage | Purpose of Research | Ref. |
|---|---|---|---|---|---|
| Tanzania | Hadza fecal samples | Firmicutes | *N. I | Sequenced DNA from diverse ecosystems for phage genomes | [112] |
| Kenya | Baboon fecal samples | Actinobacteria, Proteobacteria, Firmicutes | *N. I | Sequenced DNA from diverse ecosystems for phage genomes | [112] |
| South Africa | Thiocyanate bioreactor | Proteobacteria | *N. I | Sequenced DNA from diverse ecosystems for phage genomes | [112] |
| South Africa | Cattle feces | Non-O157 Shiga toxin-producing Escherichia coli (STEC) | Myoviridae, Siphoviridae | Isolation and characterization | [113] |
| Kenya | Environmental water samples | Ralstonia solanacearum strain GIM1.74. | Podoviridae | Evolution experiments for phage stability/storage | [114] |
| Kenya | Lake Elmentaita sediment samples | Vibrio metschnikovii, Bacillus pseudofirmus, Bacillus bogoriensis, Bacillus horikoshii, Bacillus cohnii, bacillus psedolcaliphilus, Bacillus halmapalus, Exiguobacterium aurantiacum, Exiguobacterium alkaliphilum | Myoviridae, Siphoviridae, Podoviridae | Isolation, characterization, comparative genomics | [115,116] |
| South Africa | Skin | Staphylococcus capitis, Pseudomonas | Myoviridae, Siphoviridae, Podoviridae | Metaviriome analysis | [117] |
| Tunisia | Raw and treated wastewaters of human and animal origin | Escherichia coli, Salmonella Typhimurium, Bact. fragilis, Bact. thetaiotaomicron | Somatic coliphages (SOMCPH), F-specific RNA bacteriophages (F-RNA), Bact. fragilis phages (RYC2056) and Bact. thetaiotaomicron phages | Monitor the microbial quality of water | [118] |
| South Africa | Water samples collected from taps, boreholes, and dams | V. harveyi, V. parahaemolyticus, V. cholerae, V. mimicus, V. vulnificus | Myoviridae | Isolation and characterization | [119] |
| South Africa | Carcass remnants | Bacillus anthracis | Myoviridae | Isolation and characterization | [120] |
| South Africa | Cattle feces | Shiga toxin-producing Escherichia coli (STEC) | *N. I | Isolation and characterization | [121] |
| Kenya | Lake Magadi soil sediments | Bacillus- and Paracoccus species | Myoviridae | Isolation and characterization | [122] |
| Ethiopia | Lake Chala soil sediments | Bacillus- and Paracoccus species | Myoviridae, Siphoviridae | Isolation and characterization | [122] |
| South Africa | Cattle feces | Escherichia coli O177 | Myoviridae | Isolation and characterization | [123] |
| South Africa | Vaginal swabs | Lactobacillus jensenii, Lactobacillus crispatus, Lactobacillus iners, Lactobacillus gasseri and Lactobacillus vaginalis | Myoviridae, Siphoviridae, Podoviridae | Isolation and characterization | [124] |
| Democratic Republic of Congo | *N. I | Salmonella Typhi | Myoviridae, Siphoviridae, Podoviridae | Testing of 14 Salmonella phages from the Eliava collection and commercial phage cocktail “INTESTI phage” | [125] |
| South Africa | Cattle feces | Escherichia coli O157:H7 | Podoviridae | Genome sequence | [126] |
| Côte d’Ivoire | Sewage water | Achromobacter xylosoxidans | Siphoviridae, Podoviridae | Isolation and characterization | [127] |
| Egypt | Chicken feces | Salmonella Serovars, Citrobacter freundii, Enterobacter cloacae, Escherichia coli. | Siphoviridae, Myoviridae | Isolation and characterization | [128] |
| South Africa | Human stool samples | *N. I | crAssphage | Sequencing | [129] |
| Kenya | Lake Victoria water samples | Vibrio cholerae | Myoviridae | Isolation and characterization | [130] |
| Uganda | Chicken postmortem samples | Avian Pathogenic Escherichia coli | *N. I | Isolation and characterization | [26] |
| South Africa | Cattle feces | Escherichia coli O177 | Myoviridae | Efficacy of beef decontamination and biofilm disruption | [131] |
| Côte d’Ivoire | Sewage samples | Pseudomonas aeruginosa | Myoviridae, Siphoviridae, Podoviridae | Characterization and sequencing | [132] |
| South Africa | Umhlangane River water sample | *N. I | Myoviridae, Siphoviridae, Podoviridae | Diversity of bacteriophage population | [133] |
| South Africa | *N. I | *N. I | *N. I | A predator–prey model to analyze phage–bacteria interactions | [134] |
| Senegal | Gut and water samples of Tilapia Sarotherodon melanotheron | *N. I | Myoviridae, Siphoviridae, Podoviridae | Viriome analysis | [135] |
| South Africa | Soil samples | *N. I | Escherichia coli bacteriophage Lambda W60 | Isolating new endonucleases using functional metagenomic techniques | [136] |
| South Africa | Soil samples | *N. I | Siphoviridae | Metaviromic techniques for viral diversity | [136] |
| Nigeria | Sewage water | Pseudomonas aeruginosa | Myoviridae | Genome sequencing | [137] |
| Nigeria | Human stool samples | *N. I | crAssphage | Quantitative CrAssphage analysis from multiple geographically distant populations | [138] |
| Sudan | Human stool samples | *N. I | crAssphage | Quantitative CrAssphage analysis from multiple geographically distant populations | [138] |
| Malawi | Water samples | S. Typhimurium, S. Enteritidis | Ackermannviridae, Siphoviridae | Isolation and characterization | [139] |
| Egypt | Sewage samples | Pseudomonas aeruginosa | Siphoviridae | Isolation and characterization | [140] |
| Egypt | Sewage samples | Salmonella enterica, Escherichia coli | Siphoviridae, Myoviridae | Applications in food safety | [141] |
| Egypt | Soil samples | Ralstonia solanacearum | Podoviridae | Sequencing | [142] |
| South Africa | Soil samples | *N. I | Myoviridae, Siphoviridae, Podoviridae | Metagenomic analysis of the viral community | [143] |
| Namibia | Wildlife carcass | Bacillus anthracis | Siphoviridae | Dissecting novel giant Siphovirus | [144] |
| Egypt | Soil samples | Ralstonia solanacearum | Podoviridae | Biocontrol | [145] |
| South Africa | Water samples from hot springs | *N. I | Myoviridae, Siphoviridae, Podoviridae, Fuselloviridae | Metavirome analysis | [146] |
| Egypt | Soil samples | Streptomyces flavovirens | Siphoviridae | Sequencing | [147] |
| Kenya | Sewage and wastewater | Staphylococcus aureus | N. I | Efficacy of lysis | [148] |
| Cameroon | Gorilla fecal samples | *N. I | Myoviridae, Siphoviridae | Microbiome analysis | [149] |
| South Africa | Environmental samples | Mycobacterium smegmatis | Siphoviridae | Genomics and proteomics of mycobacteriophage | [150] |
| South Africa | Soil samples | *N. I | Myoviridae, Siphoviridae, Podoviridae, Mimiviridae, Phycodnaviridae | Metaviromes of Antarctic soils | [151] |
| Egypt | Water samples from sewage systems | Escherichia coli O104: H4 Escherichia coli O157: H7 | Siphoviridae, Podoviridae | Isolation and characterization | [152] |
| South Africa | Rumen fluid | Escherichia coli O177 | Myoviridae | Viability of lytic phages under simulated rumen fermentation conditions | [153] |
| Tunisia | Sewage and waste-water treatment | Coliphages | Presence of viruses in wastewater treatment | [154] | |
| Mauritania | Soil and water samples | Prochlorococcus and Synechococcus sp. | Myoviridae | Metagenomics of viruses in the desert | [155] |
| Tunisia | Wastewater samples | Klebsiella pneumoniae | Podoviridae | Isolation and characterization | [156] |
| South Africa | Water samples | *N. I | Somatic and F-RNA Phages | Phages as an indicator of fecal contamination | |
| Kenya | Water samples | Arthrospira fusiformis | cyanophages | Cyanophages affecting an African flamingo population | [157] |
| Kenya | Poultry feces | Campylobacter jejuni | Myoviridae | Development of spray-dried biologics | [158] |
| Kenya | Poultry feces | Campylobacter jejuni Campylobacter coli | Myoviridae | Spray-dried anti-campylobacter powder suitable for global distribution | [159] |
| Kenya | Poultry feces | Campylobacter jejuni | Myoviridae | Use of Trileucine and pullulan to improve anti-campylobacter bacteriophage stability | [160] |
| Kenya | Poultry feces | Campylobacter jejuni | Myoviridae | Lyophilization process for campylobacter bacteriophage | [161] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makumi, A.; Mhone, A.L.; Odaba, J.; Guantai, L.; Svitek, N. Phages for Africa: The Potential Benefit and Challenges of Phage Therapy for the Livestock Sector in Sub-Saharan Africa. Antibiotics 2021, 10, 1085. https://doi.org/10.3390/antibiotics10091085
Makumi A, Mhone AL, Odaba J, Guantai L, Svitek N. Phages for Africa: The Potential Benefit and Challenges of Phage Therapy for the Livestock Sector in Sub-Saharan Africa. Antibiotics. 2021; 10(9):1085. https://doi.org/10.3390/antibiotics10091085
Chicago/Turabian StyleMakumi, Angela, Amos Lucky Mhone, Josiah Odaba, Linda Guantai, and Nicholas Svitek. 2021. "Phages for Africa: The Potential Benefit and Challenges of Phage Therapy for the Livestock Sector in Sub-Saharan Africa" Antibiotics 10, no. 9: 1085. https://doi.org/10.3390/antibiotics10091085
APA StyleMakumi, A., Mhone, A. L., Odaba, J., Guantai, L., & Svitek, N. (2021). Phages for Africa: The Potential Benefit and Challenges of Phage Therapy for the Livestock Sector in Sub-Saharan Africa. Antibiotics, 10(9), 1085. https://doi.org/10.3390/antibiotics10091085







