Microwave-Assisted, One-Pot Synthesis of Doxycycline under Heterogeneous Catalysis in Water
Abstract
1. Introduction
2. Materials and Methods
2.1. General Procedure for the Conventional and MW-Assisted Synthesis of Doxycycline
2.2. General Procedure for Oxytetracycline-CD Complexation and Further Hydrogenolysis Reaction
2.3. β-Doxycycline Chacterization
2.4. α-Doxycycline Chacterization
2.5. Molecular Modeling
3. Results and Discussion
3.1. A-Doxycycline Synthesis under Heterogeneous Rhodium Catalyst
3.2. Supposed Mechanism Involved in the Hydrogenation Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levy, S.B. The Antibiotic Paradox: How Miracle Drugs Are Destroying the Miracle, 1st ed.; Springer Link: New York, NY, USA, 1992. [Google Scholar] [CrossRef]
- Borghi, A.A.; Palma, M.S.A. Tetracycline: Production, waste treatment, and environmental impact assessment. Braz. J. Pharm. Sci. 2014, 50, 25–40. [Google Scholar] [CrossRef]
- Chopra, I.; Roberts, M. Tetracycline antibiotics: Mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef]
- Pickens, L.B.; Tang, Y. Oxytetracycline Biosynthesis. J. Biol. Chem. 2010, 285, 27509–27515. [Google Scholar] [CrossRef] [PubMed]
- Goodman, J.J. Fermentation and Mutational Development of the Tetracyclines. In The Tetracyclines, 1st ed.; Hlavka, J.J., Boothe, J.H., Eds.; Springer: Berlin/Heidelberg, Germany, 1985; pp. 5–57. [Google Scholar]
- Asagbra, A.E.; Sanni, A.I.; Oyewole, O.B. Solid-state fermentation production of tetracycline by Streptomyces strains using some agricultural wastes as substrate. World J. Microbiol. Biotechnol. 2005, 21, 107–114. [Google Scholar] [CrossRef]
- Shnappinger, D.; Hillen, W. Tetracyclines: Antibiotic action, uptake, and resistance mechanisms. Archiv. Microbiol. 1996, 165, 359–369. [Google Scholar] [CrossRef]
- Sloan, B.; Scheinfeld, N. The use and safety of doxycycline hydrate and other second-generation tetracyclines. Expert Opin. Drug Saf. 2008, 7, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Tariq, S.; Rizvi, S.F.A.; Anwar, U. Tetracycline: Classification, Structure-Activity Relationship and Mechanism of Action as a Theranostic Agent for Infectious Lesions-A Mini Review. Biomed. J. Sci. Tech. Res. 2018, 7, 5787–5796. [Google Scholar]
- Vardanyan, R.; Hruby, V. Synthesis of Best-Seller Drugs; Vardanyan, R., Hruby, V., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 573–643. [Google Scholar]
- Raval, J.P.; Chejara, D.R.; Ranch, K.; Joshi, P. Development of injectable in situ gelling systems of doxycycline hyclate for controlled drug delivery system. In Applications of Nanocomposite Materials in Drug Delivery, 1st ed.; Inamuddin, D., Asiri, A.M., Mohammad, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 149–162. [Google Scholar]
- Nelson, M.L.; Levy, S.B.; Ann, N.Y. The history of the tetracyclines. Annals of the N. Y. Acad. Sci. 2011, 1241, 17–32. [Google Scholar] [CrossRef]
- McCormick, J.R.D.; Jensen, E.R. Process for the Catalytic Reduction of 6-Hydroxy Hydronaphthacenes. U.S. Patent 3019260, 30 January 1962. [Google Scholar]
- Felekidis, A.; Goblet-Stachow, M.; Liégeois, J.F.; Pirotte, B.; Delarge, J.; Demonceau, A.; Fontaine, M.; Noels, A.F.; Chizhevsky, I.T.; Zinevich, T.V.; et al. Ligand effects in the hydrogenation of methacycline to doxycycline and epi-doxycycline catalysed by rhodium complexes molecular structure of the key catalyst [closo-3,3-(η2,3-C7H7CH2)-3,1,2-RhC2B9H11]. J. Organomet. Chem. 1997, 536-537, 405–412. [Google Scholar] [CrossRef]
- Charles Stephens, B.R.; Beereboom, J.J.; Rennhard, H.H.; Gordon, N.P.; Murai, K.; Blackwood, L.K.; Wittenau, M.S. 6-Deoxytetracyclines. IV.12 Preparation, C-6 Stereochemistry, and Reactions. Am. Chem. Soc. 1963, 85, 2643–2652. [Google Scholar] [CrossRef]
- Pan, Z.; Sha, Y. Effect and regeneration of heterogeneous palladium/charcoal catalysts poisoned by tetramethyl thiourea–quinoline in the stereoselective hydrogenation of methacycline to α-doxycycline. Appl. Catal. A Gen. 2003, 252, 347–352. [Google Scholar] [CrossRef]
- Wright, P.M.; Seiple, I.B.; Myers, A.G. The evolving role of chemical synthesis in antibacterial drug discovery. Angew. Chem. Int. Ed. 2014, 53, 8840–8869. [Google Scholar] [CrossRef] [PubMed]
- Pirotte, B.; Felekidis, A.; Fontaine, M.; Demonceau, A.; Noels, A.F.; Delarge, J.; Chizhevsky, I.T.; Zinevich, T.V.; Pisareva, I.V.; Bregadze, V.I. Stereoselective hydrogenation of methacycline to Doxycycline catalysed by rhodium-carborane complexes. Tetrahedron Lett. 1993, 34, 1471–1474. [Google Scholar] [CrossRef]
- Charest, M.G.; Lerner, C.D.; Brubaker, J.D.; Siegel, D.R.; Myers, A.G. A Convergent Enantioselective Route to Structurally Diverse 6-Deoxytetracycline Antibiotics. Science 2005, 308, 395–398. [Google Scholar] [CrossRef]
- Cravotto, G.; Carnaroglio, D. Microwave Chemistry, 1st ed.; De Gruyter: Berlin, Germany, 2017. [Google Scholar]
- Polshettiwar, V.; Varma, R.S. Microwave-Assisted Organic Synthesis and Transformations using Benign Reaction Media. Acc. Chem. Res. 2008, 41, 629–639. [Google Scholar] [CrossRef]
- Kappe, C.O. Controlled microwave heating in modern organic synthesis. Angew. Chem.-Int. Ed. 2004, 43, 6250–6284. [Google Scholar] [CrossRef]
- De La Hoz, A.; Ngel Díaz-Ortiz, A.; Moreno, A. Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem. Soc. Rev. 2005, 34, 164–178. [Google Scholar] [CrossRef]
- Calcio Gaudino, E.; Carnaroglio, D.; Martina, K.; Palmisano, G.; Penoni, A.; Cravotto, G. Highly Efficient Microwave-Assisted CO Aminocarbonylation with a Recyclable Pd(II)/TPP-β-Cyclodextrin Cross-Linked Catalyst. Org. Process Res. Dev. 2015, 19, 499–505. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Varma, R.S. Green chemistry by nano-catalysis. Green Chem. 2010, 12, 743–775. [Google Scholar] [CrossRef]
- Pereira, A.G.; Carpena, M.; Oliveira, P.G.; Mejuto, J.C.; Prieto, M.A.; Gandara, J.S. Main Applications of Cyclodextrins in the Food Industry as the Compounds of Choice to Form Host-Guest Complexes. Int. J. Mol. Sci. 2021, 22, 1339. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zuo, L.; Liu, H.; Zhang, L.; Guo, X. Synthesis and application of a chiral ionic liquid functionalized b-cyclodextrin as a chiral selector in capillary electrophoresis. Biomed. Chromatogr. 2013, 27, 1027–1033. [Google Scholar]
- Boffa, L.; Calcio Gaudino, E.; Martina, K.; Jicsinszky, L.; Cravotto, G. A new class of cationic cyclodextrins: Synthesis and chemico-physical properties. New J. Chem. 2010, 34, 2013–2019. [Google Scholar] [CrossRef]
- Fejos, I.; Kalydi, E.; Malanga, M.; Benkovics, G.; Béni, S. Single isomer cyclodextrins as chiral selectors in capillary electrophoresis. J. Chromat. A 2020, 1627, 461375. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.A.S.; Anconi, C.P.A.; Dos Santos, H.F.; De Almeida, W.B.; Nascimento, C.S. Inclusion process of tetracycline in β and γ-cyclodextrins: A theoretical investigation. Chem. Phys. Lett. 2015, 626, 80–84. [Google Scholar] [CrossRef]
- Lindner, K.; Saenger, W. β-Cyclodextrin dodecahydrate: Accumulation of water molecules in a hydrophobic cavity. Angew. Chem. 1978, 90, 738–740. [Google Scholar] [CrossRef]
- Barca, G.M.J.; Bertoni, C.; Carrington, L.; Datta, D.; De Silva, N.; Deustua, J.E.; Fedorov, D.G.; Gour, J.R.; Gunina, A.O.; Guidez, E.; et al. Recent developments in the general atomic and molecular electronic structure system. J. Chem. Phys. 2020, 152, 154102. [Google Scholar] [CrossRef] [PubMed]
- Auneau, F.; Michel, C.; Delbecq, F.; Pinel, C.; Sautet, P. Unravelling the Mechanism of Glycerol Hydrogenolysis over Rhodium Catalyst through Combined Experimental–Theoretical Investigations. Chem. Eur. J. 2011, 17, 14288–14299. [Google Scholar] [CrossRef]
- Wang, Z.; He, Z.; Zhang, L.; Zhang, H.; Zhang, M.; Wen, X.; Quan, G.; Huang, X.; Pan, X.; Wu, C. Optimization of a doxycycline hydroxypropyl-β-cyclodextrin inclusion complex based on computational modeling. Acta Pharm. Sin. B 2013, 3, 130–139. [Google Scholar] [CrossRef][Green Version]
- Benghodbane, S.; Khatmi, D. A theoretical study on the inclusion complexation of doxycycline with Crysmeb. C. R. Chim. 2012, 15, 371–377. [Google Scholar] [CrossRef]
- Musolino, M.G.; Mauriello, F.; Busacca, C.; Pietropaolo, R. Aromatic Alcohols as Model Molecules for Studying Hydrogenolysis Reactions Promoted by Palladium Catalysts. Top Catal. 2015, 58, 1077–1084. [Google Scholar] [CrossRef]
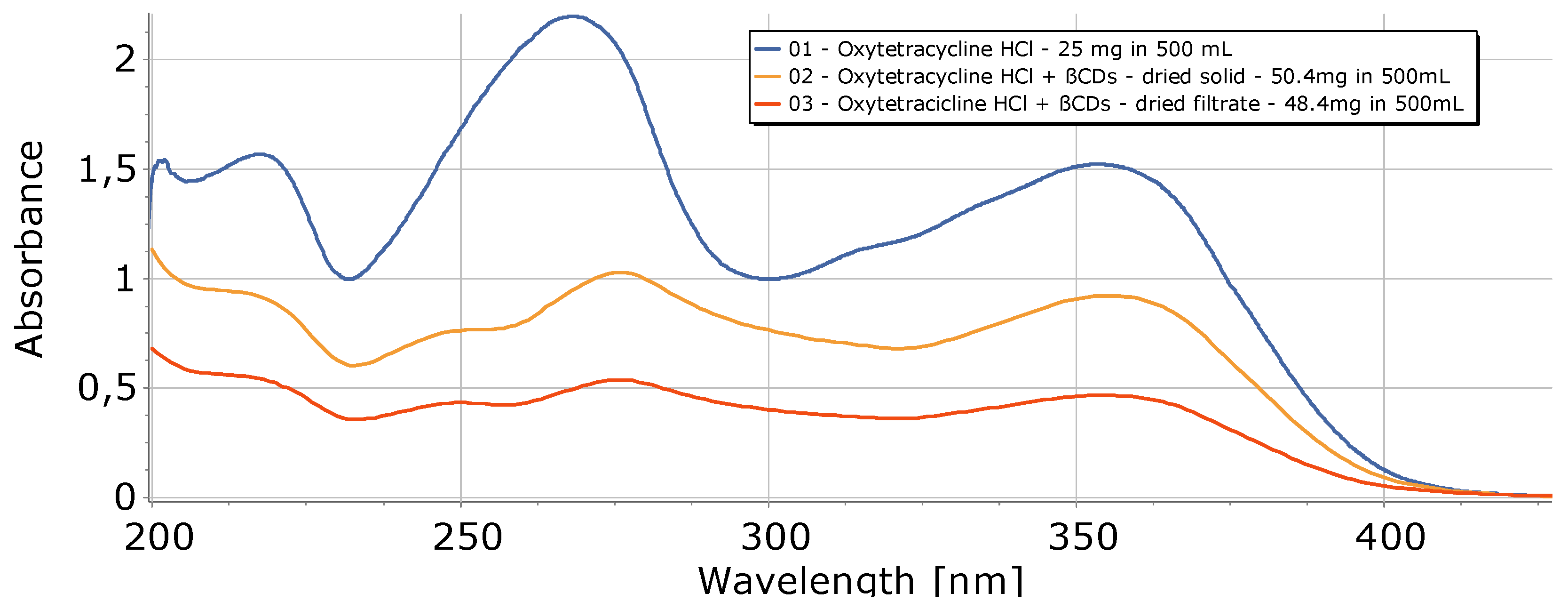
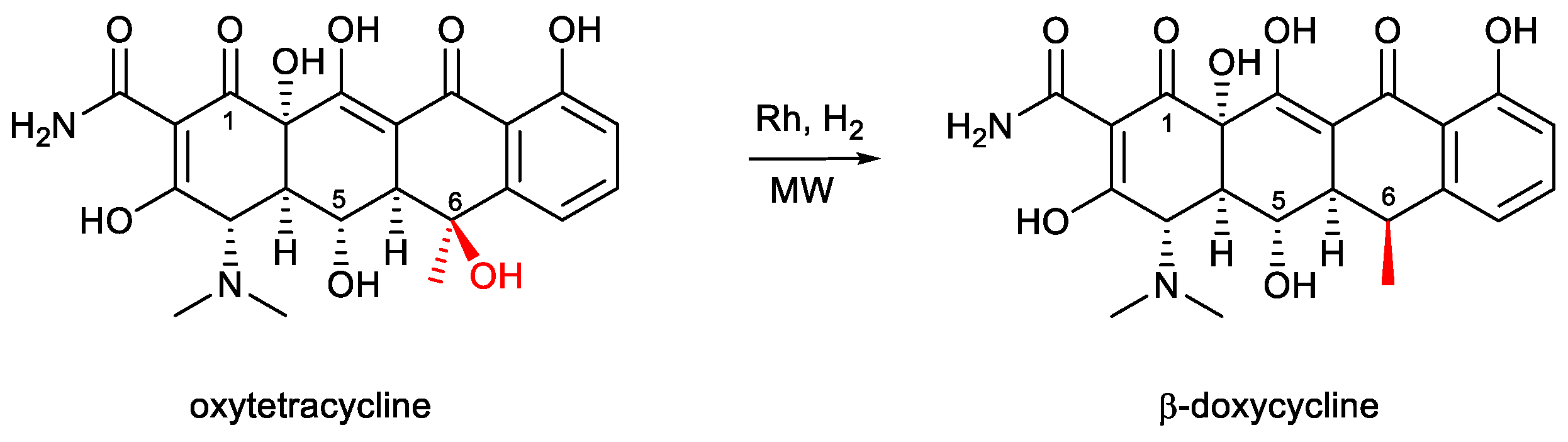
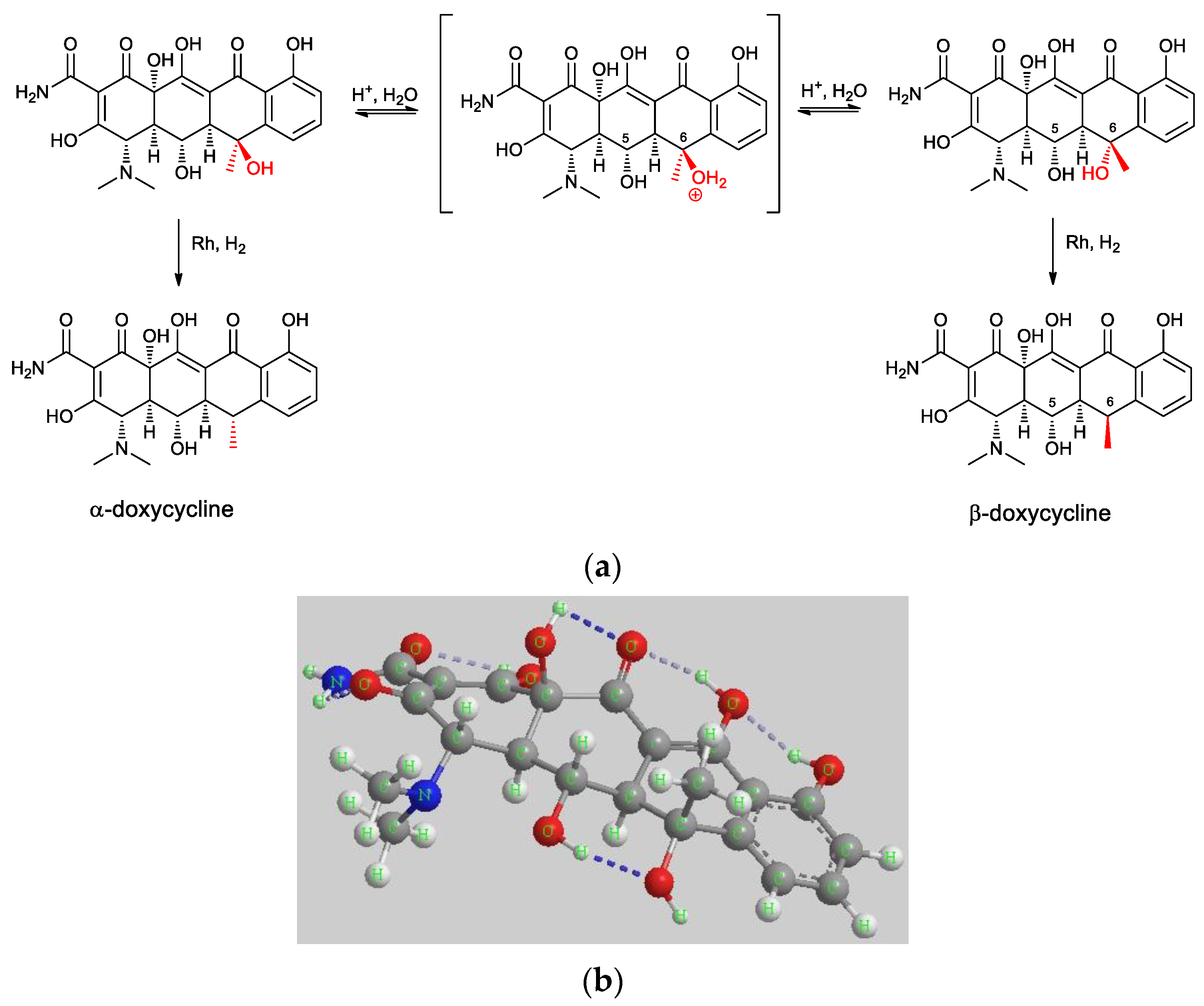

| Entry | Heating | Solvent | Catalyst | H2 (bar) | T (°C) | Time (h) | Yield β-Doxy (%) | Yield α-Doxy (%) |
|---|---|---|---|---|---|---|---|---|
| 1a | Conventional a | DMF/H2O | Rh/AC 5% | 5 | 50 | 16 | traces | n.d. |
| DMF/H2O | Rh/AC 5% | 5 | 70 | 16 | traces | n.d. | ||
| 2a | H2O | Rh/AC 5% | 10 | 50 | 16 | traces | n.d. | |
| 3a | MeOH | Rh/AC 5% | 10 | 50 | 16 | 3.00 | n.d. | |
| 4a | H2O | Rh/Al2O3 5% | 10 | 50 | 16 | 0.50 | n.d. | |
| 5a | MeOH | Rh/Al2O3 5% | 10 | 50 | 16 | traces | n.d. | |
| 6a | MW b | DMF/H2O | Rh/AC 5% | 5 | 50 | 4 | 1.20 | n.d. |
| 7a | DMF/H2O | Rh/AC 5% | 5 | 50 | 2 | 2.15 | n.d. | |
| 8a | DMF/H2O | Rh/AC 5% | 5 | 70 | 2 | 0.95 | n.d. | |
| 9a | H2O | Rh/AC 5% | 5 | 50 | 2 | traces | n.d. | |
| 10a | MeOH | Rh/AC 5% | 5 | 50 | 2 | 36.90 | n.d. | |
| 11a | H2O | Rh/Al2O3 5% | 5 | 50 | 2 | 34.20 | n.d. | |
| 12a | MeOH | Rh/Al2O3 5% | 5 | 50 | 2 | 1.10 | n.d. |
| Entry | Substrate | Conversion (%) | Yield α-Doxy (%) | Yield β-Doxy (%) |
|---|---|---|---|---|
| 1b | βCD solid a | 44.3 | 4.0 | 16.1 |
| 2b | βCD solid b | 60.0 | 14.1 | 32.2 |
| 3b | βCD filtrate b | 100 | 34.0 | n.d. |
| 4b | βCD in situ b | 100 | 19.1 | 36.2 |
| 5b | RAMEB b | 68.3 | n.d. | n.d. |
| 6b | γCD b | 100 | 26.2 | 14.3 |
| 7b | γCD in situ b | 100 | 5.2 | 6.7 |
 |  | |||||
| A− | Ai | A+ | B− | Bi | B+ | |
| βCD/OXY-HCl | −38.6 | −40.6 | −31.2 | −14.0 | −25.2 | −25.2 |
| RAMEB/OXY-HCl | −34.7 | −58.4 | −25.6 | −17.3 | −19.0 | −39.6 |
| βCD/Dehydrated-HCl | −23.6 | −20.2 | −20.0 | −13.2 | −32.4 | −22.8 |
| RAMEB/Dehydrated-HCl | −39.3 | −50.7 | −24.8 | −32.2 | −32.0 | −29.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bucciol, F.; Maffeis, E.; Calcio Gaudino, E.; Jicsinszky, L.; Tagliapietra, S.; Barge, A.; Prandi, C.; Cravotto, G. Microwave-Assisted, One-Pot Synthesis of Doxycycline under Heterogeneous Catalysis in Water. Antibiotics 2021, 10, 1084. https://doi.org/10.3390/antibiotics10091084
Bucciol F, Maffeis E, Calcio Gaudino E, Jicsinszky L, Tagliapietra S, Barge A, Prandi C, Cravotto G. Microwave-Assisted, One-Pot Synthesis of Doxycycline under Heterogeneous Catalysis in Water. Antibiotics. 2021; 10(9):1084. https://doi.org/10.3390/antibiotics10091084
Chicago/Turabian StyleBucciol, Fabio, Elia Maffeis, Emanuela Calcio Gaudino, László Jicsinszky, Silvia Tagliapietra, Alessandro Barge, Cristina Prandi, and Giancarlo Cravotto. 2021. "Microwave-Assisted, One-Pot Synthesis of Doxycycline under Heterogeneous Catalysis in Water" Antibiotics 10, no. 9: 1084. https://doi.org/10.3390/antibiotics10091084
APA StyleBucciol, F., Maffeis, E., Calcio Gaudino, E., Jicsinszky, L., Tagliapietra, S., Barge, A., Prandi, C., & Cravotto, G. (2021). Microwave-Assisted, One-Pot Synthesis of Doxycycline under Heterogeneous Catalysis in Water. Antibiotics, 10(9), 1084. https://doi.org/10.3390/antibiotics10091084











