A Qualitative and Comprehensive Analysis of Caries Susceptibility for Dental Fluorosis Patients
Abstract
1. Introduction
2. Dental Fluorosis Classification
3. Host Factor
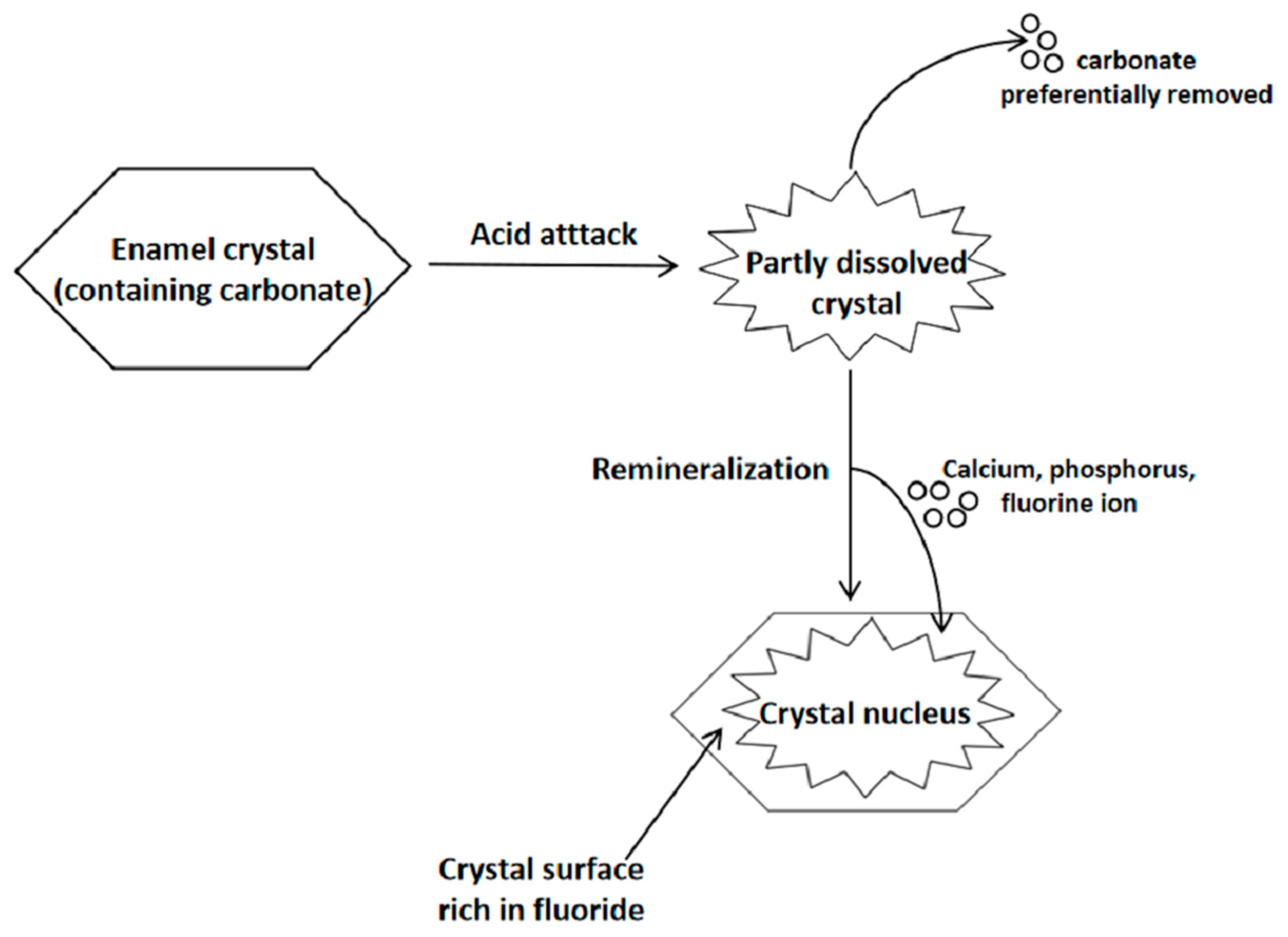
3.1. Dental Enamel
3.2. Saliva
4. Food Factor
5. Bacteria Factor
6. Post-Treatment Susceptibility
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fejerskov, O.; Manji, F.; Baelum, V. The nature and mechanisms of dental fluorosis in man. J. Dent. Res. 1990, 69, 692–700, discussion 721. [Google Scholar] [CrossRef] [PubMed]
- Bronckers, A.L.; Lyaruu, D.M.; DenBesten, P.K. The impact of fluoride on ameloblasts and the mechanisms of enamel fluorosis. J. Dent. Res. 2009, 88, 877–893. [Google Scholar] [CrossRef]
- Tenuta, L.M.; Cury, J.A. Fluoride: Its role in dentistry. Braz. Oral Res. 2010, 24 (Suppl. 1), 9–17. [Google Scholar] [CrossRef]
- Marin, L.M.; Cury, J.A.; Tenuta, L.M.; Castellanos, J.E.; Martignon, S. Higher Fluorosis Severity Makes Enamel Less Resistant to Demineralization. Caries Res. 2016, 50, 407–413. [Google Scholar] [CrossRef]
- Do, L.G.; Spencer, A.J.; Ha, D.H. Association between Dental Caries and Fluorosis among South Australian Children. Caries Res. 2009, 43, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Folayan, M.O.; El Tantawi, M.; Oginni, A.B.; Alade, M.; Adeniyi, A.; Finlayson, T.L. Malnutrition, enamel defects, and early childhood caries in preschool children in a sub-urban Nigeria population. PLoS ONE 2020, 15, e0232998. [Google Scholar] [CrossRef]
- Fejerskov, O.; Thylstrup, A.; Larsen, M.J. Clinical and structural features and possible pathogenic mechanisms of dental fluorosis. Scand J. Dent. Res. 1977, 85, 510–534. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, J.; Liu, H.; He, D.; Chen, L.; Wu, H.; Jiang, H.; Lu, Q.; Bai, S. Comparative Analysis of Gene Expression Profiles of Human Dental Fluorosis and Kashin-Beck Disease. Sci. Rep. 2018, 8, 170. [Google Scholar] [CrossRef]
- Ruan, J.P.; Wang, Z.L.; Yang, Z.Q.; Bardsen, A.; Astrom, A.N.; Bjorvatn, K. Dental fluorosis in primary teeth: A study in rural schoolchildren in Shaanxi Province, China. Int. J. Paediatr. Dent. 2005, 15, 412–419. [Google Scholar] [CrossRef]
- Warren, J.J.; Levy, S.M.; Kanellis, M.J. Prevalence of dental fluorosis in the primary dentition. J. Public Health Dent. 2001, 61, 87–91. [Google Scholar] [CrossRef]
- Jha, S.K.; Mishra, V.K.; Sharma, D.K.; Damodaran, T. Fluoride in the Environment and Its Metabolism in Humans. Rev. Environ. Contam. Toxicol. 2011, 211, 121–142. [Google Scholar]
- McGrady, M.G.; Ellwood, R.P.; Taylor, A.; Maguire, A.; Goodwin, M.; Boothman, N.; Pretty, I.A. Evaluating the use of fluorescent imaging for the quantification of dental fluorosis. BMC Oral Health 2012, 12, 1–8. [Google Scholar] [CrossRef]
- Sapov, K.; Gedalia, I.; Grobler, S.; Lewinstein, I.; Roman, I.; Shapira, L.; Hirschfeld, Z.; Teotia, S. A laboratory assessment of enamel hypoplasia of teeth with varying severities of dental fluorosis. J. Oral Rehabil. 1999, 26, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Buzalaf, M.A.R.; Pessan, J.P.; Honorio, H.M.; Ten Cate, J.M. Mechanisms of action of fluoride for caries control. Monogr. Oral Sci. 2011, 22, 97–114. [Google Scholar] [PubMed]
- Saldarriaga, A.; Rojas-Gualdron, D.F.; Restrepo, M.; Bussaneli, D.G.; Fragelli, C.; de Cassia Loiola Cordeiro, R.; Santos-Pinto, L.; Jeremias, F. Clinical changes in the severity of dental fluorosis: A longitudinal evaluation. BMC Oral Health 2021, 21, 366. [Google Scholar] [CrossRef]
- McGrady, M.G.; Ellwood, R.P.; Srisilapanan, P.; Korwanich, N.; Worthington, H.V.; Pretty, I.A. Dental fluorosis in populations from Chiang Mai, Thailand with different fluoride exposures—Paper 1: Assessing fluorosis risk, predictors of fluorosis and the potential role of food preparation. BMC Oral Health 2012, 12, 16. [Google Scholar] [CrossRef]
- Askar, H.; Krois, J.; Rohrer, C.; Mertens, S.; Elhennawy, K.; Ottolenghi, L.; Mazur, M.; Paris, S.; Schwendicke, F. Detecting white spot lesions on dental photography using deep learning: A pilot study. J. Dent. 2021, 107, 103615. [Google Scholar] [CrossRef]
- Richards, A.; Fejerskov, O.; Baelum, V. Enamel fluoride in relation to severity of human dental fluorosis. Adv. Dent. Res. 1989, 3, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Mier, E.A.; Shone, D.B.; Buckley, C.M.; Ando, M.; Lippert, F.; Soto-Rojas, A.E. Relationship between enamel fluorosis severity and fluoride content. J. Dent. 2016, 46, 42–46. [Google Scholar] [CrossRef]
- Retief, D.H.; Bradley, E.L.; Barbakow, F.H.; Friedman, M.; Vandermerwe, E.H.M.; Bischoff, J.I. Relationships among fluoride concentration in enamel, degree of fluorosis and caries incidence in a community residing in a high fluoride area. J. Oral Pathol. Med. 1979, 8, 224–236. [Google Scholar] [CrossRef]
- Brudevold, F.; Bakhos, Y.; Aasenden, R. Dental fluorosis as related to concentration of fluoride in teeth and bone. J. Am. Dent. Assoc. 1978, 96, 459–463. [Google Scholar] [CrossRef]
- Richards, A.; Likimani, S.; Baelum, V.; Fejerskov, O. Fluoride Concentrations in Unerupted Fluorotic Human Enamel. Caries Res. 1992, 26, 328–332. [Google Scholar] [CrossRef]
- Vieira, A.P.G.F.; Hancock, R.; Dumitriu, M.; Limeback, H.; Grynpas, M.D. Fluoride’s effect on human dentin ultrasound velocity (elastic modulus) and tubule size. Eur. J. Oral Sci. 2006, 114, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, S.; Saha, D. The genetic influence in fluorosis. Environ. Toxicol. Pharmacol. 2017, 56, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Richards, A.; Coote, G.E.; Pearce, E.I. Proton probe and acid etching for determining fluoride profiles in porous porcine enamel. J. Dent. Res. 1994, 73, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.J.; Fejerskov, O. Structural studies on calcium fluoride formation and uptake of fluoride in surface enamel in vitro. Scand. J. Dent. Res. 1978, 86, 337–345. [Google Scholar] [CrossRef]
- Fejerskov, O.; Larsen, M.J.; Richards, A.; Baelum, V. Dental tissue effects of fluoride. Adv. Dent. Res. 1994, 8, 15–31. [Google Scholar] [CrossRef]
- Featherstone, J.D.B. Prevention and reversal of dental caries: Role of low level fluoride. Community Dent. Oral 1999, 27, 31–40. [Google Scholar] [CrossRef]
- Hellwig, E.; Lennon, A.M. Systemic versus topical fluoride. Caries Res. 2004, 38, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Christoffersen, J. Nature and role of loosely bound fluoride in dental caries. J. Dent. Res. 1990, 69, 601–605, discussion 634–606. [Google Scholar] [CrossRef]
- Ogaard, B.; Rolla, G.; Ruben, J.; Dijkman, T.; Arends, J. Microradiographic study of demineralization of shark enamel in a human caries model. Scand. J. Dent. Res. 1988, 96, 209–211. [Google Scholar] [PubMed]
- Alhawij, H.; Lippert, F.; Martinez-Mier, E.A. Relative fluoride response of caries lesions created in fluorotic and sound teeth studied under remineralizing conditions. J. Dent. 2015, 43, 103–109. [Google Scholar] [CrossRef][Green Version]
- Fan, H.; Gao, S.; Liu, Y.; Zhu, Z.; Yu, H. The micromechanical and tribological feature of mild mottled enamel. J. Mech. Med. Biol. 2014, 14, 1450050. [Google Scholar] [CrossRef]
- Suma, R.; Shashibhushan, K.K.; Shashikiran, N.D.; Reddy, V.V.S. Progression of Artificial Caries in Fluorotic and Nonfluorotic Enamel. An in vitro Study. J. Clin. Pediatr. Dent. 2008, 33, 127–130. [Google Scholar] [PubMed]
- Duarte, M.B.S.; Carvalho, V.R.; Hilgert, L.A.; Ribeiro, A.P.D.; Leal, S.C.; Takeshita, E.M. Is there an association between dental caries, fluorosis, and molar-incisor hypomineralization? J. Appl. oral Sci. Rev. FOB 2021, 29, e20200890. [Google Scholar] [CrossRef]
- Fejerskov, O.; Silverstone, L.M.; Melsen, B.; Moller, I.J. Histological features of fluorosed human dental enamel. Caries Res. 1975, 9, 190–210. [Google Scholar] [CrossRef]
- Gal, I.; Parkinson, C.; Craft, I. Hypomineralization and increased porosity in dental fluorosis. Nutr. Rev. 1979, 37, 348–350. [Google Scholar]
- Thylstrup, A.; Fejerskov, O. Clinical appearance of dental fluorosis in permanent teeth in relation to histologic changes. Community Dent. Oral 1978, 6, 315–328. [Google Scholar] [CrossRef]
- Hu, D.; Gong, J.; He, B.; Chen, Z.; Li, M. Surface properties and Streptococcus mutans—Streptococcus sanguinis adhesion of fluorotic enamel. Arch. Oral Biol. 2021, 121, 104970. [Google Scholar] [CrossRef] [PubMed]
- Fejerskov, O.; Yanagisawa, T.; Tohda, H.; Larsen, M.J.; Josephsen, K.; Mosha, H.J. Posteruptive changes in human dental fluorosis—A histological and ultrastructural study. Proc. Finn. Dent. Soc. Suom. Hammaslaak. Toim. 1991, 87, 607–619. [Google Scholar]
- Groeneveld, A.; Van Eck, A.A.; Backer Dirks, O. Fluoride in caries prevention: Is the effect pre- or post-eruptive? J. Dent. Res. 1990, 69, 751–755, discussion 820–753. [Google Scholar] [CrossRef]
- Shinoda, H.; Ogura, H. Scanning electron microscopical study on fluorosis of enamel in rats. Calcif. Tissue Res. 1978, 25, 75–83. [Google Scholar] [CrossRef]
- Wright, J.T.; Chen, S.C.; Hall, K.I.; Yamauchi, M.; Bawden, J.W. Protein characterization of fluorosed human enamel. J. Dent. Res. 1996, 75, 1936–1941. [Google Scholar] [CrossRef]
- Giambro, N.J.; Prostak, K.; Denbesten, P.K. Characterization of fluorosed human enamel by color reflectance, ultrastructure, and elemental composition. Caries Res. 1995, 29, 251–257. [Google Scholar] [CrossRef]
- Mangum, J.E.; Crombie, F.A.; Kilpatrick, N.; Manton, D.J.; Hubbard, M.J. Surface Integrity Governs the Proteome of Hypomineralized Enamel. J. Dent. Res. 2010, 89, 1160–1165. [Google Scholar] [CrossRef]
- Robinson, C.; Connell, S.; Kirkham, J.; Brookes, S.J.; Shore, R.C.; Smith, A.M. The effect of fluoride on the developing tooth. Caries Res. 2004, 38, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Kis, V.K.; Sulyok, A.; Hegedus, M.; Kovacs, I.; Rozsa, N.; Kovacs, Z. Magnesium incorporation into primary dental enamel and its effect on mechanical properties. Acta Biomater. 2021, 120, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Gordon, L.M.; Joester, D. Mapping residual organics and carbonate at grain boundaries and the amorphous interphase in mouse incisor enamel. Front. Physiol. 2015, 6, 57. [Google Scholar] [CrossRef] [PubMed]
- Featherstone, J.D.B.; Nelson, D.G.A.; McLean, J.D. An electron-microscope study of modifications to defect regions in dental enamel and synthetic apatites. Caries Res. 1981, 15, 278–288. [Google Scholar] [CrossRef]
- Zavala-Alonso, V.; Loyola-Rodriguez, J.P.; Terrones, H.; Patino-Marin, N.; Martinez-Castanon, G.A.; Anusavice, K. Analysis of the molecular structure of human enamel with fluorosis using micro-Raman spectroscopy. J. Oral Sci. 2012, 54, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Leverett, D.H.; Featherstone, J.D.B.; Proskin, H.M.; Adair, S.M.; Eisenberg, A.D.; Mundorffshrestha, S.A.; Shields, C.P.; Shaffer, C.L.; Billings, R.J. Caries risk assessment by a cross-sectional discrimination model. J. Dent. Res. 1993, 72, 529–537. [Google Scholar] [CrossRef]
- Yao, K.; Gron, P. Fluoride concentration in duct saliva and whole saliva. Caries Res. 1970, 4, 321–331. [Google Scholar] [CrossRef]
- Machiulskiene, V.; Baelum, V.; Fejerskov, O.; Nyvad, B. Prevalence and extent of dental caries, dental fluorosis, and developmental enamel defects in Lithuanian teenage populations with different fluoride exposures. Eur. J. Oral Sci. 2009, 117, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Boros, I.; Mozsik, G.; Keszler, P. Effect of F- on major salivary-glands—The amylase activity, stimulated salivary flow response and cAMP levels in parotid-gland of rats consuming F- via drinking water. Fluoride 1984, 17, 217–223. [Google Scholar]
- Martins-Gomes, A.M.; Nicolau, J.; de Souza, D.N.; Oliveira, E. A study of some parameters in stimulated saliva from adolescents with dental fluorosis. J. Oral Sci. 2001, 43, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Guzel, K.G.U.; Kirzioglu, Z.; Adiloglu, A.K.; Erturk, M.S.O. Effect of fluoride on salivary immunoglobulins and sialic acid. Rev. Assoc. Med. Bras. 2017, 63, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Edgar, W.M. Saliva—Its secretion, composition and functions. Br. Dent. J. 1992, 172, 305–312. [Google Scholar] [CrossRef]
- Bardow, A.; Moe, D.; Nyvad, B.; Nauntofte, B. The buffer capacity and buffer systems of human whole saliva measured without loss of CO2. Arch. Oral Biol. 2000, 45, 1–12. [Google Scholar] [CrossRef]
- Proctor, G.B. The physiology of salivary secretion. Periodontology 2000 2016, 70, 11–25. [Google Scholar] [CrossRef]
- Hector, M.P.; Linden, R.W.A. The possible role of periodontal mechanoreceptors in the control of parotid secretion in man. Q. J. Exp. Physiol. Cogn. Med. Sci. 1987, 72, 285–301. [Google Scholar] [CrossRef]
- Lund, J.P. Mastication and its control by the brain stem. Critical reviews in oral biology and medicine. Off. Publ. Am. Assoc. Oral Biol. 1991, 2, 33–64. [Google Scholar]
- Aoba, T.; Fejerskov, O. Dental fluorosis: Chemistry and biology. Crit. Rev. Oral Biol. Med. 2002, 13, 155–170. [Google Scholar] [CrossRef]
- Flueck, W.T.; Smith-Flueck, J.A.M. Severe dental fluorosis in juvenile deer linked to a recent volcanic eruption in Patagonia. J. Wildl. Dis. 2013, 49, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Sheetal, A.; Hiremath, V.K.; Patil, A.G.; Sajjansetty, S.; Kumar, S.R. Malnutrition and its oral outcome—A review. J. Clin. Diagn. Res. JCDR 2013, 7, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Browne, D.; Whelton, H.; O’Mullane, D.; Tavener, J.; Flannery, E. The aesthetic impact of enamel fluorosis on Irish adolescents. Community Dent. Oral 2011, 39, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Lagerlof, F.; Oliveby, A. Caries-protective factors in saliva. Adv. Dent. Res. 1994, 8, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.M.L.; Sorensen, C.E.; Proctor, G.B.; Carpenter, G.H. Salivary functions in mastication, taste and textural perception, swallowing and initial digestion. Oral Dis. 2018, 24, 1399–1416. [Google Scholar] [CrossRef]
- Scott, J.; Flower, E.A.; Burns, J. A quantitative study of histological-changes in the human-parotid gland occurring with adult age. J. Oral Pathol. Med. 1987, 16, 505–510. [Google Scholar] [CrossRef]
- Affoo, R.H.; Foley, N.; Garrick, R.; Siqueira, W.L.; Martin, R.E. Meta-Analysis of Salivary Flow Rates in Young and Older Adults. J. Am. Geriatr. Soc. 2015, 63, 2142–2151. [Google Scholar] [CrossRef]
- Yeh, C.K.; Johnson, D.A.; Dodds, M.W.J. Impact of aging on human salivary gland function: A community-based study. Aging-Clin. Exp. Res. 1998, 10, 421–428. [Google Scholar] [CrossRef]
- Xu, F.; Laguna, L.; Sarkar, A. Aging-related changes in quantity and quality of saliva: Where do we stand in our understanding? J. Texture Stud. 2019, 50, 27–35. [Google Scholar] [CrossRef]
- Nassar, M.; Hiraishi, N.; Islam, M.S.; Otsuki, M.; Tagami, J. Age-related changes in salivary biomarkers. J. Dent. Sci. 2014, 9, 85–90. [Google Scholar] [CrossRef]
- Nagler, R.M.; Hershkovich, O. Relationships between age, drugs, oral sensorial complaints and salivary profile. Arch. Oral Biol. 2005, 50, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Godoy, F.; Hicks, M.J. Maintaining the integrity of the enamel surface—The role of dental biofilm, saliva and preventive agents in enamel demineralization and remineralization. J. Am. Dent. Assoc. 2008, 139, 25S–34S. [Google Scholar] [PubMed]
- Solomon, S.M.; Bataiosu, M.; Popescu, D.M.; Rauten, A.M.; Gheorghe, D.N.; Petrescu, R.A.; Maftei, G.A.; Maglaviceanu, C.F. Biochemical Assesment of Salivary Parameters in Young Patients with Dental Lesions. Rev. De Chim. 2019, 70, 4095–4097. [Google Scholar] [CrossRef]
- Nagler, R.M. Salivary glands and the aging process: Mechanistic aspects, health-status and medicinal-efficacy monitoring. Biogerontology 2004, 5, 223–233. [Google Scholar] [CrossRef]
- Levine, M.J.; Herzberg, M.C.; Levine, M.S.; Ellison, S.A.; Stinson, M.W.; Li, H.C.; Vandyke, T. Specificity of salivary-bacterial interactions—Role of terminal sialic-acid residues in interaction of salivary glycoproteins with Streptococcus-sanguis and Streptococcus-mutans. Infect. Immun. 1978, 19, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Nicolae, V.; Neamtu, B.; Picu, O.; Stefanache, M.A.M.; Cioranu, V.S.I. The Comparative Evaluation of Salivary Biomarkers (Calcium, Phosphate, Salivary pH) in Cario-resistance Versus Cario-activity. Rev. De Chim. 2016, 67, 821–824. [Google Scholar]
- Chaudhury, N.M.A.; Proctor, G.B.; Karlsson, N.G.; Carpenter, G.H.; Flowers, S.A. Reduced Mucin-7 (Muc7) Sialylation and Altered Saliva Rheology in Sjogren’s Syndrome Associated Oral Dryness. Mol. Cell. Proteom. 2016, 15, 1048–1059. [Google Scholar] [CrossRef]
- Gao, X.; Jiang, S.; Koh, D.; Hsu, C.-Y.S. Salivary biomarkers for dental caries. Periodontology 2016, 70, 128–141. [Google Scholar] [CrossRef]
- Dodds, M.W.J.; Johnson, D.A.; Yeh, C.K. Health benefits of saliva: A review. J. Dent. 2005, 33, 223–233. [Google Scholar] [CrossRef]
- Farooqi, A.; Masuda, H.; Firdous, N. Toxic fluoride and arsenic contaminated groundwater in the Lahore and Kasur districts, Punjab, Pakistan and possible contaminant sources. Environ. Pollut. 2007, 145, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Zhao, Y.; Liu, J.W.; Bai, X.X.; Zhou, D.Y.; Fang, S.L.; Jia, M.; Wu, J.S. Fluorine intake of a Tibetan population. Food Chem. Toxicol. 1996, 34, 755–757. [Google Scholar] [CrossRef]
- Dai, S.; Li, W.; Tang, Y.; Zhang, Y.; Feng, P. The sources, pathway, and preventive measures for fluorosis in Zhijin County, Guizhou, China. Appl. Geochem. 2007, 22, 1017–1024. [Google Scholar] [CrossRef]
- Finkelman, R.B.; Belkin, H.E.; Zheng, B.S. Health impacts of domestic coal use in China. Proc. Natl. Acad. Sci. USA 1999, 96, 3427–3431. [Google Scholar] [CrossRef]
- Lima, C.V.; Tenuta, L.M.A.; Cury, J.A. Fluoride Increase in Saliva and Dental Biofilm due to a Meal Prepared with Fluoridated Water or Salt: A Crossover Clinical Study. Caries Res. 2019, 53, 41–48. [Google Scholar] [CrossRef]
- Hedman, J.; Sjoman, R.; Sjostrom, I.; Twetman, S. Fluoride concentration in saliva after consumption of a dinner meal prepared with fluoridated salt. Caries Res. 2006, 40, 158–162. [Google Scholar] [CrossRef]
- Oliveby, A.; Lagerlof, F.; Ekstrand, J.; Dawes, C. Studies on fluoride excretion in human whole saliva and its relation to flow-rate and plasma fluoride level. Caries Res. 1989, 23, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Luo, K.-L.; Tang, Y.-G.; Liu, Y.-L. The daily fluorine and arsenic intake for residents with different dietaries and fluorosis risk in coal-burning fluorosis area, Yunnan, Southwest China. Environ. Sci. Pollut. Res. 2015, 22, 2031–2040. [Google Scholar] [CrossRef] [PubMed]
- Oliveby, A.; Ekstrand, J.; Lagerlof, F. Effect of salivary flow-rate on salivary fluoride clearance after use of a fluoride-containing chewing gum. Caries Res. 1987, 21, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Yu, P.; Xu, Z.; Li, Z.; Zhang, Q.; Yu, H.; Gao, S. Investigation on the Gradient Nanomechanical Behavior of Dental Fluorosis Enamel. Nanoscale Res. Lett. 2018, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Michel-Crosato, E.; Biazevic, M.G.H.; Crosato, E. Relationship between dental fluorosis and quality of life: A population based study. Braz. Oral Res. 2005, 19, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, X.; Hu, H.; Wei, X.; Wang, X.; Peng, Z.; Ma, R.; Zhao, Q.; Zhao, J.; Liu, J.; et al. Structural changes in the oral microbiome of the adolescent patients with moderate or severe dental fluorosis. Sci. Rep. 2021, 11, 1–9. [Google Scholar]
- Ten Cate, J.M. Fluorides in caries prevention and control: Empiricism or science. Caries Res. 2004, 38, 254–257. [Google Scholar] [CrossRef]
- Pal, K.C.; Mondal, N.K.; Chatterjee, S.; Ghosh, T.S.; Datta, J.K. Characterization of fluoride-tolerant halophilic Bacillus flexus NM25 (HQ875778) isolated from fluoride-affected soil in Birbhum District, West Bengal, India. Environ. Monit. Assess. 2014, 186, 699–709. [Google Scholar] [CrossRef]
- Eisenberg, A.D.; Mundorff, S.A.; Featherstone, J.D.B.; Leverett, D.H.; Adair, S.M.; Billings, R.J.; Proskin, H.M. Associations of microbiological factors and plaque index with caries prevalence and water fluoridation status. Oral Microbiol. Immunol. 1991, 6, 139–145. [Google Scholar] [CrossRef]
- Liao, Y.; Brandt, B.W.; Li, J.; Crielaard, W.; Van Loveren, C.; Deng, D.M. Fluoride resistance in Streptococcus mutans: A mini review. J. Oral Microbiol. 2017, 9, 1344509. [Google Scholar] [CrossRef]
- Khandelwal, V.; Nayak, U.A.; Nayak, P.A.; Ninawe, N. Aesthetic management of dental fluorosis. BMJ Case Rep. 2013, 2013. [Google Scholar] [CrossRef]
- Zotti, F.; Albertini, L.; Tomizioli, N.; Capocasale, G.; Albanese, M. Resin Infiltration in Dental Fluorosis Treatment-1-Year Follow-Up. Med.-Lith. 2021, 57, 22. [Google Scholar]
- Taraboanta, I.; Stoleriu, S.; Nica, I.; Georgescu, A.; Gamen, A.C.; Maftei, G.A.; Andrian, S. Roughness variation of a nanohybrid composite resin submitted to acid and abrasive challenges. Int. J. Med. Dent. 2020, 24, 182–187. [Google Scholar]
- Ilyinichna, K.N.; Igorevna, K.E.; Vakhtangovna, T.I.; Amurkhanovna, T.N. Change in the Mineral Composition of Mixed Saliva in Patients with Fluorosis, after Applying the Complex of Therapeutic and Prophylactic Measures. Asian J. Pharm. 2017, 11, S930–S932. [Google Scholar]
- Kebede, A.; Retta, N.; Abuye, C.; Whiting, S.J.; Kassaw, M.; Zeru, T.; Tessema, M.; Kjellevold, M. Dietary Fluoride Intake and Associated Skeletal and Dental Fluorosis in School Age Children in Rural Ethiopian Rift Valley. Int. J. Environ. Res. Public Health 2016, 13, 756. [Google Scholar] [CrossRef] [PubMed]
- Almeida, L.F.; Marin, L.M.; Martinez-Mier, E.A.; Cury, J.A. Fluoride Dentifrice Overcomes the Lower Resistance of Fluorotic Enamel to Demineralization. Caries Res. 2019, 53, 567–575. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Shen, J.; Qin, T.; Zhou, G.; Li, Y.; Chen, Z.; Li, M. A Qualitative and Comprehensive Analysis of Caries Susceptibility for Dental Fluorosis Patients. Antibiotics 2021, 10, 1047. https://doi.org/10.3390/antibiotics10091047
Li Q, Shen J, Qin T, Zhou G, Li Y, Chen Z, Li M. A Qualitative and Comprehensive Analysis of Caries Susceptibility for Dental Fluorosis Patients. Antibiotics. 2021; 10(9):1047. https://doi.org/10.3390/antibiotics10091047
Chicago/Turabian StyleLi, Qianrui, Jiaqi Shen, Tao Qin, Ge Zhou, Yifeng Li, Zhu Chen, and Mingyun Li. 2021. "A Qualitative and Comprehensive Analysis of Caries Susceptibility for Dental Fluorosis Patients" Antibiotics 10, no. 9: 1047. https://doi.org/10.3390/antibiotics10091047
APA StyleLi, Q., Shen, J., Qin, T., Zhou, G., Li, Y., Chen, Z., & Li, M. (2021). A Qualitative and Comprehensive Analysis of Caries Susceptibility for Dental Fluorosis Patients. Antibiotics, 10(9), 1047. https://doi.org/10.3390/antibiotics10091047






