A Repeated State of Acidification Enhances the Anticariogenic Biofilm Activity of Glass Ionomer Cement Containing Fluoro-Zinc-Silicate Fillers
Abstract
:1. Introduction
2. Results
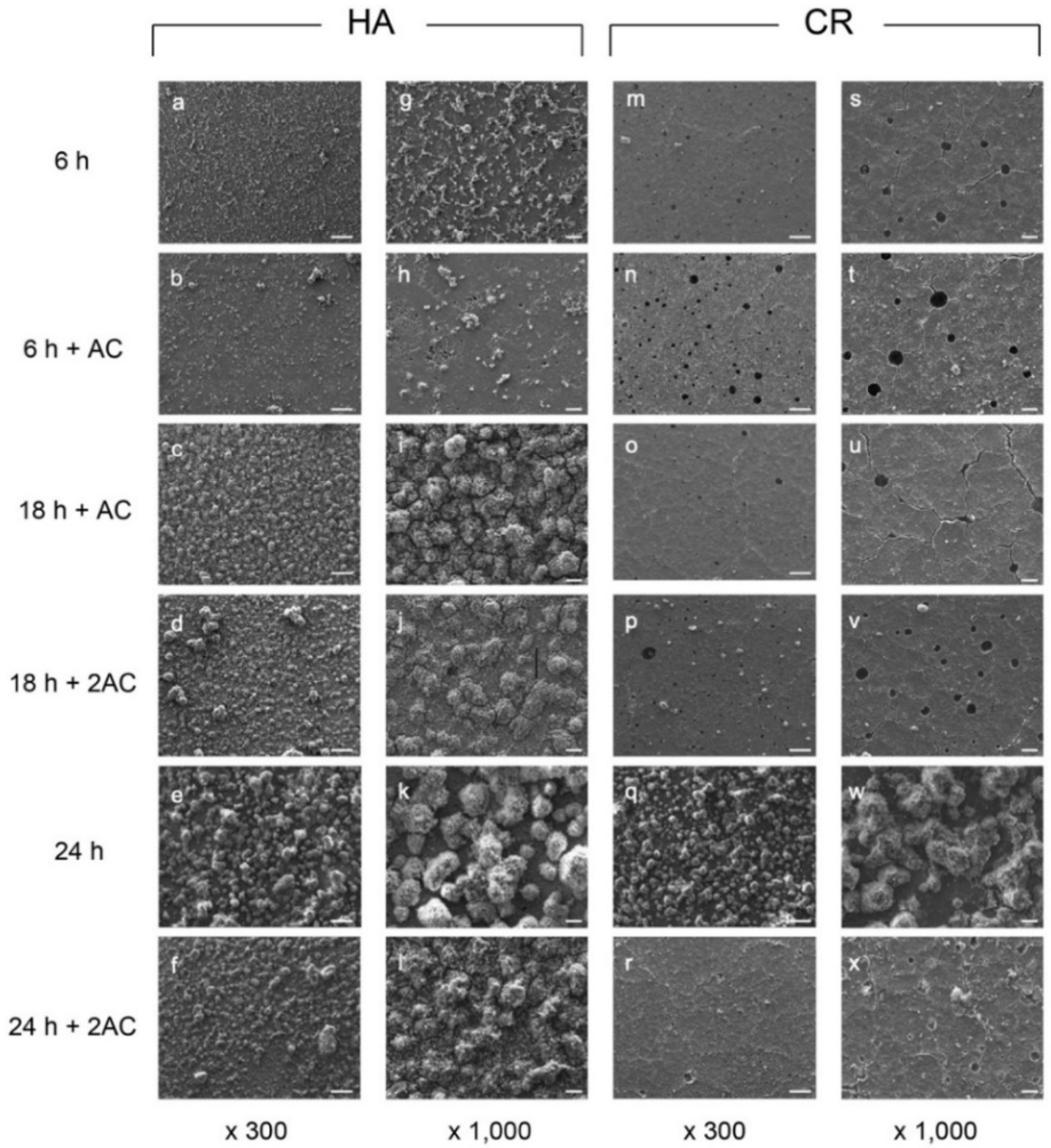
2.1. Scanning Electron Microscopy (SEM) Observations
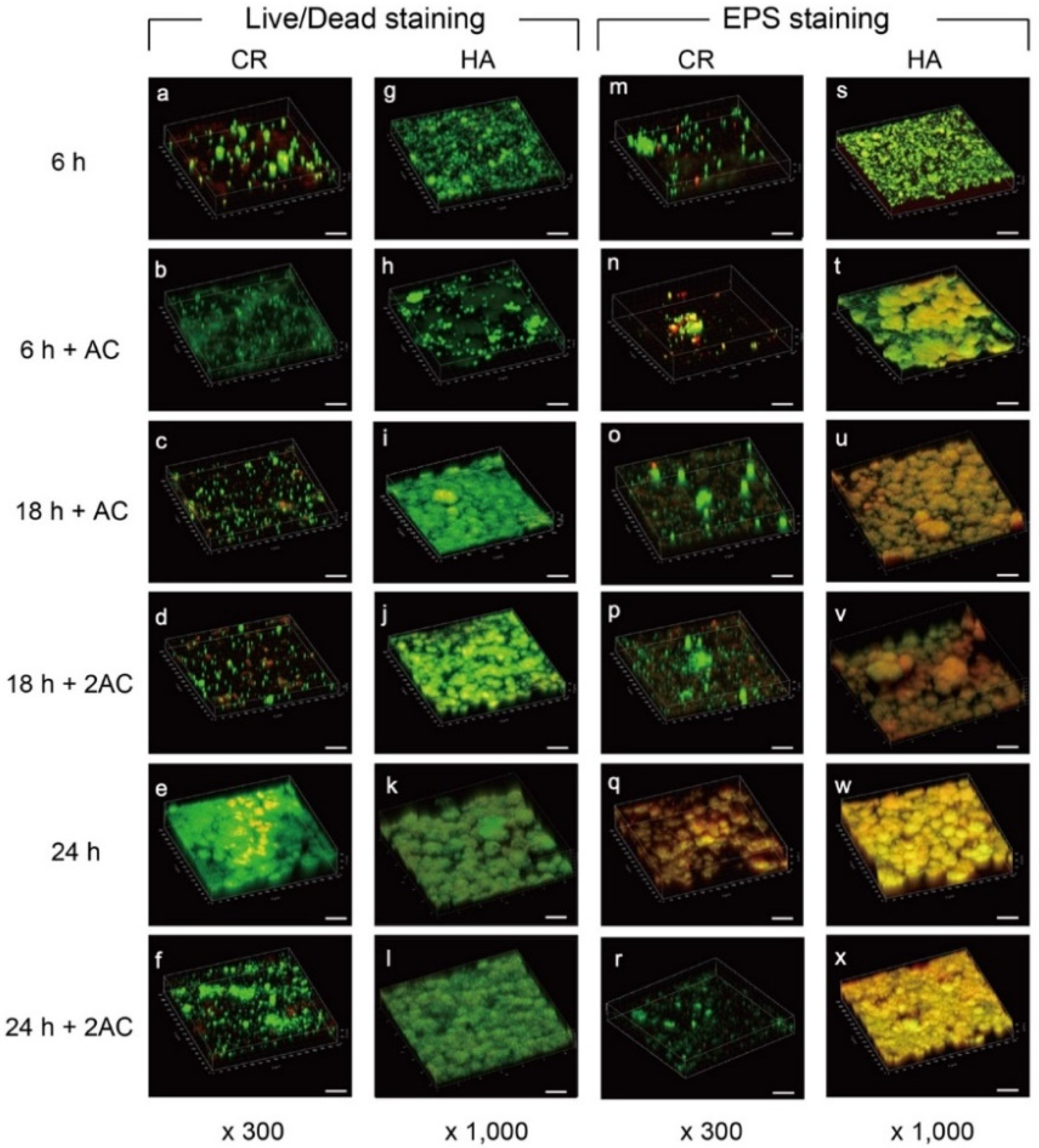
2.2. Confocal Laser Scanning Microscopy (CLSM) Observation
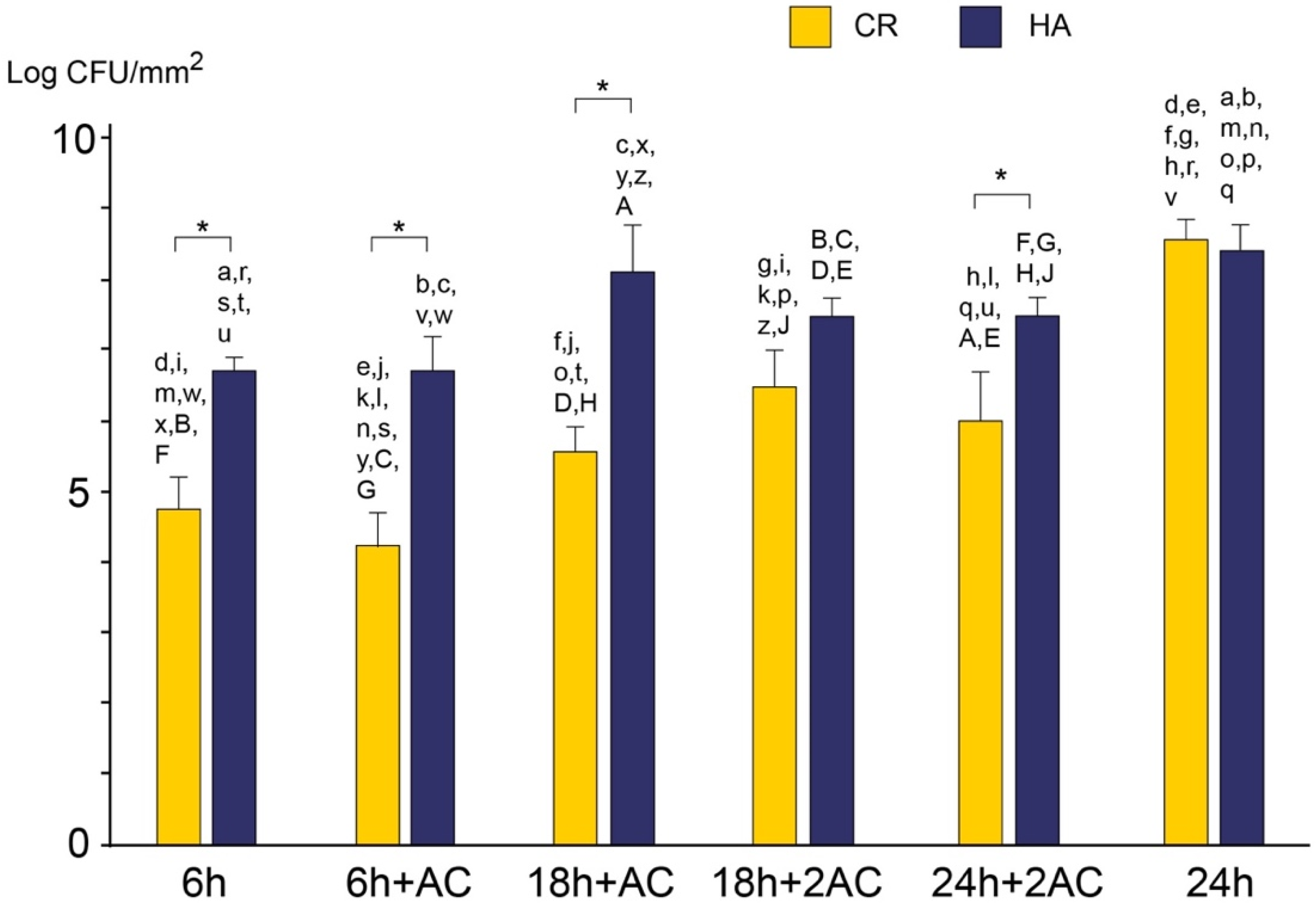
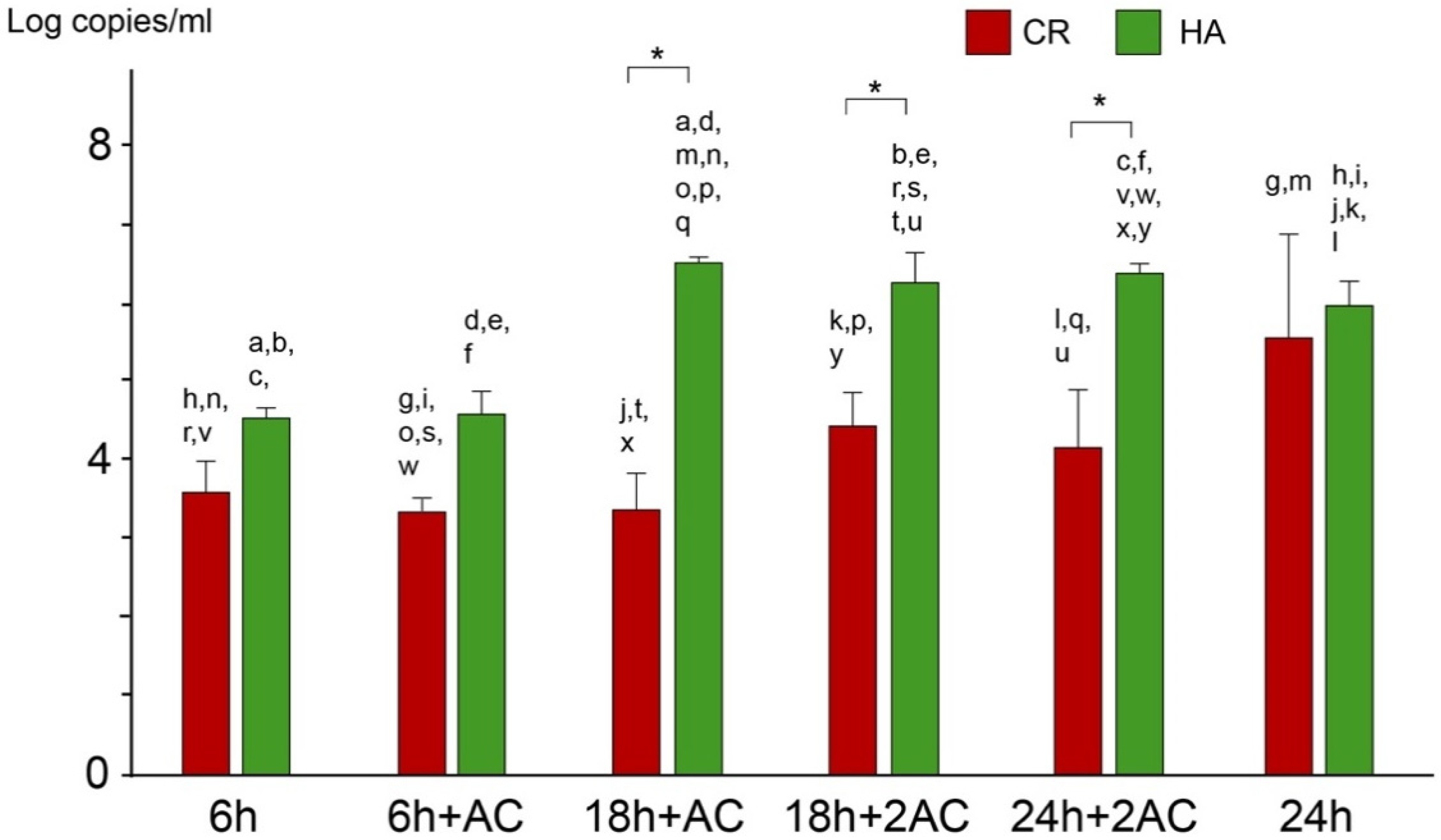
2.3. Viable and Total Cell Counts
3. Discussion
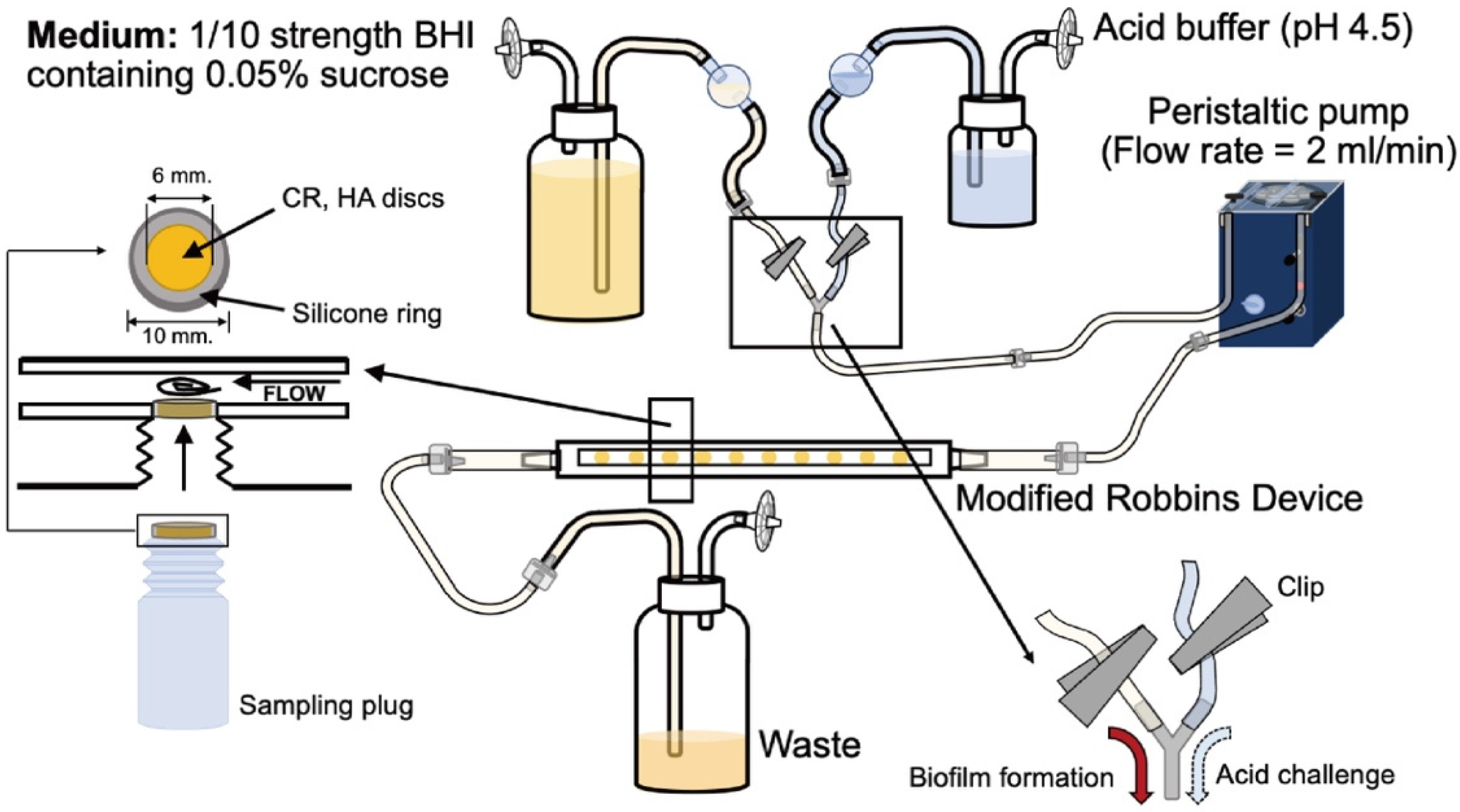
4. Materials and Methods
4.1. Specimen Preparation
4.2. Bacteria
4.3. Biofilm Formation and Acid Challenge
4.4. SEM Observation
4.5. CLSM Analysis
4.6. Viable Cell Counting
4.7. Total Cell Counting
4.8. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bowen, W.H.; Burne, R.A.; Wu, H.; Koo, H. Oral Biofilms: Pathogens, Matrix, and Polymicrobial Interactions in Microenvironments. Trends Microbiol. 2018, 26, 229–242. [Google Scholar] [CrossRef]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting Microbial Biofilms: Current and Prospective Therapeutic Strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef]
- Naha, P.C.; Liu, Y.; Hwang, G.; Huang, Y.; Gubara, S.; Jonnakuti, V.; Simon-Soro, A.; Kim, D.; Gao, L.; Koo, H.; et al. Dextran-Coated Iron Oxide Nanoparticles as Biomimetic Catalysts for Localized and pH-Activated Biofilm Disruption. ACS Nano 2019, 13, 4960–4971. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The Oral Microbiota: Dynamic Communities and Host Interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef]
- Takenaka, S.; Ohsumi, T.; Noiri, Y. Evidence-Based Strategy for Dental Biofilms: Current Evidence of Mouthwashes on Dental Biofilm and Gingivitis. Jpn. Dent. Sci. Rev. 2019, 55, 33–40. [Google Scholar] [CrossRef]
- Benelli, E.M.; Serra, M.C.; Rodrigues, A.L., Jr.; Cury, J.A. In Situ Anticariogenic Potential of Glass Ionomer Cement. Caries Res. 1993, 27, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Mickenautsch, S.; Mount, G.; Yengopal, V. Therapeutic Effect of Glass-Ionomers: An Overview of Evidence. Aust. Dent. J. 2011, 56, 10–15; quiz 103. [Google Scholar] [CrossRef] [PubMed]
- Nyvad, B.; Takahashi, N. Integrated Hypothesis of Dental Caries and Periodontal Diseases. J. Oral Microbiol. 2020, 12, 1710953. [Google Scholar] [CrossRef] [Green Version]
- Ten Cate, J.M.; Van Duinen, R.N. Hypermineralization of Dentinal Lesions Adjacent to Glass-Ionomer Cement Restorations. J. Dent. Res. 1995, 74, 1266–1271. [Google Scholar] [CrossRef]
- Wiegand, A.; Buchalla, W.; Attin, T. Review on Fluoride-Releasing Restorative Materials—Fluoride Release and Uptake Characteristics, Antibacterial Activity and Influence on Caries Formation. Dent. Mater. 2007, 23, 343–362. [Google Scholar] [CrossRef]
- Hamilton, I.R. Biochemical Effects of Fluoride on Oral Bacteria. J. Dent. Res. 1990, 69, 660–667; discussion 682. [Google Scholar] [CrossRef]
- Cury, J.A.; de Oliveira, B.H.; dos Santos, A.P.P.; Tenuta, L.M.A. Are Fluoride Releasing Dental Materials Clinically Effective on Caries Control? Dent. Mater. 2016, 32, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Weerheijm, K.L.; Kreulen, C.M.; De Soet, J.J.; Groen, H.J.; Van Amerongen, W.E. Bacterial Counts in Carious Dentine Under Restorations: 2-Year In Vivo Effects. Caries Res. 1999, 33, 130–134. [Google Scholar] [CrossRef]
- Najeeb, S.; Khurshid, Z.; Zafar, M.S.; Khan, A.S.; Zohaib, S.; Martí, J.M.; Sauro, S.; Matinlinna, J.P.; Rehman, I.U. Modifications in Glass Ionomer Cements: Nano-Sized Fillers and Bioactive Nanoceramics. Int. J. Mol. Sci. 2016, 17, 1134. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, T.; Takenaka, S.; Ohsumi, T.; Ida, T.; Ohshima, H.; Terao, Y.; Naksagoon, T.; Maeda, T.; Noiri, Y. Effect of a Novel Glass Ionomer Cement Containing Fluoro-Zinc-Silicate Fillers on Biofilm Formation and Dentin Ion Incorporation. Clin. Oral Investig. 2020, 24, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Naksagoon, T.; Ohsumi, T.; Takenaka, S.; Nagata, R.; Hasegawa, T.; Maeda, T.; Noiri, Y. Effect of Water Aging on the Anti-Biofilm Properties of Glass Ionomer Cement Containing Fluoro-Zinc-Silicate Fillers. Biofouling 2020, 36, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kohno, T.; Tsuboi, R.; Kitagawa, H.; Imazato, S. Acidity-Induced Release of Zinc Ion From BioUnionTM Filler and Its Inhibitory Effects Against Streptococcus mutans. Dent. Mater. J. 2020, 39, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.N.; Buckner, T.; Sheng, J.; Baldeck, J.D.; Marquis, R.E. Physiologic Actions of Zinc Related to Inhibition of Acid and Alkali Production by Oral Streptococci in Suspensions and Biofilms. Oral Microbiol. Immunol. 2004, 19, 31–38. [Google Scholar] [CrossRef]
- He, G.; Pearce, E.I.; Sissons, C.H. Inhibitory Effect of ZnCl(2) on Glycolysis in Human Oral Microbes. Arch. Oral Biol. 2002, 47, 117–129. [Google Scholar] [CrossRef]
- Koo, H.; Sheng, J.; Nguyen, P.T.; Marquis, R.E. Co-operative Inhibition by Fluoride and Zinc of Glucosyl Transferase Production and Polysaccharide Synthesis by mutans Streptococci in Suspension Culture and Biofilms. FEMS Microbiol. Lett. 2006, 254, 134–140. [Google Scholar] [CrossRef]
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental Caries. Nat. Rev. Dis. Primers 2017, 3, 17030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stephan, R.M.; Miller, B.F. A Quantitative Method for Evaluating Physical and Chemical Agents Which Modify Production of Acids in Bacterial Plaques on Human Teeth. J. Dent. Res. 1943, 22, 45–51. [Google Scholar] [CrossRef]
- Bowen, W.H. The Stephan Curve Revisited. Odontology 2013, 101, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Kohno, T.; Liu, Y.; Tsuboi, R.; Kitagawa, H.; Imazato, S. Evaluation of Ion Release and the Recharge Ability of Glass-Ionomer Cement Containing BioUnion Filler Using an In Vitro Saliva-Drop Setting Assembly. Dent. Mater. 2021, 37, 882–893. [Google Scholar] [CrossRef] [PubMed]
- Crusz, S.A.; Popat, R.; Rybtke, M.T.; Cámara, M.; Givskov, M.; Tolker-Nielsen, T.; Diggle, S.P.; Williams, P. Bursting the Bubble on Bacterial Biofilms: A Flow Cell Methodology. Biofouling 2012, 28, 835–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sternberg, C.; Bjarnsholt, T.; Shirtliff, M. Methods for Dynamic Investigations of Surface-Attached In Vitro Bacterial and Fungal Biofilms. Methods Mol. Biol. 2014, 1147, 3–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dashper, S.G.; Catmull, D.V.; Liu, S.-W.; Myroforidis, H.; Zalizniak, I.; Palamara, J.E.A.; Huq, N.L.; Reynolds, E.C. Casein Phosphopeptide-Amorphous Calcium Phosphate Reduces Streptococcus mutans Biofilm Development on Glass Ionomer Cement and Disrupts Established Biofilms. PLoS ONE 2016, 11, e0162322. [Google Scholar] [CrossRef]
- Hahnel, S.; Ionescu, A.C.; Cazzaniga, G.; Ottobelli, M.; Brambilla, E. Biofilm Formation and Release of Fluoride From Dental Restorative Materials in Relation to Their Surface Properties. J. Dent. 2017, 60, 14–24. [Google Scholar] [CrossRef]
- Hengtrakool, C.; Pearson, G.J.; Wilson, M. Interaction Between GIC and S. sanguis Biofilms: Antibacterial Properties and Changes of Surface Hardness. J. Dent. 2006, 34, 588–597. [Google Scholar] [CrossRef]
- Padovani, G.C.; Fúcio, S.B.; Ambrosano, G.M.; Correr-Sobrinho, L.; Puppin-Rontani, R.M. In Situ Bacterial Accumulation on Dental Restorative Materials. CLSM/COMSTAT Analysis. Am. J. Dent. 2015, 28, 3–8. [Google Scholar] [PubMed]
- Sousa, R.P.; Zanin, I.C.; Lima, J.P.; Vasconcelos, S.M.; Melo, M.A.; Beltrão, H.C.; Rodrigues, L.K. In Situ Effects of Restorative Materials on Dental Biofilm and Enamel Demineralisation. J. Dent. 2009, 37, 44–51. [Google Scholar] [CrossRef]
- Yip, H.K.; Guo, J.; Wong, W.H. Protection Offered by Root-Surface Restorative Materials Against Biofilm Challenge. J. Dent. Res. 2007, 86, 431–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiguel, M.H.; Tovo, M.F.; Kramer, P.F.; Franco, K.S.; Alves, K.M.; Delbem, A.C. Evaluation of Laser Fluorescence in the Monitoring of the Initial Stage of the De/Remineralization Process: An In Vitro and In Situ Study. Caries Res. 2009, 43, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Sedlacek, M.J.; Walker, C. Antibiotic resistance in an in vitro subgingival biofilm model. Oral Microbiol. Immunol. 2007, 22, 333–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Materials | Code | Lot No. | Composition |
|---|---|---|---|
| Caredyne Restore | CR | 1809061 | Powder: fluoro-alumino-silicate glass, fluoro-zinc-silicate glass, pigment Liquid: polyacrylic acid, distilled water, polybasic carboxylic acid |
| Hydroxyapatite | HA | 170915 | Hydroxyapatite |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naksagoon, T.; Takenaka, S.; Nagata, R.; Sotozono, M.; Ohsumi, T.; Ida, T.; Edanami, N.; Maeda, T.; Noiri, Y. A Repeated State of Acidification Enhances the Anticariogenic Biofilm Activity of Glass Ionomer Cement Containing Fluoro-Zinc-Silicate Fillers. Antibiotics 2021, 10, 977. https://doi.org/10.3390/antibiotics10080977
Naksagoon T, Takenaka S, Nagata R, Sotozono M, Ohsumi T, Ida T, Edanami N, Maeda T, Noiri Y. A Repeated State of Acidification Enhances the Anticariogenic Biofilm Activity of Glass Ionomer Cement Containing Fluoro-Zinc-Silicate Fillers. Antibiotics. 2021; 10(8):977. https://doi.org/10.3390/antibiotics10080977
Chicago/Turabian StyleNaksagoon, Traithawit, Shoji Takenaka, Ryoko Nagata, Maki Sotozono, Tatsuya Ohsumi, Takako Ida, Naoki Edanami, Takeyasu Maeda, and Yuichiro Noiri. 2021. "A Repeated State of Acidification Enhances the Anticariogenic Biofilm Activity of Glass Ionomer Cement Containing Fluoro-Zinc-Silicate Fillers" Antibiotics 10, no. 8: 977. https://doi.org/10.3390/antibiotics10080977
APA StyleNaksagoon, T., Takenaka, S., Nagata, R., Sotozono, M., Ohsumi, T., Ida, T., Edanami, N., Maeda, T., & Noiri, Y. (2021). A Repeated State of Acidification Enhances the Anticariogenic Biofilm Activity of Glass Ionomer Cement Containing Fluoro-Zinc-Silicate Fillers. Antibiotics, 10(8), 977. https://doi.org/10.3390/antibiotics10080977







