High Prevalence of Multidrug-Resistant Klebsiella pneumoniae in a Tertiary Care Hospital in Ethiopia
Abstract
1. Introduction
2. Results
2.1. Socio-Demographic and Clinical Characteristics
2.2. Antimicrobial Susceptibility Patterns of K. pneumoniae Isolates
2.3. Magnitude of Multidrug Resistance among K. pneumoniae Isolates
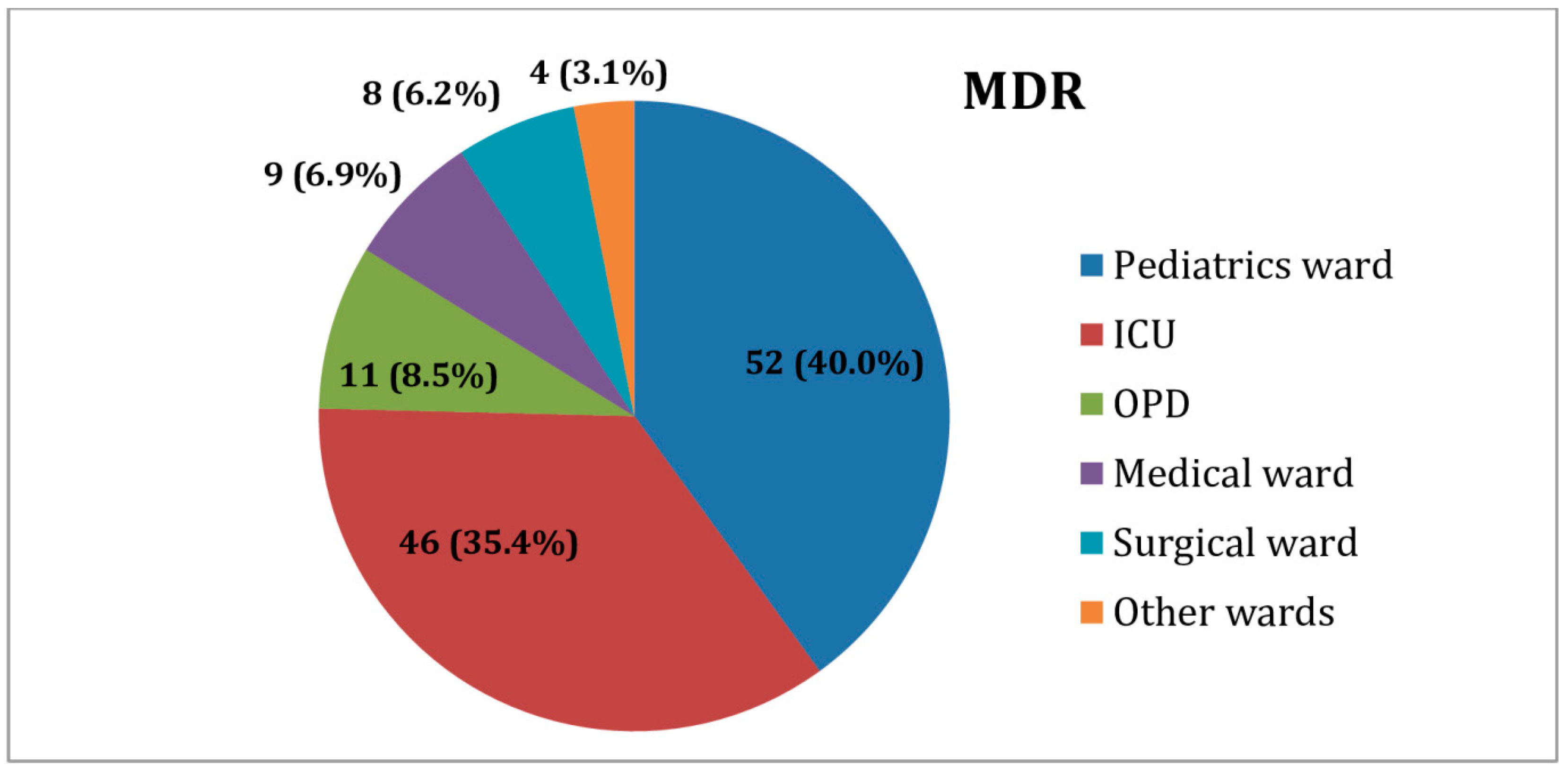
2.4. Distribution of MDR K. pneumoniae Isolates among Inpatient and Outpatient Wards
3. Discussion
3.1. Antimicrobial Resistance Patterns of K. pneumoniae Isolates
3.2. Multidrug Resistance among K. pneumoniae Isolates
4. Materials and Methods
4.1. Study Design
4.2. Bacterial Isolation and Identification
4.3. Antimicrobial Susceptibility Testing
4.4. Data Analysis
4.5. Ethics Approval and Consent to Participate
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Podschun, R.; Ullmann, U. Klebsiella spp. as Nosocomial Pathogens: Epidemiology, Taxonomy, Typing Methods, and Pathogenicity Factors. Clin. Microbiol. Rev. 1998, 11, 589–603. [Google Scholar] [CrossRef] [PubMed]
- De Jesus, M.B.; Ehlers, M.M.; Dos Santos, R.F.; Kock, M.M. Understanding β-lactamase producing Klebsiella pneumoniae. In Antimicrobial Resistance—An Open Challenge; Ossiprandi, M.C., Ed.; InTechOpen: Rijeka, Croatia, 2015; pp. 51–83. [Google Scholar]
- Navon-Venezia, S.; Kondratyeva, K.; Carattoli, A. Klebsiella pneumoniae: A major worldwide source and shuttle for antibiotic resistance. FEMS Microbiol. Rev. 2017, 41, 252–275. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; HM Government and Welcome Trust: London, UK, 2018; Available online: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (accessed on 17 August 2020).
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014; Available online: https://apps.who.int/iris/bitstream/handle/10665/112642/9789241564748_eng.pdf?sequence=1 (accessed on 5 May 2019).
- Holt, K.E.; Wertheim, H.; Zadoks, R.N.; Baker, S.; Whitehouse, C.A.; Dance, D.; Jenney, A.; Connor, T.R.; Hsu, L.Y.; Severin, J.; et al. Genomic analysis of diversity, population structure, virulence, and antimicrobial resistance in Klebsiella pneumoniae, an urgent threat to public health. Proc. Natl. Acad. Sci. USA 2015, 112, E3574–E3581. [Google Scholar] [CrossRef]
- Pendleton, J.N.; Gorman, S.P.; Gilmore, B.F. Clinical relevance of the ESKAPE pathogens. Expert Rev. Anti Infect. Ther. 2013, 11, 297–308. [Google Scholar] [CrossRef]
- Pistella, E.; Santini, C. Risk factors and outcomes of carbapenem-resistant Klebsiella pneumoniae infections. Ital. J. Med. 2016, 10, 339. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Antimicrobial resistance in the EU/EEA (EARS-Net)-Annual Epidemiological Report 2019; ECDC: Stockholm, Sweden, 2020; Available online: https://www.ecdc.europa.eu/sites/default/files/documents/surveillance-antimicrobial-resistance-Europe-2019.pdf (accessed on 12 January 2021).
- Ethiopian Public Health Institute 2020. Ethiopia Antimicrobial Resistance Surveillance. Annual Report (2nd Year) September 2018–October 2019. Available online: https://www.ephi.gov.et/images/files/Final-Annual-report---V.9.-Gebrie-1.pdf (accessed on 2 March 2021).
- Nirwati, H.; Sinanjung, K.; Fahrunissa, F.; Wijaya, F.; Napitupulu, S.; Hati, V.P.; Hakim, M.S.; Meliala, A.; Aman, A.T.; Nuryastuti, T. Biofilm formation and antibiotic resistance of Klebsiella pneumoniae isolated from clinical samples in a tertiary care hospital, Klaten, Indonesia. BMC Proc. 2019, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- da Silva, Y.; Ferrari, R.; Marin, V.A.; Junior, C.A. A Global Overview of β-lactam Resistance Genes in Klebsiella pneumoniae. Open Infect. Dis. J. 2019, 11, 22–34. [Google Scholar] [CrossRef]
- Muhie, O.A. Antibiotic use and resistance pattern in Ethiopia: Systematic review and meta-analysis. Int. J. Microbiol. 2019, 2019, 2489063. [Google Scholar] [CrossRef]
- Centres for Disease Control and Prevention. Antibiotic Resistance Threats in the United States. US Department of Health and Human Services, Centres for Disease Control and Prevention: Atlanta, GA, USA. 2019. Available online: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf (accessed on 21 September 2020).
- Teklu, D.S.; Negeri, A.A.; Legese, M.H.; Bedada, T.L.; Woldemariam, H.K.; Tullu, K.D. Extended-spectrum beta-lactamase production and multi-drug resistance among Enterobacteriaceae isolated in Addis Ababa, Ethiopia. Antimicrob. Resist. Infect. Control. 2019, 8, 39. [Google Scholar] [CrossRef]
- Moges, F.; Eshetie, S.; Abebe, W.; Mekonnen, F.; Dagnew, M.; Endale, A.; Amare, A.; Feleke, T.; Gizachew, M.; Tiruneh, M. High prevalence of extended-spectrum beta-lactamase-producing Gram-negative pathogens from patients attending Felege Hiwot Comprehensive Specialized Hospital, Bahir Dar, Amhara region. PLoS ONE 2019, 14, e0215177. [Google Scholar] [CrossRef]
- Badamchi, A.; Farahani, R.K.; Naghadalipoor, M.; Reza Etemadi, M.; Tabatabaie, A. Phenotypic and genotypic characterization of antibiotic resistance in Klebsiella pneumoniae isolated from patients admitted to a third-level hospital in Tehran, Iran. Curr. Pediatr Res. 2018, 22, 258–262. [Google Scholar]
- Gutema, G.; Håkonsen, H.; Engidawork, E.; Toverud, E.-L. Multiple challenges of antibiotic use in a large hospital in Ethiopia–a ward-specific study showing high rates of hospital-acquired infections and ineffective prophylaxis. BMC Health Serv. Res. 2018, 18, 326. [Google Scholar] [CrossRef]
- Alibi, S.; Ferjani, A.; Boukadida, J. Molecular characterization of extended spectrum beta-lactamases produced by Klebsiella pneumoniae clinical strains from a Tunisian Hospital. Med. Mal. Infect. 2015, 45, 139–143. [Google Scholar] [CrossRef]
- Chakraborty, S.; Mohsina, K.; Sarker, P.K.; Alam, M.; Karim, M.; Sayem, S. Prevalence, antibiotic susceptibility profiles and ESBL production in Klebsiella pneumoniae and Klebsiella oxytoca among hospitalized patients. Period Biol. 2016, 118, 53–58. [Google Scholar] [CrossRef]
- Nepal, K.; Pant, N.D.; Neupane, B.; Belbase, A.; Baidhya, R.; Shrestha, R.K.; Lekhak, B.; Bhatta, D.R.; Jha, B. Extended spectrum beta-lactamase and metallo beta-lactamase production among Escherichia coli and Klebsiella pneumoniae isolated from different clinical samples in a tertiary care hospital in Kathmandu, Nepal. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 1–7. [Google Scholar] [CrossRef]
- Geresu, B.; Misganaw, D.; Beyene, Y. Retrospective evaluation of cotrimoxazole use as preventive therapy in people living with HIV/AIDS in Boru Meda Hospital. BMC Pharmacol. Toxicol. 2014, 15, 4. [Google Scholar] [CrossRef][Green Version]
- Li, J.; Bi, W.; Dong, G.; Zhang, Y.; Wu, Q.; Dong, T.; Cao, T.; Zhou, T. The new perspective of old antibiotic: In vitro antibacterial activity of TMP-SMZ against Klebsiella pneumoniae. J. Microbiol. Immunol. Infect. 2020, 53, 757–765. [Google Scholar] [CrossRef]
- Magalhães, M.L.; Blanchard, J.S. Aminoglycosides: Mechanisms of action and resistance. In Antimicrobial Drug Resistance: Mechanisms of Drug Resistance; Mayers, D.L., Ed.; Humana Press: New York, NY, USA, 2009; pp. 171–181. [Google Scholar]
- El-Badawy, M.F.; Tawakol, W.M.; El-Far, S.W.; Maghrabi, I.A.; Al-Ghamdi, S.A.; Mansy, M.S.; Ashour, M.S.; Shohayeb, M.M. Molecular identification of aminoglycoside-modifying enzymes and plasmid-mediated quinolone resistance genes among Klebsiella pneumoniae clinical isolates recovered from Egyptian patients. Int. J. Microbiol. 2017, 2017, 8050432. [Google Scholar] [CrossRef] [PubMed]
- Fenta, T.; Engidawork, E.; Amogne, W.; Berha, A.B. Evaluation of current practice of antimicrobial use and clinical outcome of patients with pneumonia at a tertiary care hospital in Ethiopia: A prospective observational study. PLoS ONE 2020, 15, e0227736. [Google Scholar] [CrossRef] [PubMed]
- Eshetie, S.; Unakal, C.; Gelaw, A.; Ayelign, B.; Endris, M.; Moges, F. Multidrug resistant and carbapenemase producing Enterobacteriaceae among patients with urinary tract infection at referral Hospital, Northwest Ethiopia. Antimicrob. Resist. Infect. Control. 2015, 4, 1–8. [Google Scholar]
- Aljanaby, A.; Alhasnawi, H. Phenotypic and Molecular Characterization of Multidrug Resistant Klebsiella pneumoniae Isolated from Different Clinical Sources in Al-Najaf Province-Iraq. Pak. J. Biol. Sci. 2017, 20, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.; Carmeli, Y.; Falagas, M.; Giske, C.; Harbarth, S.; Hindle, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Breurec, S.; Guessennd, N.; Timinouni, M.; Le, T.; Cao, V.; Ngandjio, A.; Randrianirinag, J.M.; Thibergeh, A.; Kinanaa, A.; Dufougeraya, A.; et al. Klebsiella pneumoniae resistant to third-generation cephalosporins in five African and two Vietnamese major towns: Multiclonal population structure with two major international clonal groups, CG15 and CG258. Clin. Microbiol Infect. 2013, 19, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.L.; Mirrett, S.; Reller, L.B.; Weinstein, M.P.; Reimer, L.G. Recovery of clinically important microorganisms from the BacT/Alert blood culture system does not require testing for seven days. Diagn. Microbiol. Infect. Dis. 1993, 16, 31–34. [Google Scholar] [CrossRef]
- Procop, G.W.; Church, D.L.; Hall, G.S.; Janda, W.M. Koneman’s Color Atlas and Textbook of Diagnostic Microbiology, 7th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2017. [Google Scholar]
- Cheesbrough, M. Medical Laboratory Practice in Tropical Countries. Part 2, 2nd ed.; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 28th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018; CLSI supplement M100. [Google Scholar]

| Variables | Category | Frequency | Percentage |
| Gender | Male | 83 | 62.9 |
| Female | 49 | 37.1 | |
| Age | birth to <5 years | 74 | 56.1 |
| 5 years to <18 years | 20 | 15.2 | |
| 18 years to <45 years | 25 | 18.9 | |
| ≥45 years | 13 | 9.8 | |
| Specimen Type | Blood | 63 | 47.7 |
| Urine | 34 | 25.8 | |
| Wound | 21 | 15.9 | |
| Body fluids | 10 | 7.6 | |
| Sputum | 4 | 3.0 | |
| Patient Setting | Inpatient | 120 | 90.9 |
| Outpatient | 12 | 9.1 | |
| Ward type | Pediatric ward | 53 | 44.2 |
| ICU | 46 | 38.3 | |
| Medical ward | 9 | 7.5 | |
| Surgical ward | 8 | 6.7 | |
| Other wards | 4 | 3.3 |
| Antimicrobial Categories | Antimicrobial Agents | Antimicrobial Susceptibility Pattern | |||
| Susceptible n(%) | Inter Mediate n(%) | Resistant n(%) | |||
| Tetracyclines | Tetracycline | 21(15.9) | 15(11.4) | 96(72.7) | |
| Aminoglycosides | Gentamicin | 29(22.0) | 8(6) | 95(72.0) | |
| Tobramycin | 49(37.1) | 35(26.5) | 48(36.4) | ||
| Amikacin | 123(93.2) | 5(3.8) | 4(3.0) | ||
| Fluoroquinolones | Ciprofloxacin | 58(43.9) | 25(18.9) | 49(37.1) | |
| Naldixic acid | 30(22.7) | 42(31.8) | 60(45.5) | ||
| Antipseudomonal Penicillins + β-lactamase inhibitors | Piperacillin-tazobactam | 55(41.7) | 27(20.5) | 50(37.9) | |
| Penicillins + β-lactamase inhibitors | Amoxicillin-clavulanate | 18(13.6) | 32(24.2) | 82(62.1) | |
| Folate pathway inhibitors | Trimethoprim-Sulfamethoxazole | 6(4.5) | 2(1.5) | 124(94) | |
| Phenicols | Chloramphenicol | 59(44.7) | 13(9.8) | 60(45.5) | |
| Cephamycins | Cefoxitin | 62(47.0) | 12(9.1) | 58(43.9) | |
| β-lactams | 1st and 2nd generation cephalosporins | Cefuroxime | 2(1.5) | 2(1.5) | 128(97.0) |
| Cefazolin | 1(0.8) | 1(0.8) | 130(98.5) | ||
| 3rd and 4th generation cephalosporins | Cefepime | 5(3.8) | 16(12.1) | 111(84.1) | |
| Ceftriaxone | 4(3.0) | 0(0.0) | 128(97.0) | ||
| Cefotaxime | 4(3.0) | 0(0.0) | 128(97.0) | ||
| Ceftazidime | 17(12.9) | 28(21.2) | 87(65.9) | ||
| Monobactams | Aztreonam | 13(9.8) | 30(22.7) | 89(67.4) | |
| Carbapenems | Meropenem | 96(72.7) | 4(3.0) | 32(24.3) | |
| Imipenem | 107(81.1) | 9(6.8) | 16(12.1) | ||
| Ertapenem | 93(70.5) | 5(3.7) | 34(25.8) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awoke, T.; Teka, B.; Seman, A.; Sebre, S.; Yeshitela, B.; Aseffa, A.; Mihret, A.; Abebe, T. High Prevalence of Multidrug-Resistant Klebsiella pneumoniae in a Tertiary Care Hospital in Ethiopia. Antibiotics 2021, 10, 1007. https://doi.org/10.3390/antibiotics10081007
Awoke T, Teka B, Seman A, Sebre S, Yeshitela B, Aseffa A, Mihret A, Abebe T. High Prevalence of Multidrug-Resistant Klebsiella pneumoniae in a Tertiary Care Hospital in Ethiopia. Antibiotics. 2021; 10(8):1007. https://doi.org/10.3390/antibiotics10081007
Chicago/Turabian StyleAwoke, Tewachew, Brhanu Teka, Aminu Seman, Shemse Sebre, Biruk Yeshitela, Abraham Aseffa, Adane Mihret, and Tamrat Abebe. 2021. "High Prevalence of Multidrug-Resistant Klebsiella pneumoniae in a Tertiary Care Hospital in Ethiopia" Antibiotics 10, no. 8: 1007. https://doi.org/10.3390/antibiotics10081007
APA StyleAwoke, T., Teka, B., Seman, A., Sebre, S., Yeshitela, B., Aseffa, A., Mihret, A., & Abebe, T. (2021). High Prevalence of Multidrug-Resistant Klebsiella pneumoniae in a Tertiary Care Hospital in Ethiopia. Antibiotics, 10(8), 1007. https://doi.org/10.3390/antibiotics10081007






