Antibiotic-Resistant Acinetobacter baumannii in Low-Income Countries (2000–2020): Twenty-One Years and Still below the Radar, Is It Not There or Can They Not Afford to Look for It?
Abstract
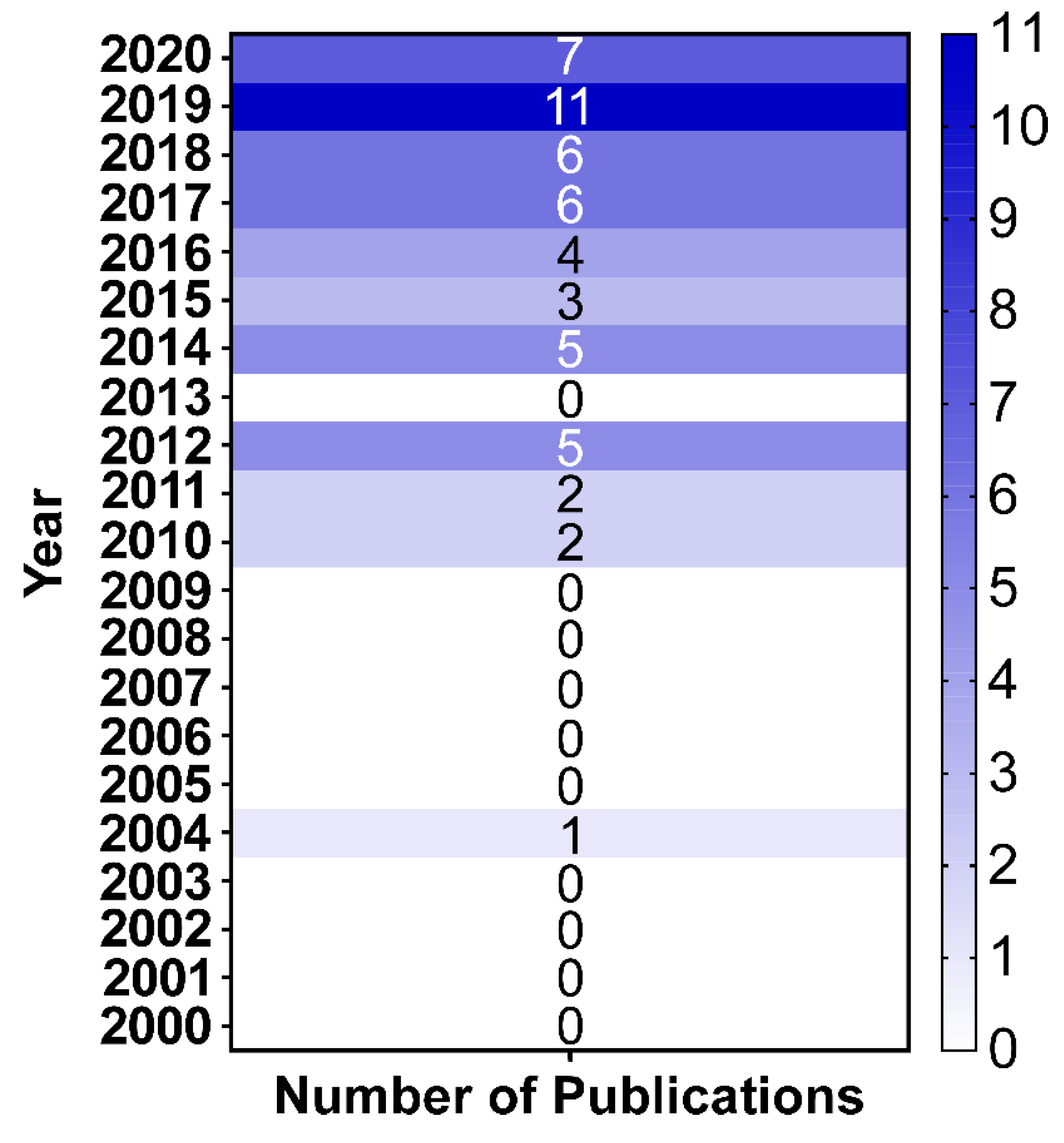
1. Introduction
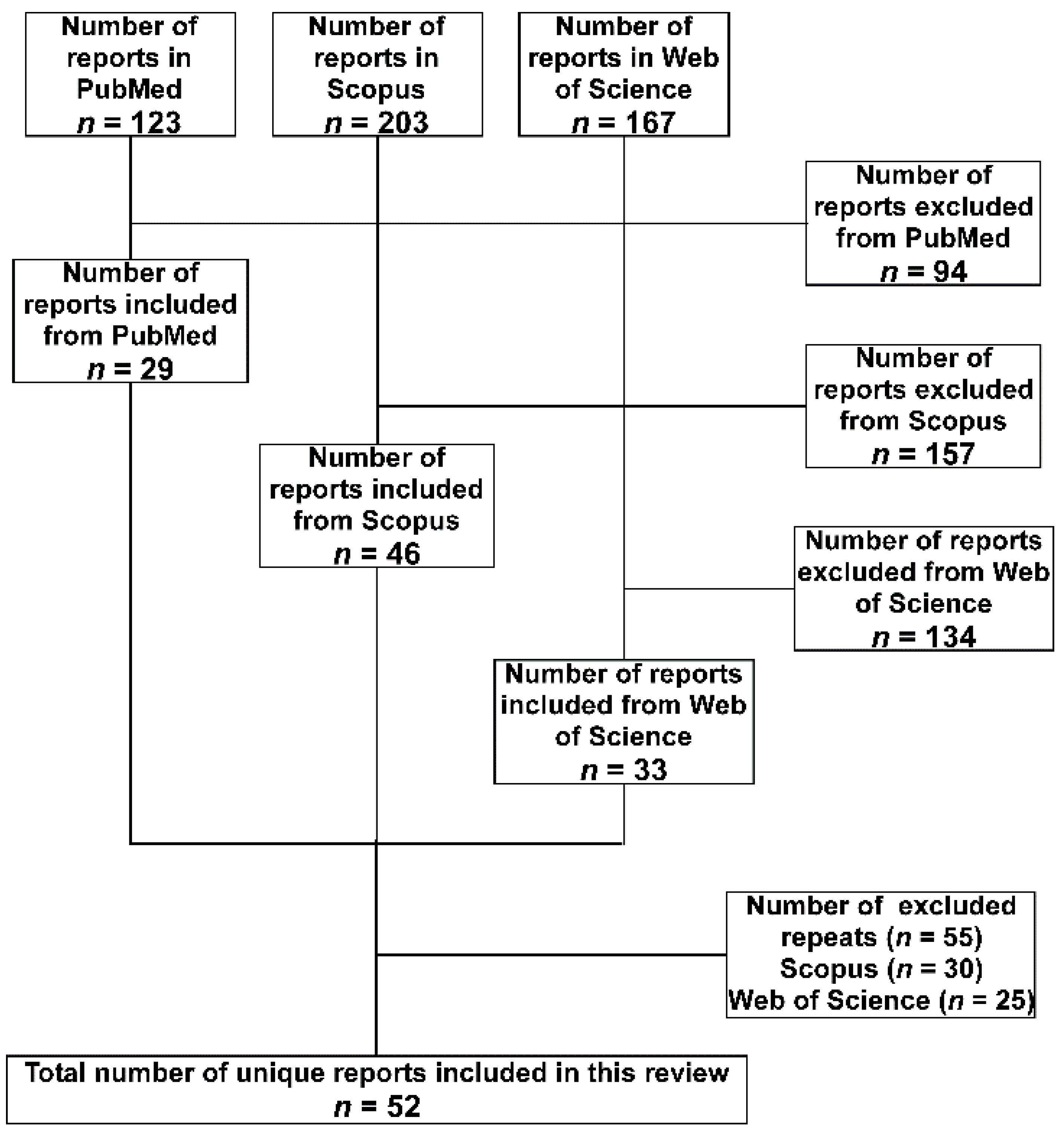
2. Methodology
3. A. baumannii in the LICs Distributed over the Different Geographical Regions
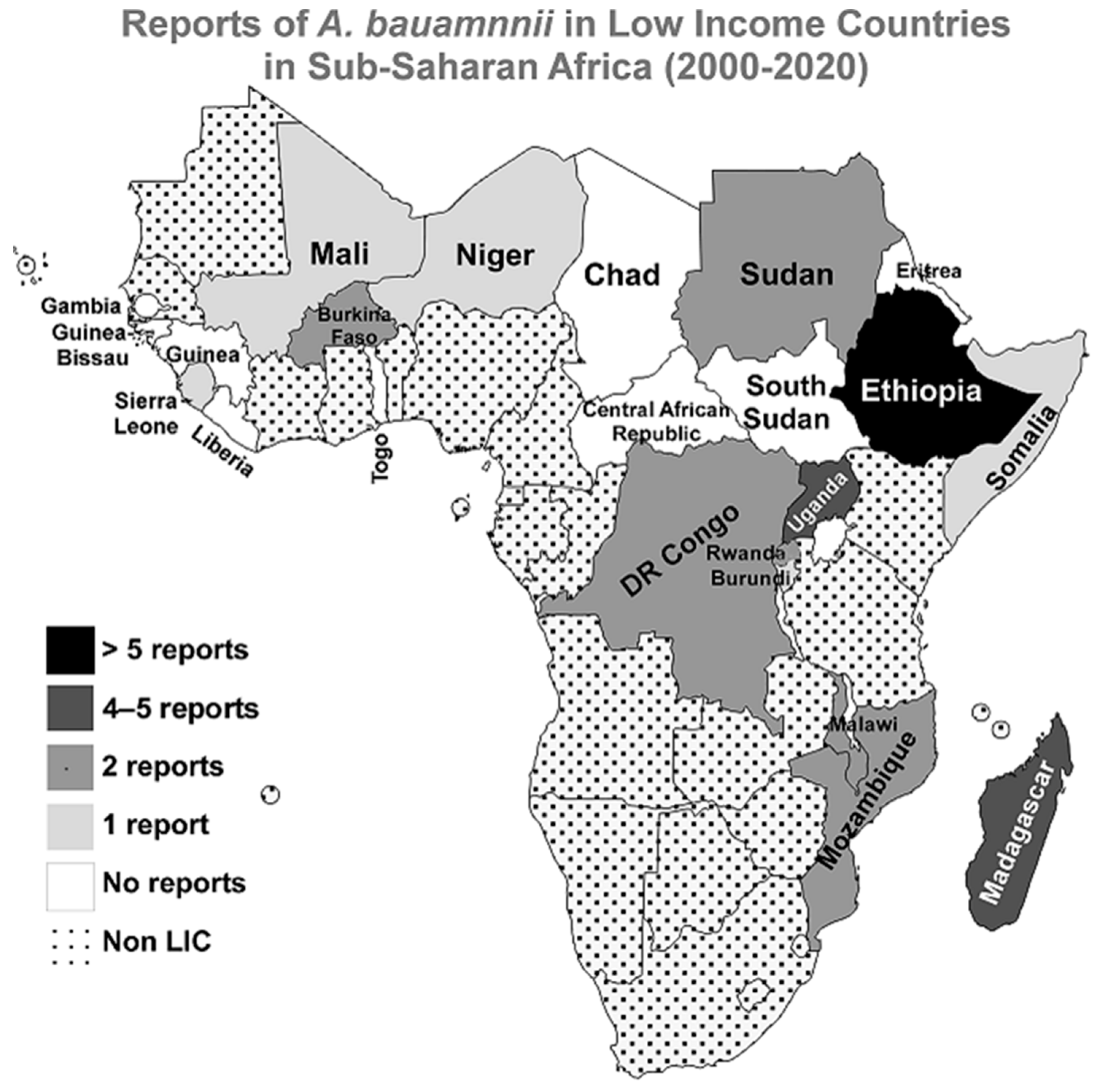
3.1. Sub-Saharan Africa
3.2. Middle East and North Africa
3.3. South Asia
3.4. Latin America and the Caribbean
3.5. Europe and Central Asia
3.6. East Asia and Pacific
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vazquez-Lopez, R.; Solano-Galvez, S.G.; Juarez Vignon-Whaley, J.J.; Abello Vaamonde, J.A.; Padro Alonzo, L.A.; Rivera Resendiz, A.; Muleiro Alvarez, M.; Vega Lopez, E.N.; Franyuti-Kelly, G.; Alvarez-Hernandez, D.A.; et al. Acinetobacter baumannii Resistance: A Real Challenge for Clinicians. Antibiotics 2020, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Howard, A.; O’Donoghue, M.; Feeney, A.; Sleator, R.D. Acinetobacter baumannii: An emerging opportunistic pathogen. Virulence 2012, 3, 243–250. [Google Scholar] [CrossRef]
- Peleg, A.Y.; Seifert, H.; Paterson, D.L. Acinetobacter baumannii: Emergence of a successful pathogen. Clin. Microbiol. Rev. 2008, 21, 538–582. [Google Scholar] [CrossRef]
- Jawad, A.; Seifert, H.; Snelling, A.M.; Heritage, J.; Hawkey, P.M. Survival of Acinetobacter baumannii on dry surfaces: Comparison of outbreak and sporadic isolates. J. Clin. Microbiol. 1998, 36, 1938–1941. [Google Scholar] [CrossRef] [PubMed]
- Espinal, P.; Marti, S.; Vila, J. Effect of biofilm formation on the survival of Acinetobacter baumannii on dry surfaces. J. Hosp. Infect. 2012, 80, 56–60. [Google Scholar] [CrossRef]
- Wagenvoort, J.H.; Joosten, E.J. An outbreak Acinetobacter baumannii that mimics MRSA in its environmental longevity. J. Hosp. Infect. 2002, 52, 226–227. [Google Scholar] [CrossRef]
- Asif, M.; Alvi, I.A.; Rehman, S.U. Insight into Acinetobacter baumannii: Pathogenesis, global resistance, mechanisms of resistance, treatment options, and alternative modalities. Infect. Drug Resist. 2018, 11, 1249. [Google Scholar] [CrossRef]
- Vaara, M. Polymyxins and Their Potential Next Generation as Therapeutic Antibiotics. Front. Microbiol. 2019, 10, 1689. [Google Scholar] [CrossRef]
- World-Bank. World Bank Country and Lending Groups-Country Classification; The World Bank Group: Washington, DC, USA, 2020. [Google Scholar]
- Kempf, M.; Abdissa, A.; Diatta, G.; Trape, J.-F.; Angelakis, E.; Mediannikov, O.; La Scola, B.; Raoult, D. Detection of Acinetobacter baumannii in human head and body lice from Ethiopia and identification of new genotypes. Int. J. Infect. Dis. 2012, 16, e680–e683. [Google Scholar] [CrossRef]
- Lema, T.; Woldeamanuel, Y.; Asrat, D.; Hunegnaw, M.; Baraki, A.; Kebede, Y.; Yamuah, L.; Aseffa, A. The pattern of bacterial isolates and drug sensitivities of infected ulcers in patients with leprosy in ALERT, Kuyera and Gambo hospitals, Ethiopia. Lepr. Rev. 2012, 83, 40–51. [Google Scholar] [CrossRef]
- Pritsch, M.; Zeynudin, A.; Messerer, M.; Baumer, S.; Liegl, G.; Schubert, S.; Löscher, T.; Hoelscher, M.; Belachew, T.; Rachow, A. First report on blaNDM-1-producing Acinetobacter baumannii in three clinical isolates from Ethiopia. BMC Infect. Dis. 2017, 17, 180. [Google Scholar] [CrossRef]
- Solomon, F.B.; Wadilo, F.; Tufa, E.G.; Mitiku, M. Extended spectrum and metalo beta-lactamase producing airborne Pseudomonas aeruginosa and Acinetobacter baumanii in restricted settings of a referral hospital: A neglected condition. Antimicrob. Resist. Infect. Control 2017, 6, 106. [Google Scholar] [CrossRef]
- Moges, F.; Eshetie, S.; Abebe, W.; Mekonnen, F.; Dagnew, M.; Endale, A.; Amare, A.; Feleke, T.; Gizachew, M.; Tiruneh, M. High prevalence of extended-spectrum beta-lactamase-producing Gram-negative pathogens from patients attending Felege Hiwot Comprehensive Specialized Hospital, Bahir Dar, Amhara region. PLoS ONE 2019, 14, e0215177. [Google Scholar] [CrossRef]
- Bitew, A.; Molalign, T.; Chanie, M. Species distribution and antibiotic susceptibility profile of bacterial uropathogens among patients complaining urinary tract infections. BMC Infect. Dis. 2017, 17, 654. [Google Scholar] [CrossRef]
- Admas, A.; Gelaw, B.; Worku, A.; Melese, A. Proportion of bacterial isolates, their antimicrobial susceptibility profile and factors associated with puerperal sepsis among post-partum/aborted women at a referral Hospital in Bahir Dar, Northwest Ethiopia. Antimicrob. Resist. Infect. Control 2020, 9, 14. [Google Scholar] [CrossRef]
- Motbainor, H.; Bereded, F.; Mulu, W. Multi-drug resistance of blood stream, urinary tract and surgical site nosocomial infections of Acinetobacter baumannii and Pseudomonas aeruginosa among patients hospitalized at Felegehiwot referral hospital, Northwest Ethiopia: A cross-sectional study. BMC Infect. Dis. 2020, 20, 92. [Google Scholar] [CrossRef] [PubMed]
- Demoz, G.T.; Alebachew, M.; Legesse, Y.; Ayalneh, B. Treatment of ventriculoperitoneal shunt infection and ventriculitis caused by Acinetobacter baumannii: A case report. J. Med. Case Rep. 2018, 12, 141. [Google Scholar] [CrossRef] [PubMed]
- Gashaw, M.; Berhane, M.; Bekele, S.; Kibru, G.; Teshager, L.; Yilma, Y.; Ahmed, Y.; Fentahun, N.; Assefa, H.; Wieser, A. Emergence of high drug resistant bacterial isolates from patients with health care associated infections at Jimma University medical center: A cross sectional study. Antimicrob. Resist. Infect. Control 2018, 7, 138. [Google Scholar] [CrossRef] [PubMed]
- Randrianirina, F.; Vaillant, L.; Ramarokoto, C.E.; Rakotoarijaona, A.; Andriamanarivo, M.L.; Razafimahandry, H.C.; Randrianomenjanahary, J.; Raveloson, J.R.; Hariniana, E.R.; Carod, J.-F. Antimicrobial resistance in pathogens causing nosocomial infections in surgery and intensive care units of two hospitals in Antananarivo, Madagascar. J. Infect. Dev. Ctries. 2010, 4, 074–082. [Google Scholar] [CrossRef] [PubMed]
- Andriamanantena, T.S.; Ratsima, E.; Rakotonirina, H.C.; Randrianirina, F.; Ramparany, L.; Carod, J.-F.; Richard, V.; Talarmin, A. Dissemination of multidrug resistant Acinetobacter baumannii in various hospitals of Antananarivo Madagascar. Ann. Clin. Microbiol. Antimicrob. 2010, 9, 17. [Google Scholar] [CrossRef]
- Rasamiravaka, T.; Sheila, H.S.; Rakotomavojaona, T.; Rakoto-Alson, A.; Rasamindrakotroka, A. Changing profile and increasing antimicrobial resistance of uropathogenic bacteria in Madagascar. Med. Mal. Infect. 2015, 45, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Tchuinte, P.L.S.; Rabenandrasana, M.A.N.; Kowalewicz, C.; Andrianoelina, V.H.; Rakotondrasoa, A.; Andrianirina, Z.Z.; Enouf, V.; Ratsima, E.H.; Randrianirina, F.; Collard, J.-M. Phenotypic and molecular characterisations of carbapenem-resistant Acinetobacter baumannii strains isolated in Madagascar. Antimicrob. Resist. Infect. Control 2019, 8, 31. [Google Scholar] [CrossRef]
- Eremeeva, M.E.; Warang, S.S.; Anderson, M.L.; Capps, D.; Zohdy, S.; Durden, L.A. Molecular Survey for Pathogens and Markers of Permethrin Resistance in Human Head Lice (Phthiraptera: Pediculidae) from Madagascar. J. Parasitol. 2019, 105, 459–468. [Google Scholar] [CrossRef]
- Moore, C.C.; Jacob, S.T.; Banura, P.; Zhang, J.; Stroup, S.; Boulware, D.R.; Scheld, W.M.; Houpt, E.R.; Liu, J. Etiology of sepsis in Uganda using a quantitative polymerase chain reaction-based TaqMan array card. Clin. Infect. Dis. 2019, 68, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Kateete, D.P.; Nakanjako, R.; Namugenyi, J.; Erume, J.; Joloba, M.L.; Najjuka, C.F. Carbapenem resistant Pseudomonas aeruginosa and Acinetobacter baumannii at Mulago Hospital in Kampala, Uganda (2007–2009). Springerplus 2016, 5, 1308. [Google Scholar] [CrossRef]
- Kateete, D.P.; Nakanjako, R.; Okee, M.; Joloba, M.L.; Najjuka, C.F. Genotypic diversity among multidrug resistant Pseudomonas aeruginosa and Acinetobacter species at Mulago Hospital in Kampala, Uganda. BMC Res. Notes 2017, 10, 1–10. [Google Scholar] [CrossRef]
- Aruhomukama, D.; Najjuka, C.F.; Kajumbula, H.; Okee, M.; Mboowa, G.; Sserwadda, I.; Mayanja, R.; Joloba, M.L.; Kateete, D.P. blaVIM-and blaOXA-mediated carbapenem resistance among Acinetobacter baumannii and Pseudomonas aeruginosa isolates from the Mulago hospital intensive care unit in Kampala, Uganda. BMC Infect. Dis. 2019, 19, 853. [Google Scholar] [CrossRef]
- Kaboré, W.A.; Konaté, A.; Bako, E.; Bagré, T.S.; Boisramé, S.; Chandad, F.; Traoré, A.S.; Barro, N.; Sangaré, L. Détection d’Acinetobacter baumannii, agent pathogène opportuniste et multirésistant dans les infections bucco-dentaires à Ouagadougou, Burkina Faso. Médecine Buccale Chir. Buccale 2016, 22, 105–112. [Google Scholar] [CrossRef]
- Sanou, S.; Ouedraogo, A.S.; Aberkane, S.; Vendrell, J.; Ouchar, O.; Bouzimbi, N.; Hema, A.; Poda, A.; Zoungrana, J.; Ouedraogo, G.A. Prevalence and Molecular Characterization of Extended Spectrum β-Lactamase, Plasmid-Mediated Quinolone Resistance, and Carbapenemase-Producing Gram-Negative Bacilli in Burkina Faso. Microb. Drug Resist. 2021, 27, 18–24. [Google Scholar] [CrossRef]
- El Far, M.Y.; El-Mahallawy, H.A.; Attia, A.S. Tracing the dissemination of the international clones of multidrug-resistant Acinetobacter baumannii among cancer patients in Egypt using the PCR-based open reading frame typing (POT) method. J. Glob. Antimicrob. Resist. 2019, 19, 210–215. [Google Scholar] [CrossRef]
- Lukuke, H.M.; Kasamba, E.; Mahuridi, A.; Ngatu, N.R.; Narufumi, S.; Mukengeshayi, A.N.; Malou, V.; Makoutode, M.; Kaj, F.M. L’incidence des infections nosocomiales urinaires et des sites opératoires dans la maternité de l’Hôpital Général de Référence de Katuba à Lubumbashi en République Démocratique du Congo. Pan Afr. Med. J. 2017, 28, 57. [Google Scholar] [CrossRef] [PubMed]
- Koyo, C.S.B.; Amanzougaghene, N.; Davoust, B.; Tshilolo, L.; Lekana-Douki, J.B.; Raoult, D.; Mediannikov, O.; Fenollar, F. Genetic diversity of human head lice and molecular detection of associated bacterial pathogens in Democratic Republic of Congo. Parasites Vectors 2019, 12, 290. [Google Scholar] [CrossRef] [PubMed]
- Bedell, R.A.; Anderson, S.T.; Van Lettow, M.; Åkesson, A.; Corbett, E.L.; Kumwenda, M.; Chan, A.K.; Heyderman, R.S.; Zachariah, R.; Harries, A.D. High prevalence of tuberculosis and serious bloodstream infections in ambulatory individuals presenting for antiretroviral therapy in Malawi. PLoS ONE 2012, 7, e39347. [Google Scholar] [CrossRef][Green Version]
- Iroh Tam, P.-Y.; Musicha, P.; Kawaza, K.; Cornick, J.; Denis, B.; Freyne, B.; Everett, D.; Dube, Q.; French, N.; Feasey, N. Emerging resistance to empiric antimicrobial regimens for pediatric bloodstream infections in Malawi (1998–2017). Clin. Infect. Dis. 2019, 69, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, J.C.; Carrilho, C.; Mandomando, I.; Martínez, M.J.; CaDMIA bacterial study group. Fatal multi-drug-resistant Acinetobacter baumannii pneumonia in Maputo, Mozambique: A case report. Enferm. Infecc. Y Microbiol. Clínica 2019, 37, 485–487. [Google Scholar] [CrossRef]
- Martínez, M.J.; Massora, S.; Mandomando, I.; Ussene, E.; Jordao, D.; Lovane, L.; Muñoz-Almagro, C.; Castillo, P.; Mayor, A.; Rodriguez, C. Infectious cause of death determination using minimally invasive autopsies in developing countries. Diagn. Microbiol. Infect. Dis. 2016, 84, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.B.; Hassan, M.; Munir, A.; Kambal, S.; Abdalla, N.I.; Hamad, A.; Mohammed, S.; Ahmed, F.; Hamid, O.; Ismail, A. Whole-Genome Sequence of Acinetobacter baumannii Strain NUBRI-A, Isolated from a Hospitalized Patient in Khartoum, Sudan. Microbiol. Resour. Announc. 2019, 8, e00542-19. [Google Scholar] [CrossRef]
- Dirar, M.; Bilal, N.; Ibrahim, M.E.; Hamid, M. Resistance Patterns and Phenotypic Detection of β-lactamase Enzymes among Enterobacteriaceae Isolates from Referral Hospitals in Khartoum State, Sudan. Cureus 2020, 12, e7260. [Google Scholar] [CrossRef]
- La Scola, B.; Raoult, D. Acinetobacter baumannii in human body louse. Emerg. Infect. Dis. 2004, 10, 1671–1673. [Google Scholar] [CrossRef] [PubMed]
- Heiden, S.E.; Kurz, M.S.; Bohnert, J.; Bayingana, C.; Ndoli, J.M.; Sendegeya, A.; Gahutu, J.B.; Eger, E.; Mockenhaupt, F.P.; Schaufler, K. Flies from a tertiary hospital in Rwanda carry multidrug-resistant Gram-negative pathogens including extended-spectrum beta-lactamase-producing E. coli sequence type 131. Antimicrob. Resist. Infect. Control 2020, 9, 1–4. [Google Scholar] [CrossRef]
- Doumbia-Singare, K.; Timbo, S.; Keita, M.; Mohamed, A.A.; Guindo, B.; Soumaoro, S. Cellulite cervico-faciale au cours de la grossesse. À propos d’une série de 10 cas au Mali. Bull. De La Société De Pathol. Exot. 2014, 107, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Lakoh, S.; Li, L.; Sevalie, S.; Guo, X.; Adekanmbi, O.; Yang, G.; Adebayo, O.; Yi, L.; Coker, J.M.; Wang, S. Antibiotic resistance in patients with clinical features of healthcare-associated infections in an urban tertiary hospital in Sierra Leone: A cross-sectional study. Antimicrob. Resist. Infect. Control 2020, 9, 1–10. [Google Scholar] [CrossRef]
- Mohamed, A.H.; Mohamud, M.F.Y.; Mohamud, H.A. Epidemiology and Antimicrobial Susceptibility Pattern of Uropathogens in Patients with the Community-and Hospital-Acquired Urinary Tract Infections at a Tertiary Hospital in Somalia. Jundishapur J. Microbiol. 2020, 13, e107453. [Google Scholar] [CrossRef]
- Louni, M.; Amanzougaghene, N.; Mana, N.; Fenollar, F.; Raoult, D.; Bitam, I.; Mediannikov, O. Detection of bacterial pathogens in clade E head lice collected from Niger’s refugees in Algeria. Parasites Vectors 2018, 11, 348. [Google Scholar] [CrossRef] [PubMed]
- Hamzeh, A.R.; Al Najjar, M.; Mahfoud, M. Prevalence of antibiotic resistance among Acinetobacter baumannii isolates from Aleppo, Syria. Am. J. Infect. Control 2012, 40, 776–777. [Google Scholar] [CrossRef]
- Teicher, C.L.; Ronat, J.-B.; Fakhri, R.M.; Basel, M.; Labar, A.S.; Herard, P.; Murphy, R.A. Antimicrobial drug–resistant bacteria isolated from Syrian war–injured patients, August 2011–March 2013. Emerg. Infect. Dis. 2014, 20, 1949–1951. [Google Scholar] [CrossRef]
- Herard, P.; Boillot, F.; Fakhri, R.M. Bone cultures from war-wounded civilians in the Middle East: A surgical prospective. Int. Orthop. 2017, 41, 1291–1294. [Google Scholar] [CrossRef]
- Fily, F.; Ronat, J.-B.; Malou, N.; Kanapathipillai, R.; Seguin, C.; Hussein, N.; Fakhri, R.M.; Langendorf, C. Post-traumatic osteomyelitis in Middle East war-wounded civilians: Resistance to first-line antibiotics in selected bacteria over the decade 2006–2016. BMC Infect. Dis. 2019, 19, 103. [Google Scholar] [CrossRef]
- Peretz, A.; Labay, K.; Zonis, Z.; Glikman, D. Disengagement does not apply to bacteria: A high carriage rate of antibiotic-resistant pathogens among Syrian civilians treated in Israeli hospitals. Clin. Infect. Dis. 2014, 59, 753–754. [Google Scholar] [CrossRef][Green Version]
- Heydari, F.; Mammina, C.; Koksal, F. NDM-1-producing Acinetobacter baumannii ST85 now in Turkey, including one isolate from a Syrian refugee. J. Med. Microbiol. 2015, 64, 1027–1029. [Google Scholar] [CrossRef]
- Hasde, A.İ.; Baran, Ç.; Gümüş, F.; Kış, M.; Özçınar, E.; Çakıcı, M.; Yazıcıoğlu, L.; Kaya, B. Effect of temporary vascular shunting as a previous intervention on lower extremity arterial injury: Single center experiences in the Syrian Civil War. Turk. J. Trauma Emerg. Surg. 2019, 25, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Rafei, R.; Dabboussi, F.; Hamze, M.; Eveillard, M.; Lemarié, C.; Mallat, H.; Rolain, J.-M.; Joly-Guillou, M.-L.; Kempf, M. First report of blaNDM-1-producing Acinetobacter baumannii isolated in Lebanon from civilians wounded during the Syrian war. Int. J. Infect. Dis. 2014, 21, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Rafei, R.; Pailhoriès, H.; Hamze, M.; Eveillard, M.; Mallat, H.; Dabboussi, F.; Joly-Guillou, M.-L.; Kempf, M. Molecular epidemiology of Acinetobacter baumannii in different hospitals in Tripoli, Lebanon using blaOXA-51-like sequence based typing. BMC Microbiol. 2015, 15, 103. [Google Scholar] [CrossRef]
- Salloum, T.; Tannous, E.; Alousi, S.; Arabaghian, H.; Rafei, R.; Hamze, M.; Tokajian, S. Genomic mapping of ST85 blaNDM-1 and blaOXA-94 producing Acinetobacter baumannii isolates from Syrian Civil War Victims. Int. J. Infect. Dis. 2018, 74, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Bakour, S.; Alsharapy, S.A.; Touati, A.; Rolain, J.-M. Characterization of Acinetobacter baumannii clinical isolates carrying blaOXA-23 carbapenemase and 16S rRNA methylase armA genes in Yemen. Microb. Drug Resist. 2014, 20, 604–609. [Google Scholar] [CrossRef]
- Sutter, D.E.; Bradshaw, L.U.; Simkins, L.H.; Summers, A.M.; Atha, M.; Elwood, R.L.; Robertson, J.L.; Murray, C.K.; Wortmann, G.W.; Hospenthal, D.R. High incidence of multidrug-resistant gram-negative bacteria recovered from Afghan patients at a deployed US military hospital. Infect. Control Hosp. Epidemiol. 2011, 32, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Potron, A.; Munoz-Price, L.S.; Nordmann, P.; Cleary, T.; Poirel, L. Genetic features of CTX-M-15-producing Acinetobacter baumannii from Haiti. Antimicrob. Agents Chemother. 2011, 55, 5946–5948. [Google Scholar] [CrossRef]
- Marra, A.R.; Martino, M.D.V.; Ribas, M.R.; Rodriguez-Taveras, C.; dos Santos, O.F.P. Microbiological findings from the Haiti disaster. Travel Med. Infect. Dis. 2012, 10, 157–161. [Google Scholar] [CrossRef]
- Murphy, R.A.; Nisenbaum, L.; Labar, A.S.; Sheridan, R.L.; Ronat, J.-B.; Dilworth, K.; Pena, J.; Kilborn, E.; Teicher, C. Invasive infection and outcomes in a humanitarian surgical burn program in Haiti. World J. Surg. 2016, 40, 1550–1557. [Google Scholar] [CrossRef]
- Chaintarli, K.; Lenglet, A.; Beauzile, B.D.; Senat-Delva, R.; Mabou, M.-M.; Martino, C.; Berthet, M.; Wong, S.; Hopman, J. High prevalence of ESBL-positive bacteria in an obstetrics emergency hospital and neonatal care unit—Haiti, 2016. Infect. Control Hosp. Epidemiol. 2018, 39, 1381–1383. [Google Scholar] [CrossRef]
- Roy, M.A.; Arnaud, J.M.; Jasmin, P.M.; Hamner, S.; Hasan, N.A.; Colwell, R.R.; Ford, T.E. A metagenomic approach to evaluating surface water quality in Haiti. Int. J. Environ. Res. Public Health 2018, 15, 2211. [Google Scholar] [CrossRef]
- Zarrilli, R.; Pournaras, S.; Giannouli, M.; Tsakris, A. Global evolution of multidrug-resistant Acinetobacter baumannii clonal lineages. Int. J. Antimicrob. Agents 2013, 41, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Yum, J.H.; Yi, K.; Lee, H.; Yong, D.; Lee, K.; Kim, J.M.; Rossolini, G.M.; Chong, Y. Molecular characterization of metallo-β-lactamase-producing Acinetobacter baumannii and Acinetobacter genomospecies 3 from Korea: Identification of two new integrons carrying the blaVIM-2 gene cassettes. J. Antimicrob. Chemother. 2002, 49, 837–840. [Google Scholar] [CrossRef] [PubMed]
- Siegman-Igra, Y.; Bar-Yosef, S.; Gorea, A.; Avram, J. Nosocomial Acinetobacter meningitis secondary to invasive procedures: Report of 25 cases and review. Clin. Infect. Dis. 1993, 17, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Duployez, C.; Guern, R.L.; Milliere, L.; Caplan, M.; Loiez, C.; Ledoux, G.; Jaillette, E.; Favory, R.; Mathieu, D.; Wallet, F. An outbreak can hide another. Jpn. J. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Wareth, G.; Brandt, C.; Sprague, L.D.; Neubauer, H.; Pletz, M.W. Spatio-Temporal Distribution of Acinetobacter baumannii in Germany-A Comprehensive Systematic Review of Studies on Resistance Development in Humans (2000–2018). Microorganisms 2020, 8, 375. [Google Scholar] [CrossRef]
- Ibrahim, M.E. Prevalence of Acinetobacter baumannii in Saudi Arabia: Risk factors, antimicrobial resistance patterns and mechanisms of carbapenem resistance. Ann. Clin. Microbiol. Antimicrob. 2019, 18, 1. [Google Scholar] [CrossRef]
- Henig, O.; Weber, G.; Hoshen, M.; Paul, M.; German, L.; Neuberger, A.; Gluzman, I.; Berlin, A.; Shapira, C.; Balicer, R. Risk factors for and impact of carbapenem-resistant Acinetobacter baumannii colonization and infection: Matched case–control study. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 2063–2068. [Google Scholar] [CrossRef] [PubMed]
- Kirby, A.; Herbert, A. Correlations between income inequality and antimicrobial resistance. PLoS ONE 2013, 8, e73115. [Google Scholar] [CrossRef]
- Isendahl, J.; Turlej-Rogacka, A.; Manjuba, C.; Rodrigues, A.; Giske, C.G.; Naucler, P. Fecal carriage of ESBL-producing E. coli and K. pneumoniae in children in Guinea-Bissau: A hospital-based cross-sectional study. PLoS ONE 2012, 7, e51981. [Google Scholar] [CrossRef] [PubMed]
- Isendahl, J.; Manjuba, C.; Rodrigues, A.; Xu, W.; Henriques-Normark, B.; Giske, C.G.; Naucler, P. Prevalence of community-acquired bacteraemia in Guinea-Bissau: An observational study. BMC Infect. Dis. 2014, 14, 3859. [Google Scholar] [CrossRef][Green Version]
- Janatova, M.; Albrechtova, K.; Petrzelkova, K.J.; Dolejska, M.; Papousek, I.; Masarikova, M.; Cizek, A.; Todd, A.; Shutt, K.; Kalousova, B.; et al. Antimicrobial-resistant Enterobacteriaceae from humans and wildlife in Dzanga-Sangha Protected Area, Central African Republic. Vet. Microbiol. 2014, 171, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Ouchar Mahamat, O.; Tidjani, A.; Lounnas, M.; Hide, M.; Benavides, J.; Somasse, C.; Ouedraogo, A.S.; Sanou, S.; Carriere, C.; Banuls, A.L.; et al. Fecal carriage of extended-spectrum beta-lactamase-producing Enterobacteriaceae in hospital and community settings in Chad. Antimicrob. Resist. Infect. Control 2019, 8, 169. [Google Scholar] [CrossRef] [PubMed]
- Fournier, P.E.; Richet, H.; Weinstein, R.A. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin. Infect. Dis. 2006, 42, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, S.; Raut, S.; Adhikari, B. Tackling antimicrobial resistance in low-income and middle-income countries. BMJ Glob. Health 2019, 4, e002104. [Google Scholar] [CrossRef] [PubMed]
- Ballereau, F.; Prazuck, T.; Schrive, I.; Lafleuriel, M.; Rozec, D.; Fisch, A.; Lafaix, C. Stability of essential drugs in the field: Results of a study conducted over a two-year period in Burkina Faso. Am. J. Trop. Med. Hyg. 1997, 57, 31–36. [Google Scholar] [CrossRef]
- Vila, J.; Pal, T. Update on antibacterial resistance in low-income countries: Factors favoring the emergence of resistance. Open Infect. Dis. J. 2010, 4, 38–54. [Google Scholar] [CrossRef]
- Xiong, W.; Sun, Y.; Zeng, Z. Antimicrobial use and antimicrobial resistance in food animals. Environ. Sci. Pollut. Res. 2018, 25, 18377–18384. [Google Scholar] [CrossRef]
- Huynh, B.-T.; Padget, M.; Garin, B.; Herindrainy, P.; Kermorvant-Duchemin, E.; Watier, L.; Guillemot, D.; Delarocque-Astagneau, E. Burden of bacterial resistance among neonatal infections in low income countries: How convincing is the epidemiological evidence? BMC Infect. Dis. 2015, 15, 127. [Google Scholar] [CrossRef]
- WHO. Antimicrobial Resistance: Global Report on Surveillance. 2014. Available online: http://www.who.int/drugresistance/documents/surveillancereport/en/ (accessed on 30 April 2021).
- Gandra, S.; Alvarez-Uria, G.; Turner, P.; Joshi, J.; Limmathurotsakul, D.; van Doorn, H.R. Antimicrobial Resistance Surveillance in Low-and Middle-Income Countries: Progress and Challenges in Eight South Asian and Southeast Asian Countries. Clin. Microbiol. Rev. 2020, 33, e00048-19. [Google Scholar] [CrossRef]
- Bebell, L.M.; Muiru, A.N. Antibiotic use and emerging resistance: How can resource-limited countries turn the tide? Glob. Heart 2014, 9, 347–358. [Google Scholar] [CrossRef] [PubMed]

| Country | Study | Isolates (n) | MDR % * | CRAB% | Isolates Characterization | References |
|---|---|---|---|---|---|---|
| Sub-Saharan Africa | ||||||
| Ethiopia | Kempf et al., 2012 | 40 | NA | NA | rpoB and recA sequencing for genotyping | [10] |
| Lema et al., 2012 | 5 | ≥20% | NA | AST with KB | [11] | |
| Pritsch et al., 2017 | 3 | 100% | 100% | AST with KB and VITEK 2, CT102 Micro-Array, real-time PCR, WGS, MLST, and detection of the blaNDM-1 | [12] | |
| Solomon et al., 2017 | 43 | 81% | 37% | AST with KB and phenotypic detection of ESBLs and MBLs | [13] | |
| Bitew et al., 2017 | 2 | 100% | NA | Identification and AST with VITEK 2 | [15] | |
| Demoz et al., 2018 | 1 | 100% | 100% | AST with KB | [18] | |
| Gashaw et al., 2018 | 2 | 50% XDR and 50% PDR | 100% | AST with KB and phenotypic detection of ESBLs and AmpC | [19] | |
| Moges et al., 2019 | 15 | ≥63% | Yes | AST with KB and phenotypic detection of ESBLs and carbapenemases | [14] | |
| Admas et al., 2020 | 6 | 100% | NA | Identification and AST with VITEK 2 | [16] | |
| Motbainor et al., 2020 | 9 | 100% | 33% | Identification with VITEK 2 and AST with KB | [17] | |
| Madagascar | Randrianirina et al., 2010 | 50 | ≥44% | 44% | AST with KB and phenotypic detection of ESBLs | [20] |
| Andriamanantena et al., 2010 | 53 | 100% | 100% | AST with KB and MIC determination, phenotypic detection of carbapenemases, ReP-PCR for genotyping and PCR for detection of; blaAmpC, blaoxa51, blaoxa23, blaoxa24, blaVIM, blaIMP, and isAba-1 | [21] | |
| Rasamiravaka et al., 2015 | 10 | ≥50% | 0% | AST with KB | [22] | |
| Tchuinte et al., 2019 | 15 | 100% | 100% | MALDI-TOF MS for identification, AST with KB and MIC determination, WGS, MLST for genotyping and WGS detecting; blaoxa51, blaoxa23, blaoxa24, blaoxa58, and isAba-1 | [23] | |
| Eremeeva et al., 2019 | 14 | NA | NA | TaqMan PCR of the rpoB for identification, and PCR for detecting: blaoxa51-like, blaoxa23, blaoxa24, blaVIM, and blaIMP | [24] | |
| Uganda | Kateete et al., 2016 | 40 | 60% | 38% | AST with Phoenix Automated Microbiology System, PCR for: blaoxa51-like, blaoxa51, blaoxa23, blaoxa24, blaoxa58, blaVIM, blaSPM, and blaIMP | [26] |
| Kateete et al., 2017 | 20 | 40% | 35% | AST with MIC determination, PAMS, Rep-PCR for genotyping and phenotypic detection of ESBLs and AmpC | [27] | |
| Moore et al., 2019 | 3 | NA | NA | qPCR TAC | [25] | |
| Aruhomukama et al., 2019 | 1077 | 3% | 3% | AST with KB, PCR for detecting: blaoxa23, blaoxa24, blaoxa58, blaVIM, blaSPM, blaKPC, and blaIMP, phenotypic detection of carbapenemases, and conjugation to show transferability of blaVIM. | [28] | |
| Burkina Faso | Kaboré et al., 2016 | 3 | 100% | NA | AST with KB and phenotypic detection of ESBLs | [29] |
| Sanou et al., 2021 # | 5 | 100% | 60% | MALDI-TOF MS for identification, AST with KB and MIC determination, phenotypic detection of ESBLs, PCR and sequencing of multiple resistance genes including; blaoxa1-like, blaoxa48-like, blaNDM, blaVIM, blaSPM, blaKPC, blaCTX-M, and blaIMP, and MLST for genotyping. | [30] | |
| DR of the Congo | Lukuke et al., 2017 | 2 | 0% | NA | API for identification and AST with KB | [32] |
| Koyo et al., 2019 | 15 | NA | NA | qPCR and phylogenetic analysis using the rpoB gene | [33] | |
| Malawi | Bedell et al., 2012 | 1 | NA | NA | Identification with standard diagnostic techniques | [34] |
| Iroh Tam et al., 2019 | 84 | ≥44% | NA | API for identification, AST with KB, and phenotypic detection of ESBLs | [35] | |
| Mozambique | Martínez et al., 2016 | 1 | NA | NA | 16S rRNA PCR and MALDI-TOF MS for identification | [37] |
| Hurtado et al., 2019 | 1 | 100% | 0% | 16S rRNA for identification and AST with KB | [36] | |
| Sudan | Mohamed et al., 2019 | 1 | NA | NA | API for identification followed by WGS | [38] |
| Dirar et al., 2020 | 12 | ≥83% | 89% | Identification with PAMS, AST with KB and phenotypic detection of ESBLs and carbapenemases. | [39] | |
| Rwanda | La Scola and Raoult 2004 | 10 | NA | NA | API for identification and recA genotyping | [40] |
| Heiden et al., 2020 | 1 | 100% | 0% | MALDI-TOF MS for identification, AST with VITEK 2, phenotypic detection of ESBLs and carbapenemases, and WGS | [41] | |
| Burundi | La Scola and Raoult 2004 | 3 | NA | NA | API for identification and recA genotyping | [40] |
| Mali | Doumbia-Singare et al., 2014 | 1 | NA | NA | Not mentioned | [42] |
| Sierra Leone | Lakoh et al., 2020 | 14 | ≥40% | 10% | Identification and AST with VITEK 2 | [43] |
| Somalia | Mohamed et al., 2020 | 7 | 100% | 100% | AST with KB | [44] |
| Niger | Louni et al., 2018 | 29 | NA | NA | qPCR and rpoB PCR for identification and phylogenetic analysis | [45] |
| Central African Republic | No Reports | |||||
| Chad | No Reports | |||||
| Eritrea | No Reports | |||||
| Gambia | No Reports | |||||
| Guinea | No Reports | |||||
| Guinea-Bissau | No Reports | |||||
| Liberia | No Reports | |||||
| South Sudan | No Reports | |||||
| Togo | No Reports | |||||
| Middle East and North Africa | ||||||
| Syria | Hamzeh et al., 2012 | 260 | ≥65% | 65% | Identification and AST with PAMS | [46] |
| Teicher et al., 2014 | 6 | 100% | 80% | API for identification and AST with MicroScan Walk-Away System | [47] | |
| Peretz et al., 2014 | 5 | 100% | NA | Not mentioned | [50] | |
| Rafei et al., 2014 | 4 | 100% | 100% | Identification with rpoB sequencing and blaoxa51, PCR, AST with KB and Etest, PCR for: blaoxa23-like, blaoxa24-like, blaoxa58-like, and blaNDM, and PFGE for genotyping | [53] | |
| Heydari et al., 2015 | 1 | 100% | 100% | Identification and AST with VITEK 2, phenotypic detection of ESBLs and carbapenemases, PCR for the blaNDM, PFGE and MLST for typing | [51] | |
| Rafei et al., 2015 | 59 | Yes | 74% | Identification with MALDI-TOF MS, rpoB sequencing and blaoxa51 PCR, AST with KB and Etest, PCR for detecting: blaoxa23, blaoxa24, blaoxa58, blaNDM-1, blaVIM, blaoxa143, and blaIMP, and MLST for typing | [54] | |
| Herard and Fakhri 2017 | 38 | NA | NA | Not mentioned | [48] | |
| Salloum et al., 2018 | 2 | 100% | 100% | AST with KB and Etest, PCR for blaoxa58 and blaNDM, plasmid typing with PBRT, MLST, and WGS | [55] | |
| Fily et al., 2019 | 6 | NA | 67% | AST with KB | [49] | |
| Hasde et al., 2019 | 5 | NA | NA | Not mentioned | [52] | |
| Yemen | Bakour et al., 2014 | 3 | 100% | 100% | API and MALDI-TOF MS for identification, AST with KB and E-test, phenotypic detection of carbapenemases, PCR detection of: blaoxa23, blaoxa24, blaoxa58, blaNDM, blaVIM, blaSIM, blaoxa48-like, blaIMP and others, and MLST | [56] |
| Fily et al., 2019 | 1 | NA | 100% | AST with KB | [49] | |
| South Asia | ||||||
| Afghanistan | Sutter et al., 2011 | 57 ¥ | ≥75% | 76% | Identification and AST with MicroScan autoSCAN-4 | [57] |
| Latin America and The Caribbean | ||||||
| Haiti | Potron et al., 2011 | 3 | 66.7% | 0% | API and 16sRNA for identification, AST with KB and E-test, phenotypic detection of ESBLs, PCR for detection of: blaTEM, blaSHV, blaPER-1, blaVEB-1, blaGES-1, and blaCTX-M | [58] |
| Marra et al., 2012 | 1 | 100% | 0% | Identification and AST with VITEK 2 | [59] | |
| Murphy et al., 2016 | 4 | ≥25% | 25% | AST but the method was not indicated | [60] | |
| Chaintarli et al., 2018 | 2 | 0% | 0% | Identification and AST with VITEK 2 and phenotypic detection of ESBLs | [61] | |
| Roy et al., 2018 | 0 ϕ | NA | NA | Metagenomic analyses of water samples | [62] | |
| Europe and Central Asia | ||||||
| Tajikistan | No Reports | |||||
| East Asia and Pacific | ||||||
| Democratic People’s Republic of Korea | No Reports | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizk, S.S.; Elwakil, W.H.; Attia, A.S. Antibiotic-Resistant Acinetobacter baumannii in Low-Income Countries (2000–2020): Twenty-One Years and Still below the Radar, Is It Not There or Can They Not Afford to Look for It? Antibiotics 2021, 10, 764. https://doi.org/10.3390/antibiotics10070764
Rizk SS, Elwakil WH, Attia AS. Antibiotic-Resistant Acinetobacter baumannii in Low-Income Countries (2000–2020): Twenty-One Years and Still below the Radar, Is It Not There or Can They Not Afford to Look for It? Antibiotics. 2021; 10(7):764. https://doi.org/10.3390/antibiotics10070764
Chicago/Turabian StyleRizk, Soha S., Wafaa H. Elwakil, and Ahmed S. Attia. 2021. "Antibiotic-Resistant Acinetobacter baumannii in Low-Income Countries (2000–2020): Twenty-One Years and Still below the Radar, Is It Not There or Can They Not Afford to Look for It?" Antibiotics 10, no. 7: 764. https://doi.org/10.3390/antibiotics10070764
APA StyleRizk, S. S., Elwakil, W. H., & Attia, A. S. (2021). Antibiotic-Resistant Acinetobacter baumannii in Low-Income Countries (2000–2020): Twenty-One Years and Still below the Radar, Is It Not There or Can They Not Afford to Look for It? Antibiotics, 10(7), 764. https://doi.org/10.3390/antibiotics10070764







