Effect of Antibacterial Root Canal Sealer on Persistent Apical Periodontitis
Abstract
1. Introduction
2. Results
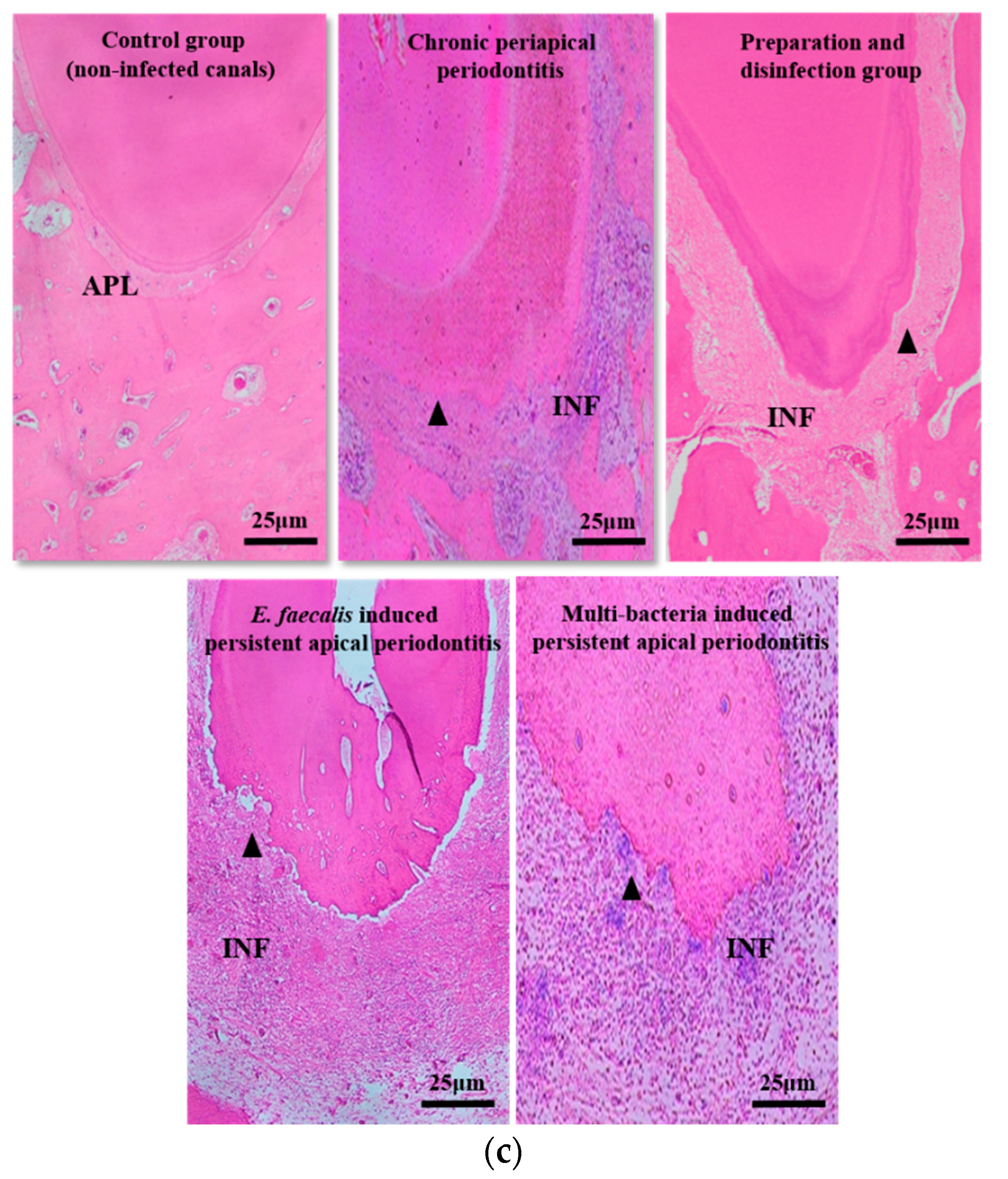
2.1. The Volume and Inflammatory Grade of Apical Lesions of Persistent AP
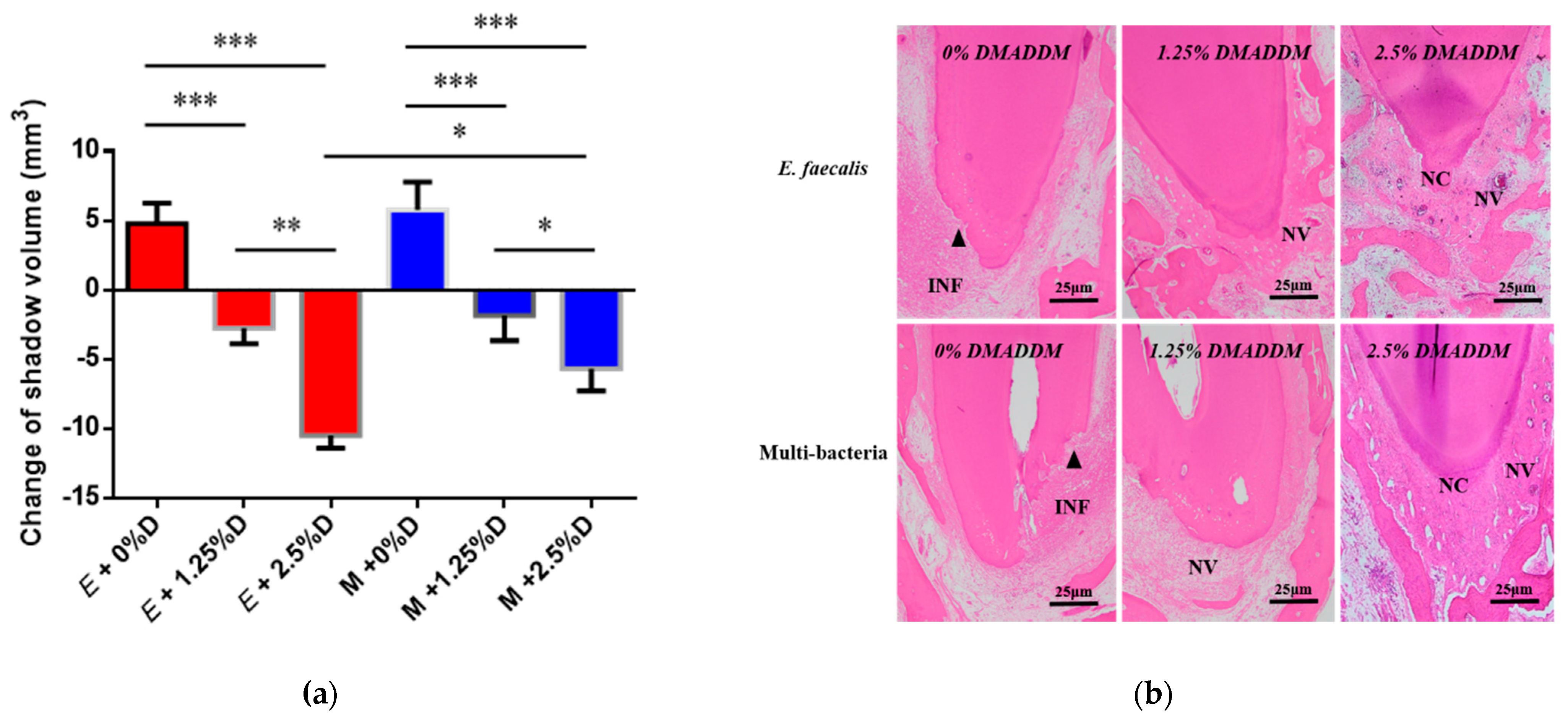
2.2. Analysis of the Antibacterial Effect of DMADDM-Modified Sealer on Persistent AP
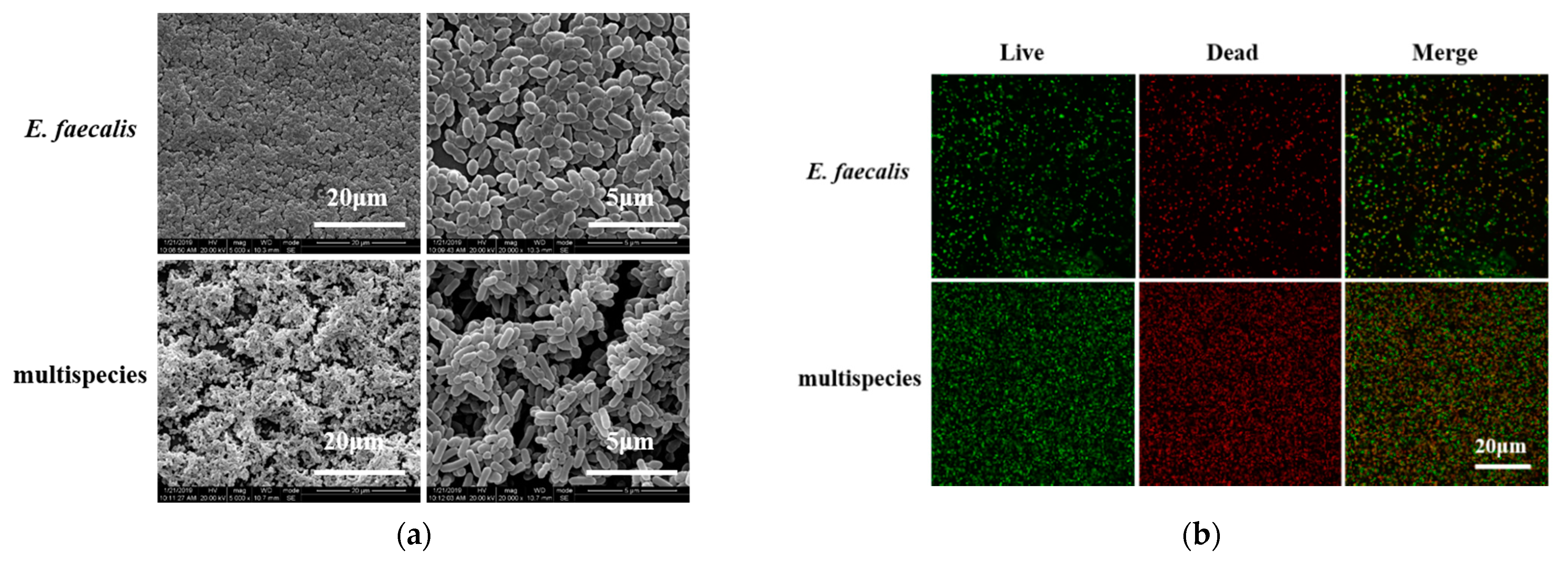
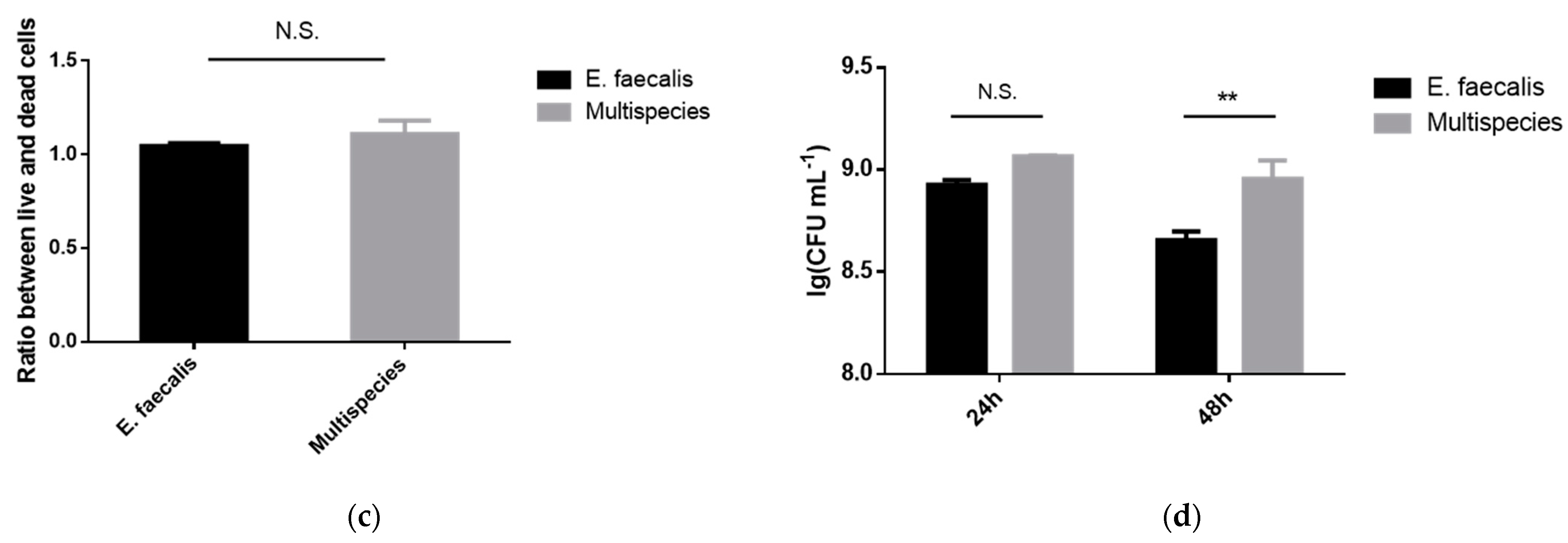
2.3. Morphology and Proliferation Activity of Biofilm Models In Vitro
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Culture Conditions
4.2. Synthesis of DMADDM and Modified Root Canal Sealer Preparation
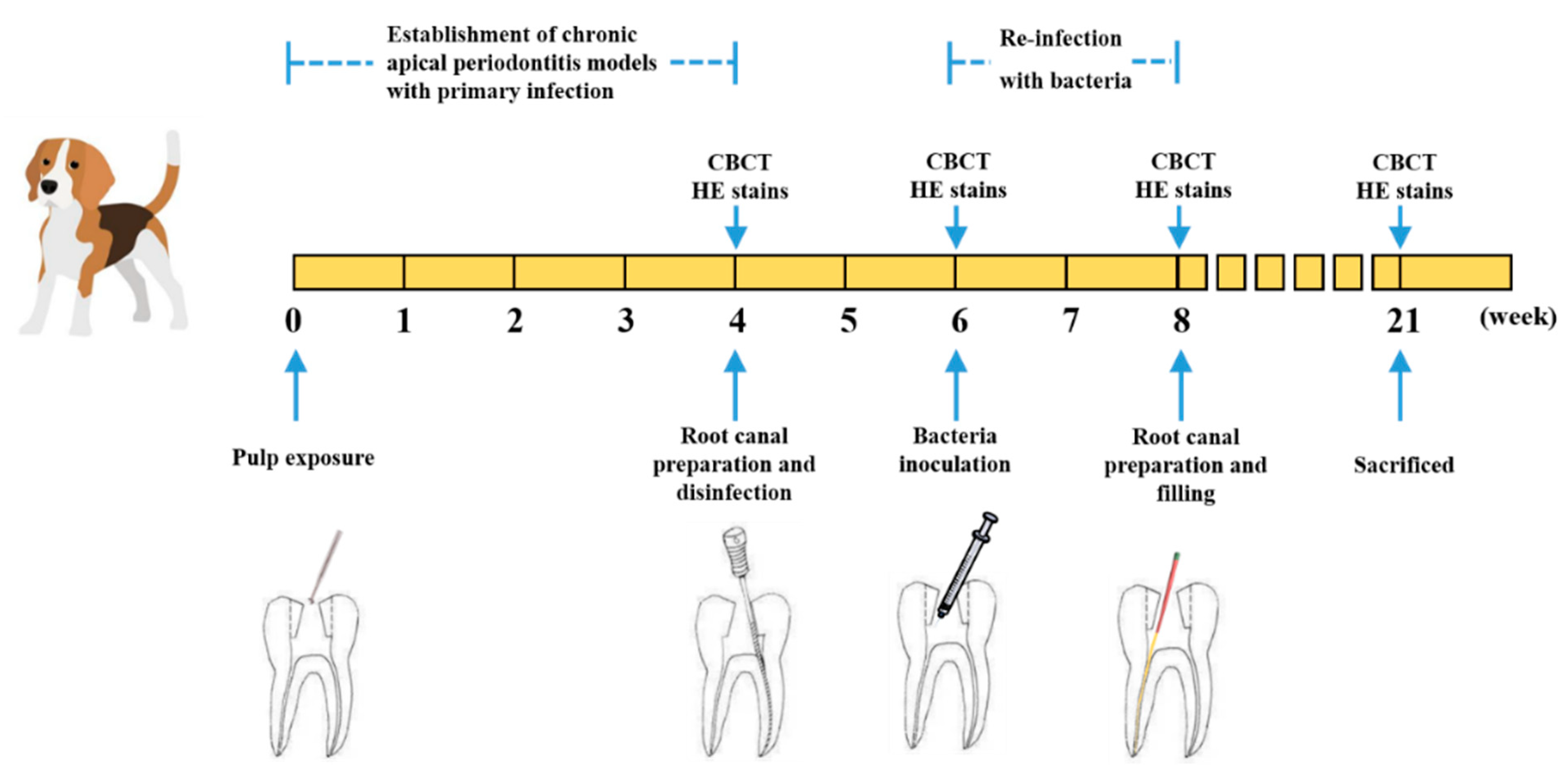
4.3. Animal Preparations
4.4. Establishment of Persistent AP Models
4.5. Antibacterial Efficiency of Modified Sealer on Persistent AP
4.6. Biofilm Formation and Imaging
4.7. DNA Isolation and Real-Time Polymerase Chain Reaction
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Siqueira, J.F., Jr.; Rôças, I.N.; Ricucci, D.; Hülsmann, M. Causes and management of post-treatment apical periodontitis. Br. Dent. J. 2014, 216, 305–312. [Google Scholar] [CrossRef]
- Estrela, C.; Freitas Silva, B.S.; Silva, J.A.; Yamamoto-Silva, F.P.; Pinto-Júnior, D.D.; Gomez, R.S. Stem Cell Marker Expression in Persistent Apical Periodontitis. J. Endod. 2017, 43, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.N. On the causes of persistent apical periodontitis: A review. Int. Endod. J. 2006, 39, 249–281. [Google Scholar] [CrossRef]
- Chow, A.T.; Quah, S.Y.; Bergenholtz, G.; Lim, K.C.; Yu, V.S.H.; Tan, K.S. Bacterial species associated with persistent apical periodontitis exert differential effects on osteogenic differentiation. Int. Endod. J. 2019, 52, 201–210. [Google Scholar] [CrossRef]
- Pinheiro, E.T.; Gomes, B.P.; Ferraz, C.C.; Sousa, E.L.; Teixeira, F.B.; Souza-Filho, F.J. Microorganisms from canals of root-filled teeth with periapical lesions. Int. Endod. J. 2003, 36, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F., Jr.; Rôças, I.N. Polymerase chain reaction-based analysis of microorganisms associated with failed endodontic treatment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 97, 85–94. [Google Scholar] [CrossRef]
- Bouillaguet, S.; Manoil, D.; Girard, M.; Louis, J.; Gaïa, N.; Leo, S.; Schrenzel, J.; Lazarevic, V. Root Microbiota in Primary and Secondary Apical Periodontitis. Front. Microbiol. 2018, 9, 2374. [Google Scholar] [CrossRef] [PubMed]
- Stuart, C.H.; Schwartz, S.A.; Beeson, T.J.; Owatz, C.B. Enterococcus faecalis: Its role in root canal treatment failure and current concepts in retreatment. J. Endod. 2006, 32, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Athanassiadis, B.; Abbott, P.V.; Walsh, L.J. The use of calcium hydroxide, antibiotics and biocides as antimicrobial medicaments in endodontics. Aust. Dent. J. 2007, 52, S64–S82. [Google Scholar] [CrossRef]
- De Paz, L.E.C. Development of a multispecies biofilm community by four root canal bacteria. J. Endod. 2012, 38, 318–323. [Google Scholar] [CrossRef]
- Corotti, M.V.; Zambuzzi, W.F.; Paiva, K.B.; Menezes, R.; Pinto, L.C.; Lara, V.S.; Granjeiro, J.M. Immunolocalization of matrix metalloproteinases-2 and -9 during apical periodontitis development. Arch. Oral Biol. 2009, 54, 764–771. [Google Scholar] [CrossRef]
- Lin, S.K.; Kok, S.H.; Kuo, M.Y.; Wang, T.J.; Wang, J.T.; Yeh, F.T.; Hsiao, M.; Lan, W.H.; Hong, C.Y. Sequential expressions of MMP-1, TIMP-1, IL-6, and COX-2 genes in induced periapical lesions in rats. Eur. J. Oral Sci. 2002, 110, 246–253. [Google Scholar] [CrossRef]
- Cheung, G.S.; Ho, M.W. Microbial flora of root canal-treated teeth associated with asymptomatic periapical radiolucent lesions. Oral Microbiol. Immunol. 2001, 16, 332–337. [Google Scholar] [CrossRef]
- Lu, B.; Zhang, J.; Huang, X.; Xiao, S.; Zhang, M.; Cai, Z. Expression of Interleukin-1β and Matrix Metalloproteinase-8 in Cytolytic and Noncytolytic Enterococcus faecalis-induced Persistent Apical Periodontitis: A Comparative Study in the Rat. J. Endod. 2015, 41, 1288–1293. [Google Scholar] [CrossRef]
- Yamauchi, N.; Yamauchi, S.; Nagaoka, H.; Duggan, D.; Zhong, S.; Lee, S.M.; Teixeira, F.B.; Yamauchi, M. Tissue engineering strategies for immature teeth with apical periodontitis. J. Endod. 2011, 37, 390–397. [Google Scholar] [CrossRef]
- Leonardo, M.R.; Flores, D.S.; de Paula, E.S.F.W.; de Toledo Leonardo, R.; da Silva, L.A. A comparison study of periapical repair in dogs’ teeth using RoekoSeal and AH plus root canal sealers: A histopathological evaluation. J. Endod. 2008, 34, 822–825. [Google Scholar] [CrossRef]
- Fan, W.; Li, Y.; Sun, Q.; Tay, F.R.; Fan, B. Quaternary ammonium silane, calcium and phosphorus-loaded PLGA submicron particles against Enterococcus faecalis infection of teeth: An in vitro and in vivo study. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110856. [Google Scholar] [CrossRef]
- Del Carpio-Perochena, A.; Kishen, A.; Shrestha, A.; Bramante, C.M. Antibacterial Properties Associated with Chitosan Nanoparticle Treatment on Root Dentin and 2 Types of Endodontic Sealers. J. Endod. 2015, 41, 1353–1358. [Google Scholar] [CrossRef]
- Kitagawa, R.; Kitagawa, H.; Izutani, N.; Hirose, N.; Hayashi, M.; Imazato, S. Development of an antibacterial root canal filling system containing MDPB. J. Dent. Res. 2014, 93, 1277–1282. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, K.; Zhou, X.; Xu, N.; Xu, H.H.; Weir, M.D.; Ge, Y.; Wang, S.; Li, M.; Li, Y.; et al. Antibacterial effect of dental adhesive containing dimethylaminododecyl methacrylate on the development of Streptococcus mutans biofilm. Int. J. Mol. Sci. 2014, 15, 12791–12806. [Google Scholar] [CrossRef]
- Wang, S.P.; Ge, Y.; Zhou, X.D.; Xu, H.H.; Weir, M.D.; Zhang, K.K.; Wang, H.H.; Hannig, M.; Rupf, S.; Li, Q.; et al. Effect of anti-biofilm glass-ionomer cement on Streptococcus mutans biofilms. Int. J. Oral Sci. 2016, 8, 76–83. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, K.; Zhang, N.; Melo, M.A.S.; Weir, M.D.; Zhou, X.D.; Bai, Y.X.; Reynolds, M.A.; Xu, H.H.K. Developing a New Generation of Antimicrobial and Bioactive Dental Resins. J. Dent. Res. 2017, 96, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Peng, X.; Wang, S.; Han, Q.; Li, B.; Zhou, X.; Ren, B.; Xu, H.H.K.; Weir, M.D.; Li, M.; et al. A novel antibacterial resin-based root canal sealer modified by Dimethylaminododecyl Methacrylate. Sci. Rep. 2019, 9, 10632. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Feng, Y.; Zhang, R.; Liu, W.; Lei, L.; Hu, T. The extent of pyroptosis varies in different stages of apical periodontitis. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 226–237. [Google Scholar] [CrossRef]
- Bletsa, A.; Virtej, A.; Berggreen, E. Vascular endothelial growth factors and receptors are up-regulated during development of apical periodontitis. J. Endod. 2012, 38, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Jiang, X.; Lin, D.; Chen, Y.; Tong, Z. The Starvation Resistance and Biofilm Formation of Enterococcus faecalis in Coexistence with Candida albicans, Streptococcus gordonii, Actinomyces viscosus, or Lactobacillus acidophilus. J. Endod. 2016, 42, 1233–1238. [Google Scholar] [CrossRef]
- Vengerfeldt, V.; Špilka, K.; Saag, M.; Preem, J.K.; Oopkaup, K.; Truu, J.; Mändar, R. Highly diverse microbiota in dental root canals in cases of apical periodontitis (data of illumina sequencing). J. Endod. 2014, 40, 1778–1783. [Google Scholar] [CrossRef]
- Murad, C.F.; Sassone, L.M.; Faveri, M.; Hirata, R., Jr.; Figueiredo, L.; Feres, M. Microbial diversity in persistent root canal infections investigated by checkerboard DNA-DNA hybridization. J. Endod. 2014, 40, 899–906. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Dummer, P.M. Properties and applications of calcium hydroxide in endodontics and dental traumatology. Int. Endod. J. 2011, 44, 697–730. [Google Scholar] [CrossRef]
- Kayaoglu, G.; Erten, H.; Bodrumlu, E.; Ørstavik, D. The resistance of collagen-associated, planktonic cells of Enterococcus faecalis to calcium hydroxide. J. Endod. 2009, 35, 46–49. [Google Scholar] [CrossRef]
- Ge, Y.; Wang, S.; Zhou, X.; Wang, H.; Xu, H.H.; Cheng, L. The Use of Quaternary Ammonium to Combat Dental Caries. Materials 2015, 8, 3532–3549. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, K.; Melo, M.A.; Weir, M.D.; Zhou, X.; Xu, H.H. Anti-biofilm dentin primer with quaternary ammonium and silver nanoparticles. J. Dent. Res. 2012, 91, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Grandini, S.; Ames, J.M.; Gu, L.S.; Kim, S.K.; Pashley, D.H.; Gutmann, J.L.; Tay, F.R. Critical review on methacrylate resin-based root canal sealers. J. Endod. 2010, 36, 383–399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, S.; Zhou, X.; Xu, H.H.; Weir, M.D.; Ge, Y.; Li, M.; Wang, S.; Li, Y.; Xu, X.; et al. Effect of antibacterial dental adhesive on multispecies biofilms formation. J. Dent. Res. 2015, 94, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Coburn, P.S.; Gilmore, M.S. The Enterococcus faecalis cytolysin: A novel toxin active against eukaryotic and prokaryotic cells. Cell. Microbiol. 2003, 5, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.S.; Seo, J.G.; Lee, G.S.; Kim, J.H.; Kim, S.Y.; Han, Y.W.; Kang, H.; Kim, H.O.; Rhee, J.H.; Chung, M.J.; et al. Antimicrobial activity of enterocins from Enterococcus faecalis SL-5 against Propionibacterium acnes, the causative agent in acne vulgaris, and its therapeutic effect. J. Microbiol. 2009, 47, 101–109. [Google Scholar] [CrossRef] [PubMed]
- De Paz, L.E.C.; Davies, J.R.; Bergenholtz, G.; Svensäter, G. Strains of Enterococcus faecalis differ in their ability to coexist in biofilms with other root canal bacteria. Int. Endod. J. 2015, 48, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Weir, M.D.; Zhang, K.; Arola, D.D.; Zhou, X.; Xu, H.H. Dental primer and adhesive containing a new antibacterial quaternary ammonium monomer dimethylaminododecyl methacrylate. J. Dent. 2013, 41, 345–355. [Google Scholar] [CrossRef]
- Liang, J.; Li, M.; Ren, B.; Wu, T.; Xu, H.H.K.; Liu, Y.; Peng, X.; Yang, G.; Weir, M.D.; Zhang, S.; et al. The anti-caries effects of dental adhesive resin influenced by the position of functional groups in quaternary ammonium monomers. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2018, 34, 400–411. [Google Scholar] [CrossRef]
- Cheng, L.; Weir, M.D.; Xu, H.H.; Antonucci, J.M.; Kraigsley, A.M.; Lin, N.J.; Lin-Gibson, S.; Zhou, X. Antibacterial amorphous calcium phosphate nanocomposites with a quaternary ammonium dimethacrylate and silver nanoparticles. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2012, 28, 561–572. [Google Scholar] [CrossRef]
- Liang, J.; Liu, F.; Zou, J.; Xu, H.H.K.; Han, Q.; Wang, Z.; Li, B.; Yang, B.; Ren, B.; Li, M.; et al. pH-Responsive Antibacterial Resin Adhesives for Secondary Caries Inhibition. J. Dent. Res. 2020, 99, 1368–1376. [Google Scholar] [CrossRef] [PubMed]

| Groups | The Categories of Inflammation | The Average Number of Inflammatory Cells |
|---|---|---|
| Initial chronic AP | 3 | 196 |
| After root canal disinfection for 2 weeks | 1 | 42 |
| E.faecalis-induced persistent AP | 3 | 210 |
| Multi-bacteria-induced persistent AP | 3 | 193 |
| Groups | The Categories of Inflammation | The Average Number of Inflammatory Cells |
|---|---|---|
| E.faecalis + 0% DMADDM | 3 | 207 |
| E.faecalis + 1.25% DMADDM | 2 | 88 |
| E.faecalis + 2.5% DMADDM | 1 | 30 |
| Multi-bacteria + 0% DMADDM | 3 | 201 |
| Multi-bacteria + 1.25% DMADDM | 2 | 89 |
| Multi-bacteria + 2.5% DMADDM | 1 | 35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Yang, G.; Ren, B.; Gao, Y.; Peng, X.; Li, M.; H.K.Xu, H.; Han, Q.; Li, J.; Zhou, X.; et al. Effect of Antibacterial Root Canal Sealer on Persistent Apical Periodontitis. Antibiotics 2021, 10, 741. https://doi.org/10.3390/antibiotics10060741
Wang Z, Yang G, Ren B, Gao Y, Peng X, Li M, H.K.Xu H, Han Q, Li J, Zhou X, et al. Effect of Antibacterial Root Canal Sealer on Persistent Apical Periodontitis. Antibiotics. 2021; 10(6):741. https://doi.org/10.3390/antibiotics10060741
Chicago/Turabian StyleWang, Zheng, Ge Yang, Biao Ren, Yuan Gao, Xian Peng, Mingyun Li, Hockin H.K.Xu, Qi Han, Jiyao Li, Xuedong Zhou, and et al. 2021. "Effect of Antibacterial Root Canal Sealer on Persistent Apical Periodontitis" Antibiotics 10, no. 6: 741. https://doi.org/10.3390/antibiotics10060741
APA StyleWang, Z., Yang, G., Ren, B., Gao, Y., Peng, X., Li, M., H.K.Xu, H., Han, Q., Li, J., Zhou, X., & Cheng, L. (2021). Effect of Antibacterial Root Canal Sealer on Persistent Apical Periodontitis. Antibiotics, 10(6), 741. https://doi.org/10.3390/antibiotics10060741








