Abstract
Colistin has a long story of safe use in animals for the treatment and prevention of certain bacterial diseases. Nevertheless, the first description of the mcr-1 gene showed that colistin resistance can spread by horizontal gene transfer and changed the landscape. This study aimed to assess the effect of colistin administration on the dispersion of resistance in the microbiota of day-old broiler chicks and how the presence of mcr-1 genes influences the spread of colistin resistance determinants. In this study, 100 one-day-old chicks were divided into four groups of 25 animals (G1, G2, G3, and G4). Animals from G3/G4 were challenged with mcr-1-carrying Salmonella (day 7), while colistin (600 mg/L) was administered daily to G2/G4 animals through drinking water (from day 8 to day 15). Two quantitative PCR assays were performed to compare the amount of Salmonella and mcr-1 that were present in the caecal samples. We observed that levels of mcr-1 were higher in G3/G4 animals, especially G4, due to the spread of mcr-1-carrying Salmonella. On day 21, Salmonella levels decreased in G4, reaching similar values as those for G3, but mcr-1 levels remained significantly higher, suggesting that colistin may accelerate the spreading process when mcr-1-carrying bacteria reach the gut.
Keywords:
colistin; mcr-1; Salmonella; in vivo assay; real-time PCR quantification; microbiota; chicks 1. Introduction
The gut microbiome constitutes a rich and diverse microbial ecosystem that influences host nutrition and physiology, developing important functions that are beneficial for host health [1]. The microbiota of new-born chicks, first colonised by bacteria of the facultative anaerobic Proteobacteria phylum, progressively changes during the first 19 days of life [2]. Commensal Enterobacteriaceae is a major constituent of Proteobacteria and plays an important role in the protection of the gut against colonisation by pathogens, such as Salmonella enterica (especially serovars Typhimurium and Enteritidis), obligate anaerobic spore-forming bacteria, such as Clostridia species, and facultative anaerobic bacteria from the Streptococcus and Staphylococcus genera [3]. However, Proteobacteria, especially Enterobacteriaceae, play a critical role in the emergence and maintenance of antimicrobial resistance, as the genes responsible are more prone to be found in this phylum (and also in Firmicutes) than in other phyla of the gut microbiome [4]. For example, plasmid-encoded resistance genes can move between gut bacteria via horizontal gene transfer. Thus, commensal Enterobacteriaceae become an excellent reservoir for antimicrobial resistance genes, making them accessible to other pathogenic or commensal bacteria [4,5].
Antimicrobial resistance is a public health concern worldwide and has become an increasing threat to human and animal health over the last decade, limiting the therapeutic alternatives available to treat infections caused by multidrug-resistant (MDR) pathogens. The increase of human infections due to multidrug-resistant Gram-negative bacteria, especially those producing extended-spectrum beta-lactamases and carbapenemases, has forced the reintroduction of colistin to treat infections caused by these microorganisms in human medicine, as it is often the only effective antimicrobial against them [6,7,8]. Consequently, colistin has been classified among the antibiotic groups with activity against “Critical Priority” or “High Priority” pathogens identified by the World Health Organisation (WHO) [9] and is considered to be the last line to treat these infections.
The prevalence of colistin resistance in Enterobacteriaceae from food animals has been increasing annually and is thought to be related to the use of colistin in veterinary medicine on a global scale [10,11]. Colistin has been used for decades in animals to treat and prevent infectious diseases [6,12,13], usually administered orally in feed or drinking water [14,15]. The Committee for Medicinal Products for Veterinary Use (CVMP) of the European Medicines Agency (EMA) has restricted the administration of colistin sulphate to poultry to 3 to 7 days to treat gastrointestinal infections [16].
Until 2015, all described colistin resistance mechanisms were related to chromosomal point mutations, mainly involving the two-component regulatory system (pmrAB/phoPQ) and its negative regulator mgrB. However, a new plasmid-mediated gene, called mcr-1, was first described in that year, which meant that horizontal transfer was possible [8,17]. Thus, due to the increase of colistin resistance and the emergence of the mcr-1 gene, the EMA recommended minimising the use of colistin in animals in the European Union (EU) in 2016, aiming to limit its use to 5 mg/population correction unit (PCU) at the national level, with a desirable level of 1 mg/PCU [15]. Since then, colistin resistance levels have appeared to drop with the decrease in its use. We also observed this trend in a recent study carried out in Spain [18].
There have been no studies of the effects of colistin administration on the intestinal microbiota of the neonatal gut and its ability to select colistin-resistant bacteria. Thus, we aimed to assess the effect of colistin administration on the dispersion of resistance in the microbiota of day-old broiler chicks and how the presence of mcr-1 genes influences the spread of colistin resistance determinants using a monophasic Salmonella Typhimurium strain carrying a mcr-1 gene.
2. Results
2.1. Clinical Signs and Pathological Findings
Four animals (two animals from G1, one from G3, and one from G4) died during the first week during the adjustment period. Post-mortem examination revealed fibrinous pericarditis and perihepatitis (septicaemic colibacillosis) as the cause of death. We collected samples from these animals, as their deaths occurred the day before the first sampling. Thus, none of the samples were excluded from the experiment. There were no significant changes found in any animal included in the study during the post-mortem examinations throughout the experiment.
2.2. Salmonella Counts in the Chick Samples
The amount of Salmonella in the chick caecal samples was estimated by plate count. Only animals from the Salmonella-challenged groups (G3 and G4) showed growth of Salmonella on SMID2 plates (Figure 1). The mean values were non-statistically different (p < 0.05): from <5.4 × 101 to 1.2 × 105 CFU/mg (mean of 2.0 × 104 CFU/mg) for G3 (without colistin) and from <5.4 × 101 to 3.6 × 105 CFU/mg (mean of 2.3 × 104 CFU/mg) for G4 (colistin treated). Chicks from G4 had higher Salmonella counts from D9 to D14, at which time G4 reached the highest value. Finally, after the withdrawal of colistin (D14), both G3 and G4 showed similar CFU counts on D21 (Figure 1). All recovered Salmonella isolates were mcr-1-positive by conventional PCR.
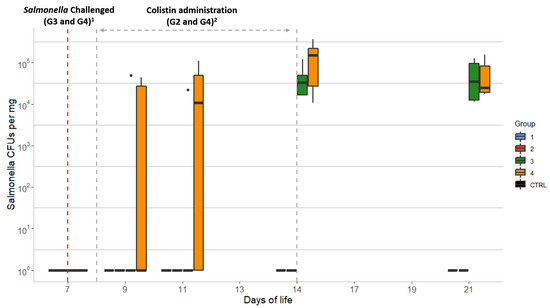
Figure 1.
Evolution of Salmonella counts over 21 days in chick caecal samples: CTRL: control group; Group 1 (G1): animals without colistin nor Salmonella challenge; Group 2 (G2): colistin administered; Group 3 (G3): Salmonella challenged; Group 4 (G4): colistin administered and Salmonella challenged. Dots represent outliers; 1 Salmonella challenge started at D7 for groups G3 and G4 (sampling from D7 was performed before the challenge was started); 2 Colistin was provided to chicks from groups G2 and G4 from D8 to D14.
2.3. Quantitative qPCR of the mcr-1 Gene
In total, 87 of 100 caecal samples were analysed on D7 (7, 20, 20, 20, and 20 samples from the control group, G1, G2, G3, and G4, respectively). Overall, 13 samples were excluded because it was impossible to obtain enough samples (at least 100 mg) for DNA extraction.
Quantitative PCR data for the mcr-1 gene of the 7 animals from the CTRL group and 21 animals from G1 (neither receiving colistin nor challenged with Salmonella) showed high inter-individual variability. Two animals from the CTRL group showed the barely detectable presence of the mcr-1 gene, which remained below the quantification limit, and four animals from G1 showed quantifiable levels ranging from 6.7 × 103 to 1.6 × 106 mcr-1 gene copies per mg caecal content, establishing a slight background. The remaining 17 chicks showed no qPCR signals or showed mcr-1 values below the quantitation limit. The values for the mcr-1 levels from the groups not challenged with Salmonella (G1 and G2) remained similar throughout most of the study, reaching their peaks on D11 (3.2 × 105 and 2.6 × 106 gene copies per mg caecal content, respectively) (Table 1). Concerning the Salmonella-challenged groups (G3 and G4), chicks from G3 (colistin untreated) showed an increase from less than 1.0 × 102 to 1.9 × 105 mcr-1 copies/mg from D9 to D21. The number of mcr-1 copies/mg for chicks from G4 (colistin treated) increased from 1.5 × 103 to 2.1 × 105 for the same period. The greatest differences between the four groups were observed on D11 and D14, coinciding with the end of colistin administration, with G4 showing significantly higher mcr-1 values, as the median of these values was outside the interquartile range of the other groups (Figure 2). In addition, chickens from G1 and G2 showed a non-significant increase in the median from D9 to D21 (p = 0.23), whereas mcr-1 levels increased throughout the study in the challenged groups, G3 and G4.

Table 1.
Quantitative PCR data for mcr-1 gene copies/mg per day of life (expressed in Log10).
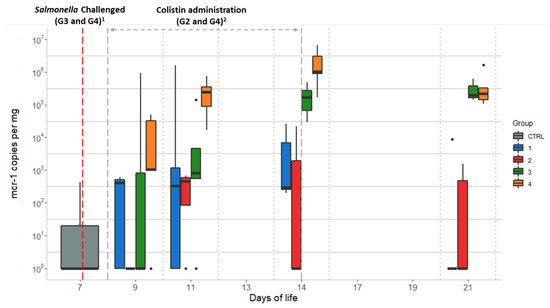
Figure 2.
Quantitative qPCR data of mcr-1 gene copies/mg obtained per day of life of the chicks for each studied group: Group 1 (G1): animals without colistin nor Salmonella challenge; Group 2 (G2): colistin administered; Group 3 (G3): Salmonella challenged; Group 4 (G4): colistin administered and Salmonella challenged. Dots represent outliers; 1 Salmonella challenge started at D7 for groups G3 and G4 (sampling from D7 was performed before starting the challenge); 2 Colistin was provided to chicks from groups G2 and G4 from D8 to D14.
2.4. Comparison of Salmonella Spp. and mcr-1 Quantitative qPCR Data
Quantitative PCR data for Salmonella quantification for the groups, including chicks inoculated with mcr-1-carrying Salmonella, showed median values for G3 (without colistin) of <3.3 × 102, 5.1 × 103, 1.5 ×1 03, 1.3 × 104, and 1.1 × 104 copies per mg for days 7, 9, 11, 14, and 21, respectively. For G4 (colistin administered), the corresponding median values were <3.3×102, 1.7 × 103, 1.7 × 106, 4.7 × 104, and 1.6 × 104 copies/mg (Figure 3). Quantification of mcr-1 and Salmonella showed similar values on D9 (p < 0.05, Table 2), indicating that most copies of the mcr-1 genes in the samples came from the inoculated S. Typhimurium strain. The levels of mcr-1 and Salmonella increased thereafter in G4, during the colistin administration period, whereas the levels remained approximately the same in G3. After the withdrawal of colistin, Salmonella levels in G4 decreased, reaching G3-equivalent values on D21. However, mcr-1 levels of both groups remained significantly higher (Table 2), suggesting that mcr-1 may have been transferred to other intestinal bacterial species (Figure 3).
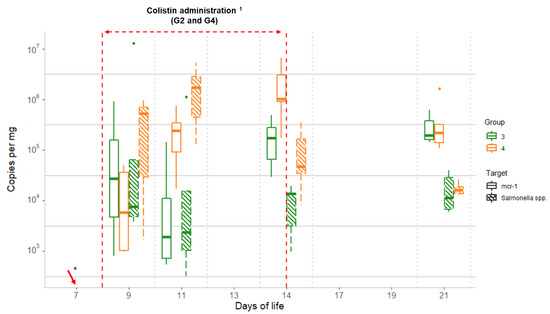
Figure 3.
Comparison between qPCR data for Salmonella and mcr-1 obtained throughout the study for the challenged groups (G3 and G4): Group 3 (G3): Salmonella challenged; Group 4 (G4): colistin administered and Salmonella challenged. Dots represent outliers; 1 Colistin was provided to chicks from group G4, from D8 to D14; * Salmonella challenge started on D7 for groups G3 and G4 (sampling from D7 was performed before starting the challenge).

Table 2.
T-test p-values obtained from a comparative analysis of mcr-1 and Salmonella qPCR quantitative data per sampling day.
3. Discussion
Food-producing animals have been highlighted as potential reservoirs for the dissemination of colistin-resistance determinants, especially since late 2015, when Liu et al. identified the plasmid-mediated colistin resistance gene, mcr-1, in China [17]. Horizontal gene transfer processes have been proposed to play a critical role in the spreading of the mcr-1 gene [19,20]. In the present study, colistin administration had a significant effect on the spread of mcr-mediated colistin resistance in the chicks’ microbiota. Its effect was greater when mcr-1-carrying bacteria were introduced into the gut environment in the presence of colistin, in which case mcr-1 colistin resistance appeared to become widespread. We used a Salmonella enterica serovar Typhimurium strain because neonatal chicks are highly susceptible to colonisation by Salmonella serovars [21] and it is easily identified in chicks because it is not a component of their early microbiota. We studied the effect of colistin by administering it for seven days, as recommended by the EMA for treatment of enteric infections caused by susceptible non-invasive E. coli [15], and focused on the mcr-1 gene because it is the mobile gene most frequently related to colistin resistance worldwide [17].
This scenario has been described in other studies, in which a link was proposed between colistin use and the spread of the mcr-1 gene [12,18,22]. Comparison of the two groups to which colistin was administered (G2 and G4) showed colistin treatment to significantly increase mcr-1 levels in G4 (in which the chicks were challenged with Salmonella) but not G2 (in which the chicks were not challenged with Salmonella).
This study shows the importance of the presence of an intestinal bacterial population that carries the mcr-1 gene for colistin resistance for the dispersion associated with its use. This is clear from the data of the mcr-1 challenged chicks which were not administered colistin (G3). Chicks from non-challenged groups (G1 and G2) showed a baseline presence of mcr-1 from the first day of sampling, which may have been due to the pre-existence of these genes in certain bacterial groups different from Salmonella. However, chicks from these groups (G1 and G2) showed parallel progression, with no significant differences in their mcr-1 outcomes. Chicks from G3, despite not having been given colistin, showed an increase in mcr-1 levels, which became significantly different from those of the animals from G1 and G2 by D14 and D21. Similarly, in assessing the effect of colistin administration, chicks challenged with mcr-1-carrying Salmonella (G4) showed significantly more (p-value < 0.01) mcr-1 copies/mg relative to the non-challenged group (G2) (Figure 2), demonstrating that the presence of gene-carrying Salmonella in the gut is more decisive in gene dispersal than the effect of the antibiotic, as has been seen in other studies [23].
Salmonella counts in chicks from group G4 were higher than those in animals from group G3, as observed for the Salmonella qPCR results. Harbouring mcr genes usually suppose a negative fitness cost to the carrier bacteria [24] and maybe this fact was responsible for lower levels of Salmonella detected in G3 than those from G4, since Salmonella in G4 had a selective advantage because of colistin treatment. Thus, data from group G4 support the initial hypothesis about the effect of colistin use on the spread of colistin resistance caused by mcr family genes. These data are consistent with the high dispersion capacity of the mcr-1 gene, especially in the presence of colistin, as previously described [12,13].
Therefore, the mcr-1 levels of group G4 (colistin administered–Salmonella challenged) began to decline after exposure to colistin stopped, but slower than those of Salmonella, remaining similar to the values of group G3, which suggests that the reduction corresponded to mcr-1 harboured by the Salmonella and that the remaining mcr-1 quantified on D21 mainly came from other bacterial species of the microbiota that had been selected by the administration of colistin. Thus, our results highlight the possible horizontal transfer of genes from Salmonella to other intestinal bacteria, which may have allowed mcr-1 levels to remain higher for a longer period in both groups (G3 and G4). Although the clonal spread of mcr-1-carrying Salmonella is common, there is a close association between certain serotypes of Salmonella enterica, as serovar Typhimurium, and mcr-1 gene and different types of plasmid also play an important role in the conjugation phenomenon [8,25]. Therefore, further studies are needed to determine the type of plasmid where mcr-1 was located and confirm this hypothesis.
4. Methods
4.1. Ethical Approval
Experimental procedures were approved by the University Complutense of Madrid Animal Care and Ethics Committee (date of approval: 31/07/2019; registration number: 99/107262.9/19) in compliance with the regulations of the Community of Madrid (PROEX 152/19). Animal experiments took place in the biosafety level 3 (BSL-3) facilities of the VISAVET Health Surveillance Centre and animals were housed according to the European legislation on animal welfare (Directive 2010/63/EU).
4.2. Experimental Design
In total, 100 one-day-old broiler chicks (Ross 308) were obtained from a commercial hatchery and housed at the VISAVET Surveillance Centre facilities for the 21 days of the experiment under the same environmental conditions described previously by Herrero-Encinas et al. [26]. On the first day, when the animals arrived, box litter samples were collected for Salmonella spp. detection following ISO 6579:2017 standards. All samples were found negative. Chicks were divided into four groups of 25 animals. All chicks were housed in different cages until day (D) 7 but under the same environmental and feeding conditions. Thus, they were considered to be a single group for data analysis (control group: CTRL). From D7, chicks from each cage were exposed to different conditions to form four groups: animals without colistin nor Salmonella challenge (G1), colistin administered (G2), Salmonella challenged (G3), and colistin administered and Salmonella challenged (G4). On D7, chicks from G3 and G4 were challenged with a Salmonella enterica subsp. enterica serovar Typhimurium (monophasic variant) strain that was positive for mcr-1 through their drinking water (3.3 × 105 CFU/mL). Starting from D8, colistin (colistin sulphate 1,025,000 IU, Acolan, Spain) was administered for seven days to chickens of groups G2 and G4 through their drinking water (600 mg/L) adjusting it to a concentration of 75,000 IU recommended in poultry by the EMA [15], replacing the water and corresponding dose of colistin every 24 h.
4.3. Sampling and Sample Preparation
On days 7, 9, 11, 14, and 21, five randomly selected animals from each group (CTRL and G1 to G4) were sedated intramuscularly with diazepam and euthanised with an overdose of sodium pentobarbital by intraperitoneal injection. Caecum faeces were collected during the autopsy, and an aliquot of 1.5 g was stored at 4 °C for analysis using microbiological methods in the following 24 h. The remaining caecal faeces collected from each animal was preserved at −80 °C for molecular analysis.
Salmonella Counting Using Selective Media
A gram of each fresh aliquot was mixed with 2 mL saline (0.85% NaCl). Then, 50 µL was diluted into 9 mL brain–heart infusion broth (BHI) supplemented with a 10 µg colistin disk and incubated at 37 °C for 4 h. Then, six 10-fold serial dilutions were carried out in BHI. ChromID selective medium Salmonella Agar (SMID2) (bioMérieux, Marcy-l’Étoile, France) was used for Salmonella counting. SMID2 plates were inoculated with 100 µL of −3 and −4 BHI sample dilutions and incubated at 37 °C for 24 h. After incubation, all colonies suspected to be Salmonella were counted according to the manufacturer’s specifications. One colony of each SMID2 plate was streaked onto blood agar plates and incubated at 37 °C for 24 h for subsequent species confirmation by mass spectrometry using a Bruker Daltonics UltrafleXtrem MALDI TOF/TOF instrument (Bruker Daltonics, Bremen, Germany). Conventional PCR was performed to confirm the presence of the mcr-1 gene [27].
4.4. Quantitative Assay for Mcr-1
Direct DNA extraction from chick caecal samples was carried out using a commercial kit (FASTI001-1 FavorPrep Stool DNA Isolation Mini Kit, Favorgen-Europe, Vienna, Austria) following the manufacturer’s specifications (elution volume of 200 µL), coupled with a specific SYBRGreen (Thermo Fisher Scientific, Vilnius) real-time PCR assay for quantitative detection of the mcr-1 gene (qPCR), as described previously by Li J et al. [28] and further validated in our previous work [29]. Samples were considered positive if quantitative values were >1.00 × 102 fg/µL (equivalent to 1.58 × 103 copies/mg caecal content).
4.5. Quantitative Real-Time PCR Assay for Salmonella
Quantitative real-time PCR was carried out for the quantitative detection of Salmonella spp. in each sample using a commercial “Salmonella spp. DNA extraction and real-time PCR detection” kit (Kylt® Salmonella spp. (FS), Oldenburg, Germany). Two microliters of each DNA elute were also run in triplicate. A sample was considered to be positive when its cycle threshold (CT) was ≤42.
4.6. Statistical Analysis
The data obtained by qPCR were analysed using a t-test for two related samples after normalisation by logarithmic transformation into Log10. A Kruskal–Wallis test was used to analyse differences in SMID2 Salmonella counts among experimental groups. A difference was considered significant when the p-value was <0.05 for both statistical tests.
4.7. Data Visualisation
All figures included in this study were illustrated with R (core team 2019) [30] using the ggplot2 package (H. Wickham, 2016) [31].
5. Conclusions
The presence of mcr-1-carrying S. enterica serovar Typhimurium in the gut resulted in the spread of the mcr-1 gene, probably due to horizontal gene transfer. The administration of colistin accelerated this process by selecting the colistin-resistant bacteria present in the gut microbiota, keeping mcr-1 levels constant after the withdrawal of colistin.
Author Contributions
Conceptualization, P.M.-V., M.A.M., L.D., and M.U.-R.; methodology, P.M.-V., A.R.-M., A.R.-B., M.H., and D.R.-L.; software, M.H., D.R.-L., and A.Q.; validation, M.H., D.R.-L., and A.Q.; formal analysis, P.M.-V., M.H., M.A.M., D.R.-L., A.G., A.Q., J.G., L.D., and M.U.-R.; investigation P.M-V., M.A.M., L.D., and M.U-R.; resources, P.M-V., M.A.M., L.D., and M.U-R.; data curation, P.M.-V., M.A.M., L.D., and M.U.-R.; writing—original draft preparation, P.M.-V., M.A.M., L.D., and M.U.-R.; writing—review and editing, P.M.-V., M.H., M.A.M., D.R.-L., A.G., A.Q., J.G., L.D., and M.U.-R.; visualization, P.M.-V., M.H., M.A.M., D.R.-L., A.G., A.Q., J.G., L.D., and M.U.-R.; supervision, P.M.-V., M.H., M.A.M., D.R.-L., A.G., A.Q., J.G., L.D., and M.U.-R.; project administration, P.M.-V., M.A.M., L.D., and M.U.-R.; funding acquisition, P.M.-V., M.A.M., L.D., and M.U.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Spanish Ministry of Economy, Industry, and Competitiveness (AGL2016-74882-C3), the Spanish Ministry of Agriculture, Fishing, and Food, and the Autonomous Community of Madrid (S2013/ABI-2747). Pedro Miguela-Villoldo was supported by the FPI Programme (BES-2017-080264) from the Spanish Ministry of Science, Innovation, and Universities and Estefanía Martínez Fernández by a grant co-funded by the European Social Fund and Youth Employment Initiative (YEI) (PEJ-2017-TL/BIO- 7114). Work in the laboratory of Alberto Quesada is also funded by the Junta de Extremadura and FEDER (IB16073 and GR15075) in Spain.
Institutional Review Board Statement
Experimental procedures were approved by the University Complutense of Madrid Animal Care and Ethics Committee (date of approval: 31/07/2019; registration number: 99/107262.9/19) in compliance with the regulations of the Community of Madrid (PROEX 152/19). Animal experiments took place in the biosafety level 3 (BSL-3) facilities of the VISAVET Health Surveillance Centre and animals were housed according to the European legislation on animal welfare (Directive 2010/63/EU).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors wish to thank the technicians María García, Estefanía Rivero, Nisrin Maasoumi, and Estefanía Martínez for their excellent technical assistance at the Foodborne Zoonoses and Antibiotic Resistance Unit, the staff of the Quality and Biosafety Unit, María Mazariegos, Laura Delgado, David Duque, and Pedro Alcubilla, for their technical support at the BSL3 facilities and Red de Investigación en Sanidad Animal (RISA).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rakoff-Nahoum, S.; Paglino, J.; Eslami-Varzaneh, F.; Edberg, S.; Medzhitov, R. Recognition of Commensal Microflora by Toll-Like Receptors Is Required for Intestinal Homeostasis. Cell 2004, 118, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Crhanova, M.; Hradecka, H.; Faldynova, M.; Matulova, M.; Havlickova, H.; Sisak, F.; Rychlik, I. Immune response of chicken gut to natural colonization by gut microflora and to Salmonella enterica serovar enteritidis infection. Infect. Immun. 2011, 79, 2755–2763. [Google Scholar] [CrossRef]
- Litvak, Y.; Mon, K.K.Z.; Nguyen, H.; Chanthavixay, G.; Liou, M.; Velazquez, E.M.; Kutter, L.; Alcantara, M.A.; Byndloss, M.X.; Tiffany, C.R.; et al. Commensal Enterobacteriaceae Protect against Salmonella Colonization through Oxygen Competition. Cell Host Microbe 2019, 25, 128–139.e125. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yang, X.; Qin, J.; Lu, N.; Cheng, G.; Wu, N.; Pan, Y.; Li, J.; Zhu, L.; Wang, X.; et al. Metagenome-wide analysis of antibiotic resistance genes in a large cohort of human gut microbiota. Nat. Commun. 2013, 4, 2151. [Google Scholar] [CrossRef] [PubMed]
- Sommer, M.O.A.; Dantas, G.; Church, G.M. Functional Characterization of the Antibiotic Resistance Reservoir in the Human Microflora. Science 2009, 325, 1128–1131. [Google Scholar] [CrossRef]
- Catry, B.; Cavaleri, M.; Baptiste, K.; Grave, K.; Grein, K.; Holm, A.; Jukes, H.; Liebana, E.; Lopez Navas, A.; Mackay, D.; et al. Use of colistin-containing products within the European Union and European Economic Area (EU/EEA): Development of resistance in animals and possible impact on human and animal health. Int. J. Antimicrob. Agents 2015, 46, 297–306. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Wang, Y.; Zhang, S.; Shen, Z.; Wang, S. Emergence of the colistin resistance gene mcr-1 and its variant in several uncommon species of Enterobacteriaceae from commercial poultry farm surrounding environments. Vet. Microbiol. 2018, 219, 161–164. [Google Scholar] [CrossRef]
- Lima, T.; Domingues, S.; Da Silva, G.J. Plasmid-Mediated Colistin Resistance in Salmonella enterica: A Review. Microorganisms 2019, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Model Lists of Essential Medicines. Available online: https://www.who.int/groups/expert-committee-on-selection-and-use-of-essential-medicines/essential-medicines-lists (accessed on 26 April 2021).
- Poirel, L.; Madec, J.Y.; Lupo, A.; Schink, A.K.; Kieffer, N.; Nordmann, P.; Schwarz, S. Antimicrobial Resistance in Escherichia coli. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef]
- Borowiak, M.; Fischer, J.; Hammerl, J.A.; Hendriksen, R.S.; Szabo, I.; Malorny, B. Identification of a novel transposon-associated phosphoethanolamine transferase gene, mcr-5, conferring colistin resistance in d-tartrate fermenting Salmonella enterica subsp. enterica serovar Paratyphi B. J. Antimicrob. Chemother. 2017, 72, 3317–3324. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, H.; Liu, Y.H.; Feng, Y. Towards Understanding MCR-like Colistin Resistance. Trends Microbiol. 2018, 26, 794–808. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Jayol, A.; Nordmann, P. Polymyxins: Antibacterial Activity, Susceptibility Testing, and Resistance Mechanisms Encoded by Plasmids or Chromosomes. Clin. Microbiol. Rev. 2017, 30, 557–596. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2017. EFSA J. 2019, 17, 5598. [Google Scholar] [CrossRef]
- Agency, E.M. Updated Advice on the Use of Colistin Products in Animals within the European Union: Development of Resistance and Possible Impact on Human and Animal Health. Available online: https://www.ema.europa.eu/documents/scientific-guideline/updated-advice-use-colistin-products-animals-within-european-union-development-resistance-possible_en-0.pdf (accessed on 26 April 2021).
- EMA/CVMP. Article 35. Community Interest Referral: Initiated in Cases Involving the Interests of the Community or Concerns Relating to the Protection of Human or Animal Health or the Environment. [European Commission Final Decision]. 2015. Available online: https://www.ema.europa.eu/en/documents/referral/colistin-oral-article-35-referral-annex-i-ii-iii_en.pdf (accessed on 26 April 2021).
- Liu, Y.Y.; Wang, Y.; Walsh, T.R.; Yi, L.X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Miguela-Villoldo, P.; Hernández, M.; Moreno, M.A.; Rodríguez-Lázaro, D.; Quesada, A.; Domínguez, L.; Ugarte-Ruiz, M. National colistin sales versus colistin resistance in Spanish pig production. Res. Vet. Sci. 2019, 123, 141–143. [Google Scholar] [CrossRef]
- Poirel, L.; Kieffer, N.; Nordmann, P. In Vitro Study of ISApl1-Mediated Mobilization of the Colistin Resistance Gene mcr-1. Antimicrob. Agents Chemother. 2017, 61, e00127-17. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zeng, X.; Li, X.-P.; Liao, X.-P.; Liu, Y.-H.; Lin, J. Plasmid-mediated colistin resistance in animals: Current status and future directions. Anim. Health Res. Rev. 2017, 18, 136–152. [Google Scholar] [CrossRef]
- Sadler, W.W.; Brownell, J.R.; Fanelli, M.J. Influence of Age and Inoculum Level on Shed Pattern of Salmonella typhimurium in Chickens. Avian Dis. 1969, 13, 793–803. [Google Scholar] [CrossRef]
- Skov, R.L.; Monnet, D.L. Plasmid-mediated colistin resistance (mcr-1 gene): Three months later, the story unfolds. Euro Surveill. Eur. Commun. Dis. Bull. 2016, 21, 30155. [Google Scholar] [CrossRef]
- Ahmed, S.; Hansen, C.; Dahlkilde, A.L.; Herrero-Fresno, A.; Pedersen, K.S.; Nielsen, J.P.; Olsen, J.E. The Effect of Colistin Treatment on the Selection of Colistin-Resistant Escherichia coli in Weaner Pigs. Antibiotics 2021, 10, 465. [Google Scholar] [CrossRef] [PubMed]
- Nang, S.C.; Morris, F.C.; McDonald, M.J.; Han, M.L.; Wang, J.; Strugnell, R.A.; Velkov, T.; Li, J. Fitness cost of mcr-1-mediated polymyxin resistance in Klebsiella pneumoniae. J. Antimicrob. Chemother. 2018, 73, 1604–1610. [Google Scholar] [CrossRef]
- Cui, M.; Zhang, J.; Zhang, C.; Li, R.; Wai-Chi Chan, E.; Wu, C.; Wu, C.; Chen, S. Distinct mechanisms of acquisition of mcr-1 -bearing plasmid by Salmonella strains recovered from animals and food samples. Sci. Rep. 2017, 7, 13199. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Encinas, J.; Blanch, M.; Pastor, J.J.; Mereu, A.; Ipharraguerre, I.R.; Menoyo, D. Effects of a bioactive olive pomace extract from Olea europaea on growth performance, gut function, and intestinal microbiota in broiler chickens. Poultry Sci. 2020, 99, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, A.R.; Bortolaia, V.; Kjeldgaard, J.S.; Pedersen, S.K.; Leekitcharoenphon, P.; Hansen, I.M.; Guerra, B.; Malorny, B.; Borowiak, M.; Hammerl, J.A.; et al. Multiplex PCR for detection of plasmid-mediated colistin resistance determinants, mcr-1, mcr-2, mcr-3, mcr-4 and mcr-5 for surveillance purposes. Euro Surveill. Eur. Commun. Dis. Bull. 2018, 23, 17-00672. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shi, X.; Yin, W.; Wang, Y.; Shen, Z.; Ding, S.; Wang, S. A Multiplex SYBR Green Real-Time PCR Assay for the Detection of Three Colistin Resistance Genes from Cultured Bacteria, Feces, and Environment Samples. Front. Microbiol. 2017, 8, 2078. [Google Scholar] [CrossRef] [PubMed]
- Miguela-Villoldo, P.; Moreno, M.A.; Hernández, M.; Rodríguez-Lázaro, D.; Gallardo, A.; Borge, C.; Quesada, A.; Domínguez, L.; Ugarte-Ruiz, M. Complementarity of Selective Culture and qPCR for Colistin Resistance Screening in Fresh and Frozen Pig Cecum Samples. Front. Microbiol. 2020, 11, 572712. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 26 April 2021).
- Wickham, H. ggplot2; Use R! Springer International Publishing; Available online: https://link.springer.com/book/10.1007%2F978-3-319-24277-4 (accessed on 26 April 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).