D-Penicillamine: The State of the Art in Humans and in Dogs from a Pharmacological and Regulatory Perspective
Abstract
1. Introduction
2. Absorption, Distribution, Metabolism and Excretion (ADME)
Mechanism of Action
3. Clinical Applications in Humans and Companion Animals
3.1. In Humans
3.1.1. D-Penicillamine in Wilson Disease and Metal Accumulation
3.1.2. Neonatal Period
3.1.3. Dermatological Application and Cutaneous Adverse Effects
3.2. In Animals
4. Adverse Effect in Humans and Animals
4.1. In Humans
4.2. In Animals
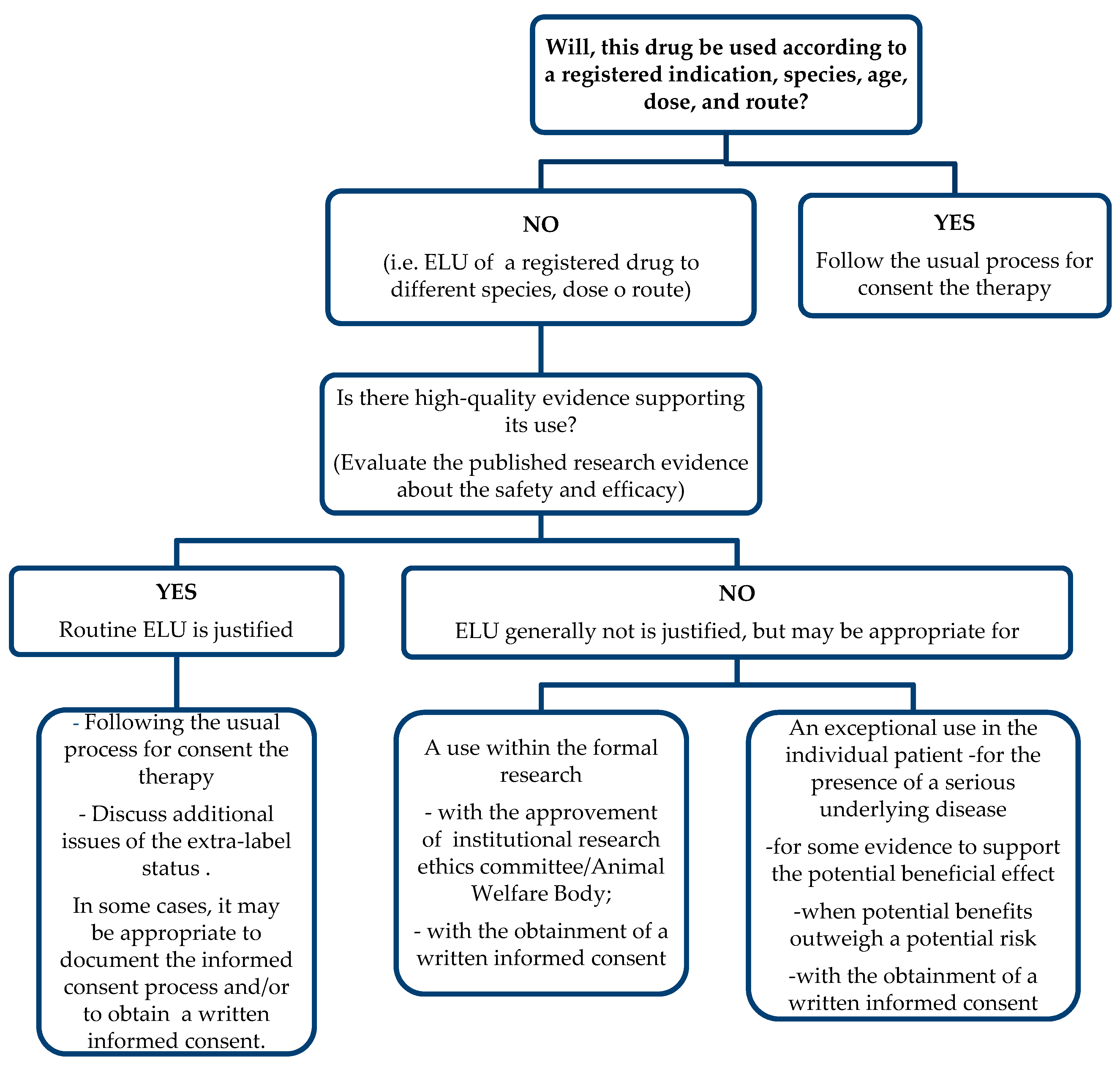
5. Prescription in Veterinary Medicine from a Regulatory Perspective
Extra-Label Drug Use (ELU)
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weigert, W.M.; Offermanns, H.; Degussa, P.S. D-Penicillamine? Production and Properties. Angew. Chem. Int. Ed. 1975, 14, 330–336. [Google Scholar] [CrossRef]
- Lipsky, P.E.; Ziff, M. The effect of D-penicillamine on mitogen-induced human lymphocyte proliferation: Synergistic inhibition by D-penicillamine and copper salts. J. Immunol. 1978, 120, 1006–1013. [Google Scholar]
- Lipsky, P.E.; Ziff, M. Inhibition of Human Helper T Cell Function In Vitro by d-Penicillamine and CuSO4. J. Clin. Investig. 1980, 65, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.C. Collagen cross-linking. Effect of D-penicillamine on cross-linking in vitro. J. Biol. Chem. 1977, 252, 254–259. [Google Scholar] [CrossRef]
- Uitto, J.; Tan, E.M.L.; Ryhanen, L. Inhibition of Collagen Accumulation in Fibrotic Processes: Review of Pharmacologic Agents and New Approaches with Amino Acids and Their Analogues. J. Investig. Dermatol. 1982, 79, 113s–120s. [Google Scholar] [CrossRef] [PubMed]
- Abraham, E.P.; Chain, E.; Baker, W.; Robinson, R. Penicillamine, a Characteristic Degradation Product of Penicillin. Nat. Cell Biol. 1943, 151, 107. [Google Scholar] [CrossRef]
- Birker, P.J.; Freeman, H.C. Structure, properties, and function of a copper (I)-copper(II) complex of D-penicillamine: Pentathallium (I) µ8-chloro-dodeca(D-penicillaminato)octacuprate(I)hexacuprate(II) n-hydrate. J. Am. Chem. Soc. 1977, 99, 6890–6899. [Google Scholar] [CrossRef]
- Ishak, R.; Abbas, O. Penicillamine Revisited: Historic Overview and Review of the Clinical Uses and Cutaneous Adverse Effects. Am. J. Clin. Dermatol. 2013, 14, 223–233. [Google Scholar] [CrossRef]
- Levy, R.S.; Fisher, M.; Alter, J.N. Penicillamine: Review and cutaneous manifestations. J. Am. Acad. Dermatol. 1983, 8, 548–558. [Google Scholar] [CrossRef]
- Medici, V.; Rossaro, L.; Sturniolo, G. Wilson disease—A practical approach to diagnosis, treatment and follow-up. Dig. Liver Dis. 2007, 39, 601–609. [Google Scholar] [CrossRef]
- Lyle, W.H. Penicillamine in metal poisoning. J. Rheumatol. 1981, 7, 96–99. [Google Scholar]
- Chow, G.K.; Streem, S.B. Medical Treatment of Cystinuria: Results of Contemporary Clinical Practice. J. Urol. 1996, 156, 1576–1578. [Google Scholar] [CrossRef]
- Suarez-Almazor, M.E.; Belseck, E.; Spooner, C. Penicillamine for treating rheumatoid arthritis. Cochrane Database Syst. Rev. 2000, CD001460. [Google Scholar] [CrossRef]
- Fieten, H.; Dirksen, K.; Ingh, T.S.V.D.; Winter, E.A.; Watson, A.L.; Leegwater, P.A.; Rothuizen, J. D-penicillamine treatment of copper-associated hepatitis in Labrador retrievers. Vet. J. 2013, 196, 522–527. [Google Scholar] [CrossRef]
- Langlois, D.K.; Querubin, J.R.; Schall, W.D.; Nelson, N.C.; Smedley, R.C. Ammonium tetrathiomolybdate treatment of copper-associated hepatopathy in dogs. J. Vet. Intern. Med. 2019, 33, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- Ramli, F.F. Clinical management of chronic mercury intoxication secondary to skin lightening products: A proposed algorithm. Bosn. J. Basic Med. Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kalia, K.; Flora, S. Strategies for Safe and Effective Therapeutic Measures for Chronic Arsenic and Lead Poisoning. J. Occup. Health 2005, 47, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Kang, M.H.; Park, H.M. Treatment of zinc toxicosis in a dog with chelation using d-penicillamine. J. Vet. Emerg. Crit. Care 2016, 26, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Mandigers, P.; Van Den Ingh, T.; Bode, P.; Rothuizen, J. Improvement in liver pathology after 4 months of D-penicillamine in 5 Doberman Pinschers with subclinical hepatitis. J. Vet. Intern. Med. 2005, 19, 40–43. [Google Scholar] [CrossRef]
- Humann-Ziehank, E.; Bickhardt, K. Effects of D-Penicillamine on Urinary Copper Excretion in High-Copper Supplemented Sheep. J. Vet. Med. Ser. A 2001, 48, 537–544. [Google Scholar] [CrossRef]
- Perrett, D. The metabolism and pharmacology of D-penicillamine in man. J. Rheumatol. 1981, 7, 41–50. [Google Scholar]
- Kyogoku, K.; Inoue, K.; Otake, T.; Noda, K.; Ohzeki, M. Determination of D-Penicillamine and Its Metabolites in Blood and Urine. J. Pharm. Soc. Jpn. 1982, 102, 322–327. [Google Scholar] [CrossRef]
- Waring, R.H.; Mitchell, S.C. The metabolism of 35S-D-penicillamine in man. Xenobiotica 1988, 18, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, S.; Mumper, R.J. D-penicillamine and other low molecular weight thiols: Review of anticancer effects and related mechanisms. Cancer Lett. 2013, 337, 8–21. [Google Scholar] [CrossRef]
- Bialy-Golan, A.; Brenner, S. Penicillamine-induced bullous dermatoses. J. Am. Acad. Dermatol. 1996, 35, 732–742. [Google Scholar] [CrossRef]
- Taylor, H.G.; Samanta, A. Penicillamine in Rheumatoid Arthritis. Drug Saf. 1992, 7, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.; Sjoerdsma, A. Effect of penicillamine on human collagen and its possible application to treatment of scleroderma. Lancet 1966, 288, 996–999. [Google Scholar] [CrossRef]
- Cao, Y.; Skaug, M.A.; Andersen, O.; Aaseth, J. Chelation therapy in intoxications with mercury, lead and copper. J. Trace Elem. Med. Biol. 2015, 31, 188–192. [Google Scholar] [CrossRef]
- Nagler, R.; Cohen, S.; Savulescu, D.; Leschiner, S.; Otradnov, I.; Gavish, M. Penicillamine as a Potent Protector against Injurious Effects of Cigarette Smoke in Aerodigestive Tract Cancer. Oncology 2010, 78, 12–19. [Google Scholar] [CrossRef]
- Nimni, M.E. Mechanism of Inhibition of Collagen Crosslinking by Penicillamine. Proc. R. Soc. Med. 1977, 70, 65–72. [Google Scholar] [CrossRef]
- Chong, C.R.; Auld, D.S. Inhibition of carboxypeptidase A by D-penicillamine: Mechanism and implications for drug design. Biochemistry 2000, 39, 7580–7588. [Google Scholar] [CrossRef]
- Peisach, J.; Blumberg, W.E. A mechanism for the action of penicillamine in the treatment of Wilson’s disease. Mol. Pharmacol. 1969, 5, 200–209. [Google Scholar]
- Munro, R.; Capell, H.A. Penicillamine. Rheumatology 1997, 36, 104–109. [Google Scholar] [CrossRef]
- Jaffe, I.A. Penicillamine: An anti-rheumatoid drug. Am. J. Med. 1983, 75, 63–68. [Google Scholar] [CrossRef]
- Kaya, T.I.; Kokturk, A.; Türsen, Ü.; Ikizoglu, G.; Polat, A. D-Penicillamine Treatment for Lipoid Proteinosis. Pediatr. Dermatol. 2002, 19, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Levene, C.I.; Sharman, D.F.; Callingham, B.A. Inhibition of chick embryo lysyl oxidase by various lathyrogens and the antagonistic effect of pyridoxal. Int. J. Exp. Pathol. 1992, 73, 613–624. [Google Scholar] [PubMed]
- Omori, K.; Fujiseki, Y.; Omori, K.; Suzukawa, J.; Inagaki, C. Regulation of the expression of lysyl oxidase mRNA in cultured rabbit retinal pigment epithelium cells. Matrix Biol. 2002, 21, 337–348. [Google Scholar] [CrossRef]
- Dootson, G.; Sarkany, I. D-penicillamine induced dermopathy in Wilson’s disease. Clin. Exp. Dermatol. 1987, 12, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T.; Hirohata, K. Suppression of human fibroblast proliferation by d-penicillamine and copper sulfate in vitro. Arthritis Rheum. 1988, 31, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Derk, C.; Huaman, G.; Jimenez, S. A retrospective randomly selected cohort study of D-penicillamine treatment in rapidly progressive diffuse cutaneous systemic sclerosis of recent onset. Br. J. Dermatol. 2008, 158, 1063–1068. [Google Scholar] [CrossRef]
- Jain, S.; Samourian, S.; Scheuer, P.; McGee, J.; Sherlock, S. A controlled trial of d-penicillamine therapy in primary biliary cirrhosis. Lancet 1977, 309, 831–834. [Google Scholar] [CrossRef]
- Phelps, D.; Lakatos, L.; Watts, J. D-Penicillamine for preventing retinopathy of prematurity in preterm infants. Cochrane Database Syst. Rev. 2001, CD001073. [Google Scholar] [CrossRef]
- Tandon, M.; Dutta, S.; Dogra, M.R.; Gupta, A. Oral D-penicillamine for the prevention of retinopathy of prematurity in very low birth weight infants: A randomized, placebo-controlled trial. Acta Paediatr. 2010, 99, 1324–1328. [Google Scholar] [CrossRef] [PubMed]
- Laakso, M.; Mutru, O.; Isomaki, H.; Koota, K. Mortality from amyloidosis and renal diseases in patients with rheumatoid arthritis. Ann. Rheum. Dis. 1986, 45, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Savage, A.; Hinton, C.; Tribe, C.R. Experimental murine amyloidosis II: Effect of penicillamine therapy. Br. J. Exp. Pathol. 1980, 61, 471–473. [Google Scholar] [PubMed]
- Staite, N.D.; Zoschke, D.C.; Messner, R.P. Scavenging of Hydrogen Peroxide—A New Mechanism of Action for d-Penicillamine in Rheumatoid Arthritis? N. Engl. J. Med. 1984, 311, 538–539. [Google Scholar] [CrossRef]
- Maines, M.D. Zinc protoporphyrin is a selective inhibitor of heme oxygenase activity in the neonatal rat. Biochim. Biophys. Acta Gen. Subj. 1981, 673, 339–350. [Google Scholar] [CrossRef]
- Squitti, R.; Rossini, P.M.; Cassetta, E.; Moffa, F.; Pasqualetti, P.; Cortesi, M.; Colloca, A.; Rossi, L.; Finazzi-Agro′, A. D-penicillamine reduces serum oxidative stress in Alzheimer’s disease patients. Eur. J. Clin. Investig. 2002, 32, 51–59. [Google Scholar] [CrossRef]
- Brem, S. Angiogenesis and Cancer Control: From Concept to Therapeutic Trial. Cancer Control. 1999, 6, 1–18. [Google Scholar] [CrossRef]
- Togashi, Y.; Li, Y.; Kang, J.-H.; Takeichi, N.; Fujioka, Y.; Nagashima, K.; Kobayashi, H. D-penicillamine prevents the development of hepatitis in long-evans cinnamon rats with abnormal copper metabolism. Hepatology 1992, 15, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Gupte, A.; Mumper, R.J. Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treat. Rev. 2009, 35, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Brem, S.; Grossman, S.A.; Carson, K.A.; New, P.; Phuphanich, S.; Alavi, J.B.; Mikkelsen, T.; Fisher, J.D. Phase 2 trial of copper depletion and penicillamine as antiangiogenesis therapy of glioblastoma. Neuro-Oncology 2005, 7, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Font, L.; Aragon, C.M.G.; Miquel, M. Ethanol-induced conditioned place preference, but not aversion, is blocked by treatment with d-penicillamine, an inactivation agent for acetaldehyde. Psychopharmacology 2006, 184, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Mayou, B.J. D-Penicillamine in the treatment of keloids. Br. J. Dermatol. 1981, 105, 87–89. [Google Scholar] [CrossRef]
- Corrigan, J., Jr.; Damiano, M.; Leissinger, C.; Wulff, K. Treatment of chronic haemophilic synovitis in humans with D-penicillamine. Haemophilia 2003, 9, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, S.L.; Chen, W. D-penicillamine for primary sclerosing cholangitis. Cochrane Database Syst. Rev. 2006, CD004182. [Google Scholar] [CrossRef]
- Beer, W.; Lyle, W. Penicillamine for the treatment of Darier’s disease and other disorders of keratin formation. Lancet 1966, 288, 1337–1340. [Google Scholar] [CrossRef]
- Litwin, T.; Członkowska, A.; Socha, P. Oral Chelator Treatment of Wilson Disease: D-penicillamine. In Clinical and Translational Perspectives on Wilson Disease; Elsevier: Amsterdam, The Netherlands, 2019; pp. 357–364. [Google Scholar]
- Walshe, J. Treatment of Wilson’s disease with penicillamine. Lancet 1960, 275, 188–192. [Google Scholar] [CrossRef]
- Niedermeier, W.; Creitz, E.E.; Holley, H.L. Trace metal composition of synovial fluid from patients with rheumatoid arthritis. Arthritis Rheum. 1962, 5, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Payne, R.W.; Cahill, C.L. Therapeutic studies of D-penicillamine in the treatment of rheumatoid arthritis. J. Okla. State Med. Assoc. 1969, 62, 487–491. [Google Scholar]
- Graziano, J.H.; Leong, J.K.; Friedheim, E. 2,3-Dimercaptosuccinic acid: A new agent for the treatment of lead poisoning. J. Pharmacol. Exp. Ther. 1978, 206, 696–700. [Google Scholar]
- Ren, M.-S.; Zhang, Z.; Wu, J.-X.; Li, F.; Xue, B.-C.; Yang, R.-M. Comparison of long-lasting therapeutic effects between succimer and penicillamine on hepatolenticular degeneration. World J. Gastroenterol. 1998, 4, 530–532. [Google Scholar] [CrossRef] [PubMed]
- Bavdekar, A.R.; Bhave, S.A.; Pradhan, A.M.; Pandit, A.N.; Tanner, M.S. Long term survival in Indian childhood cirrhosis treated with D-penicillamine. Arch. Dis. Child. 1996, 74, 32–35. [Google Scholar] [CrossRef]
- Hunt, A.H.; Parr, R.M.; Taylor, D.M.; Trott, N.G. Relation Between Cirrhosis and Trace Metal Content of Liver. BMJ 1963, 2, 1498–1501. [Google Scholar] [CrossRef] [PubMed]
- Deering, T.B.; Dickson, E.R.; Fleming, C.R.; Geall, M.G.; McCall, J.T.; Baggenstoss, A.H. Effect of d-Penicillamine on Copper Retention in Patients with Primary Biliary Cirrhosis. Gastroenterology 1977, 72, 1208–1212. [Google Scholar] [CrossRef]
- LaRusso, N.F.; Wiesner, R.H.; Ludwig, J.; Maccarty, R.L.; Beaver, S.J.; Zinsmeister, A.R. Prospective trial of penicillamine in primary sclerosing cholangitis. Gastroenterology 1988, 95, 1036–1042. [Google Scholar] [CrossRef]
- Lakatos, L.; Kövér, B.; Péter, F. D-penicillamine therapy of neonatal hyperbilirubinaemia. Acta Paediatr. Acad. Sci. Hung. 1974, 15, 77–85. [Google Scholar]
- Brückmann, G.; Zondek, S.G. Iron, copper and manganese in human organs at various ages. Biochem. J. 1939, 33, 1845–1857. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.-L.; Sauble, E.N.; Cabrera, A.; Roth, A.; Ackland, M.L.; Mercer, J.F.B.; Linder, M.C. Lack of ceruloplasmin expression alters aspects of copper transport to the fetus and newborn, as determined in mice. BioMetals 2011, 25, 373–382. [Google Scholar] [CrossRef]
- Lakatos, L.; Balla, G.; Pataki, I.; Vekerdy-Nagy, Z.; Oroszlán, G. D-Penicillamine in the Neonatal Period; Lambert Academic Publishing: Düsseldorf, Germany, 2016. [Google Scholar]
- Jimenez, S.; Sigal, S.H. A 15-year prospective study of treatment of rapidly progressive systemic sclerosis with D-penicillamine. J. Rheumatol. 1991, 18, 1496–1503. [Google Scholar]
- Falanga, V.; Medsger, T.A. D-penicillamine in the treatment of localized scleroderma. Arch. Dermatol. 1990, 126, 609–612. [Google Scholar] [CrossRef] [PubMed]
- Moynahan, E. D(-)penicillamine in morphœa (localised scleroderma). Lancet 1973, 301, 428–429. [Google Scholar] [CrossRef]
- Sharifian, M.; Sari-Aslani, F.; Hemmatinejad, B.; Fallahzadeh, M.K.; Kasraee, B.; Khoshandish, M.J.; Miri, R.; Mohammadi-Samani, S.; Jowkar, F.; Namazi, M.R. D-penicillamine, a potent melanogenesis inhibitor, lacks any depigmenting effect on black guinea pig skin: The first randomized, evaluator-blinded, vehicle-controlled, in vivo study. Acta Dermatovenerol. Alp. Pannonica Adriat. 2011, 20, 51–53. [Google Scholar]
- Brożyna, A.A.; Van Middlesworth, L.; Slominski, A.T. Inhibition of melanogenesis as a radiation sensitizer for melanoma therapy. Int. J. Cancer 2008, 123, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Stork, J.; Nĕmcová, D.; Hoza, J.; Kodetová, D. Eosinophilic fasciitis in an adolescent girl with lymphadenopathy and vitiligo-like and linear scleroderma-like changes: A case report. Clin. Exp. Rheumatol. 1996, 14, 337–341. [Google Scholar]
- Bischoff, L.; Derk, C.T. Eosinophilic fasciitis: Demographics, disease pattern and response to treatment: Report of 12 cases and review of the literature. Int. J. Dermatol. 2007, 47, 29–35. [Google Scholar] [CrossRef]
- Farrington, M.L.; Haas, J.E.; Nazar-Stewart, V.; Mellins, E.D. Eosinophilic fasciitis in children frequently progresses to scleroderma-like cutaneous fibrosis. J. Rheumatol. 1993, 20, 128–132. [Google Scholar]
- Caspi, D.; Fishel, R.; Varon, M.; Yona, E.; Baratz, M.; Yaron, M. Multisystem presentationof eosinophilic fasciitis. Rheumatology 1982, 21, 218–221. [Google Scholar] [CrossRef]
- Schnabel, A.; Arlt, A.C.; Gross, W.L. Treatment of chronic eosinophilia-myalgia syndrome—Effective therapy regimens become evident. Z. Rheumatol. 1992, 51, 155–157. [Google Scholar]
- Rekha, A. Keloids—A frustrating hurdle in wound healing. Int. Wound J. 2004, 1, 145–148. [Google Scholar] [CrossRef]
- Peacock, E.E., Jr. Biologic basis for the treatment of keloids and hypertrophic scars. South Med. J. 1970, 63, 755–760. [Google Scholar] [CrossRef]
- Paller, A.S. Histology of lipoid proteinosis. JAMA 1994, 272, 564–565. [Google Scholar] [CrossRef]
- Hoffmann, G. Copper-Associated Liver Diseases. Vet. Clin. N. Am. Small Anim. Pract. 2009, 39, 489–511. [Google Scholar] [CrossRef] [PubMed]
- Dirksen, K.; Fieten, H. Canine Copper-Associated Hepatitis. Vet. Clin. N. Am. Small Anim. Pract. 2017, 47, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Webster, C.R.L.; Center, S.A.; Cullen, J.M.; Penninck, D.G.; Richter, K.P.; Twedt, D.C.; Watson, P.J. ACVIM consensus statement on the diagnosis and treatment of chronic hepatitis in dogs. J. Vet. Intern. Med. 2019, 33, 1173–1200. [Google Scholar] [CrossRef] [PubMed]
- Twedt, D.C.; Sternlieb, I.; Gilbertson, S.R. Clinical, morphologic, and chemical studies on copper toxicosis of Bedlington Terriers. J. Am. Vet. Med Assoc. 1979, 175, 269–275. [Google Scholar]
- Thornburg, L.P.; Shaw, D.; Dolan, M.; Raisbeck, M.; Crawford, S.; Dennis, G.L.; Olwin, D.B. Hereditary Copper Toxicosis in West Highland White Terriers. Vet. Pathol. 1986, 23, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Haywood, S.; Rutgers, H.C.; Christian, M.K. Hepatitis and Copper Accumulation in Skye Terriers. Vet. Pathol. 1988, 25, 408–414. [Google Scholar] [CrossRef]
- Webb, C.B.; Twedt, D.C.; Meyer, D.J. Copper-Associated Liver Disease in Dalmatians: A Review of 10 Dogs (1998–2001). J. Vet. Intern. Med. 2002, 16, 665. [Google Scholar] [CrossRef] [PubMed]
- Mandigers, P.J.J.; van den Ingh, T.S.G.A.M.; Bode, P.; Teske, E.; Rothuizen, J. Association between liver copper concentration and subclinical hepatitis in Doberman Pinschers. J. Vet. Intern. Med. 2004, 18, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, G.; van den Ingh, T.S.G.A.M.; Bode, P.; Rothuizen, J. Copper-associated chronic hepatitis in Labrador Retrievers. J. Vet. Intern. Med. 2006, 20, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Fieten, H.; Leegwater, P.A.J.; Watson, A.L.; Rothuizen, J. Canine models of copper toxicosis for understanding mammalian copper metabolism. Mamm. Genome 2011, 23, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Van De Sluis, B.; Rothuizen, J.; Pearson, P.L.; Van Oost, B.A.; Wijmenga, C. Identification of a new copper metabolism gene by positional cloning in a purebred dog population. Hum. Mol. Genet. 2002, 11, 165–173. [Google Scholar] [CrossRef]
- Favier, R.; Spee, B.; Penning, L.; Rothuizen, J. Copper-induced hepatitis: The COMMD1 deficient dog as a translational animal model for human chronic hepatitis. Vet. Q. 2011, 31, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Fieten, H.; Hooijer-Nouwens, B.; Biourge, V.; Leegwater, P.; Watson, A.; Ingh, T.V.D.; Rothuizen, J. Association of Dietary Copper and Zinc Levels with Hepatic Copper and Zinc Concentration in Labrador Retrievers. J. Vet. Intern. Med. 2012, 26, 1274–1280. [Google Scholar] [CrossRef]
- Guilford, W.G.; Center, S.A.; Strombeck, D.R.; Williams, D.A.; Meyer, D.J. Strombeck’s Small Animal Gastroenterology; Saunders: Philadelphia, PA, USA, 1996. [Google Scholar]
- Walshe, J. Penicillamine, a new oral therapy for Wilson’s disease. Am. J. Med. 1956, 21, 487–495. [Google Scholar] [CrossRef]
- Foruny, J.R.; Boixeda, D.; López-Sanroman, A.; Vázquez-Sequeiros, E.; Villafruela, M.; Vázquez-Romero, M.; Rodríguez-Gandía, M.; De Argila, C.M.; Camarero, C.; Milicua, J.M. Usefulness of penicillamine-stimulated urinary copper excretion in the diagnosis of adult Wilson’s disease. Scand. J. Gastroenterol. 2008, 43, 597–603. [Google Scholar] [CrossRef]
- Kean, W.F.; Lock, C.J.L.; Howard-Lock, H.E.; Buchanan, W.W. Prior gold therapy does not influence the adverse effects of D-penicillamine in rheumatoid arthritis. Arthritis Rheum. 1982, 25, 917–922. [Google Scholar] [CrossRef]
- Walshe, J. Wilson’s disease: New oral therapy. Lancet 1956, 267, 25–26. [Google Scholar] [CrossRef]
- Walshe, J.M. Copper Chelation in Patients with Wilson’s Disease: A comparision of penicillamine and triethylene tetramine dihydrochloride. QJM Int. J. Med. 1973, 42, 441–452. [Google Scholar] [CrossRef]
- Grand, R.J.; Vawter, G.F. Juvenile Wilson disease: Histologic and functional studies during penicillamine therapy. J. Pediatr. 1975, 87, 1161–1170. [Google Scholar] [CrossRef]
- Pan, H.Y.M.; Lau, J.Y.N.; Lai, C.L.; Wu, P.C.; Lin, H.J.; Todd, D. Wilson’s Disease: 35 Years’ Experience. QJM Int. J. Med. 1990, 75, 597–605. [Google Scholar] [CrossRef]
- Medici, V.; Trevisan, C.P.; D’Incà, R.; Barollo, M.; Zancan, L.; Fagiuoli, S.; Martines, D.; Irato, P.; Sturniolo, G.C. Diagnosis and management of Wilson’s disease: Results of a single center experience. J. Clin. Gastroenterol. 2006, 40, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Walshe, J.M. Wilson’s Disease Presenting with Features of Hepatic Dysfunction: A Clinical Analysis of Eighty-seven Patients. QJM Int. J. Med. 1989, 70, 253–263. [Google Scholar] [CrossRef]
- Roberts, E. American Association for Study of Liver Diseases (AASLD). Diagnosis and treatment of Wilson disease: An update. Hepatology 2008, 47, 2089–2111. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL clinical practice guidelines: Wilson’s disease. J. Hepatol. 2012, 56, 671–685. [Google Scholar] [CrossRef]
- Iorio, R.; D’Ambrosi, M.; Marcellini, M.; Barbera, C.; Maggiore, G.; Zancan, L.; Giacchino, R.; Vajro, P.; Marazzi, M.G.; Francavilla, R. Serum transaminases in children with Wilson’s disease. J. Pediatric Gastroenterol. Nutr. 2004, 39, 331–336. [Google Scholar] [CrossRef]
- Aggarwal, A.; Bhatt, M. Complete Neurological Recovery in Wilson Disease: Experience With 100 Consecutive Patients Seen From 2005–2013. Neurology 2014, 82, S47.007. [Google Scholar]
- Kalita, J.; Kumar, V.; Chandra, S.; Kumar, B.; Misra, U.K. Worsening of Wilson Disease following Penicillamine Therapy. Eur. Neurol. 2014, 71, 126–131. [Google Scholar] [CrossRef]
- Moores, A.; Fox, S.; Lang, A.; Hirschfield, G.M. Wilson disease: Canadian perspectives on presentation and outcomes from an adult ambulatory setting. Can. J. Gastroenterol. 2012, 26, 333–339. [Google Scholar] [CrossRef]
- Brewer, G.J.; Terry, C.A.; Aisen, A.M.; Hill, G.M. Worsening of Neurologic Syndrome in Patients with Wilson’s Disease with Initial Penicillamine Therapy. Arch. Neurol. 1987, 44, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Svetel, M.; Šternić, N.; Pejović, S.; Kostić, V.S. Penicillamine-induced lethal status dystonicus in a patient with Wilson’s disease. Mov. Disord. 2001, 16, 568–569. [Google Scholar] [CrossRef]
- Harpey, J.; Caille, B.; Moulias, R.; Goust, J. Lupus-like syndrome induced by d-penicillamine in Wilson’s disease. Lancet 1971, 297, 292. [Google Scholar] [CrossRef]
- Dourmishev, L.A.; Stomonjakova, S.R.; Dourmishev, A.L. D-penicillamine induced polymyositis and morphea in a woman with Hashimoto thyroiditis. J. Eur. Acad. Dermatol. Venereol. 2002, 16, 538–539. [Google Scholar] [CrossRef]
- Jaffe, I.A. Induction of auto-immune syndromes by penicillamine therapy in rheumatoid arthritis and other diseases. Semin. Immunopathol. 1981, 4, 193–207. [Google Scholar] [CrossRef]
- Hakoda, M.; Taniguchi, A.; Kamatani, N.; Akahoshi, T.; Kashiwazaki, S. Intermittent treatment with D-penicillamine is effective in lower doses and with fewer adverse effects in patients with rheumatoid arthritis. J. Rheumatol. 1994, 21, 1637–1641. [Google Scholar]
- Camp, A.V. Hematologic toxicity from penicillamine in rheumatoid arthritis. J. Rheumatol. 1981, 7, 164–165. [Google Scholar]
- Manzini, C.; Sebastiani, M.; Giuggioli, D.; Manfredi, A.; Colaci, M.; Cesinaro, A.; Ferri, C. D-penicillamine in the treatment of eosinophilic fasciitis: Case reports and review of the literature. Clin. Rheumatol. 2011, 31, 183–187. [Google Scholar] [CrossRef]
- Langlois, D.; Lehner, A.; Buchweitz, J.; Ross, D.; Johnson, M.; Kruger, J.; Bailie, M.; Hauptman, J.; Schall, W. Pharmacokinetics and Relative Bioavailability of d -Penicillamine in Fasted and Nonfasted Dogs. J. Vet. Intern. Med. 2013, 27, 1071–1076. [Google Scholar] [CrossRef]
- Miller, A.D.; Leslie, R.A. The Area Postrema and Vomiting. Front. Neuroendocr. 1994, 15, 301–320. [Google Scholar] [CrossRef] [PubMed]
- Scherk, M. Toxic, Metabolic, Infectious, and Neoplastic Liver Disease. In Textbook of Veterinary Internal Medicine; Elsevier: Amsterdam, The Netherlands, 2005; pp. 1464–1478. [Google Scholar]
- Gloyd, J.S. FDA tightens screws on extra-label drug use, liberalizes policy on use of human drugs in animals. J. Am. Vet. Med Assoc. 1992, 201, 676–677. [Google Scholar]
- Drew, M.L. Update on the Animal Medicinal Drug Use Clarification Act of 1994 Regulations for Wildlife Veterinarians. In Proceedings of the Annual Conference-American Association of Zoo Veterinarians, Omaha, NE, USA, 17–22 October 1998; pp. 163–167. [Google Scholar]
- Kirkpatrick, D. The veterinary drugs directorate to discuss extra-label drug use in Halifax. Can. Vet. J. 2002, 43, 425–426. [Google Scholar]
- Veterinary Pharmaceuticals World Market Study, 2015–2019 & 2020–2030. Available online: https://www.globenewswire.com/news-release/2020/01/13/1969779/0/en/Veterinary-Pharmaceuticals-World-Market-Study-2015-2019-2020-2030.html (accessed on 27 May 2021).
- Welser, J.R. Extra-label drug use—Pharmaceutical industry view. J. Am. Vet. Med. Assoc. 1993, 202, 1635–1658. [Google Scholar]
- Cleland, J.L. Extra-label drug use—Veterinary practitioner views: Companion animals. J. Am. Vet. Med. Assoc. 1993, 202, 1642–1658. [Google Scholar]
- Geyer, R.E. Extralabel drug use and compounding in veterinary medicine. Food Drug Law J. 1997, 52, 291–295. [Google Scholar]
- Hart, B.; Cliff, K.D. Interpreting published results of extra-label drug use with special reference to reports of drugs used to correct problem behavior in animals. J. Am. Vet. Med. Assoc. 1996, 209, 1382–1385. [Google Scholar]
- Gillick, M.R. Controlling off-label medication use. Ann. Intern. Med. 2009, 150, 344–347. [Google Scholar] [CrossRef]
- Heauner, J.E.; Teske, R. Legal Implications of the Extra-Label Use of Drugs in Food Animals. Vet. Clin. N. Am. Food Anim. Pr. 1986, 2, 517–525. [Google Scholar] [CrossRef]
- Riviere, J.E.; Webb, A.I.; Craigmill, A.L. Primer on estimating withdrawal times after extralabel drug use. J. Am. Vet. Med. Assoc. 1998, 213, 966–968. [Google Scholar]
- Payne, M.A.; Baynes, R.E.; Sundlof, S.E.; Webb, A.I.; Riviere, J.E. Drugs prohibited from extralabel use in food animals. J. Am. Vet. Med. Assoc. 1999, 215, 28–32. [Google Scholar]
- Kelly, M.; Gazarian, M.; McPhee, J. Off-label prescribing. Aust. Prescr. 2005, 28, 7. [Google Scholar] [CrossRef]
- Bergstrom, R.F.; Kay, D.R.; Wagner, J.G. The pharmacokinetics of penicillamine in a female mongrel dog. J. Pharmacokinet. Biopharm. 1981, 9, 603–621. [Google Scholar] [CrossRef] [PubMed]
- Lehner, A.F.; Dirikolu, L.; Johnson, M.; Buchweitz, J.P.; Langlois, D.K. Liquid chromatography/tandem mass spectrometric analysis of penicillamine for its pharmacokinetic evaluation in dogs. Toxicol. Mech. Methods 2020, 30, 1–40. [Google Scholar] [CrossRef] [PubMed]
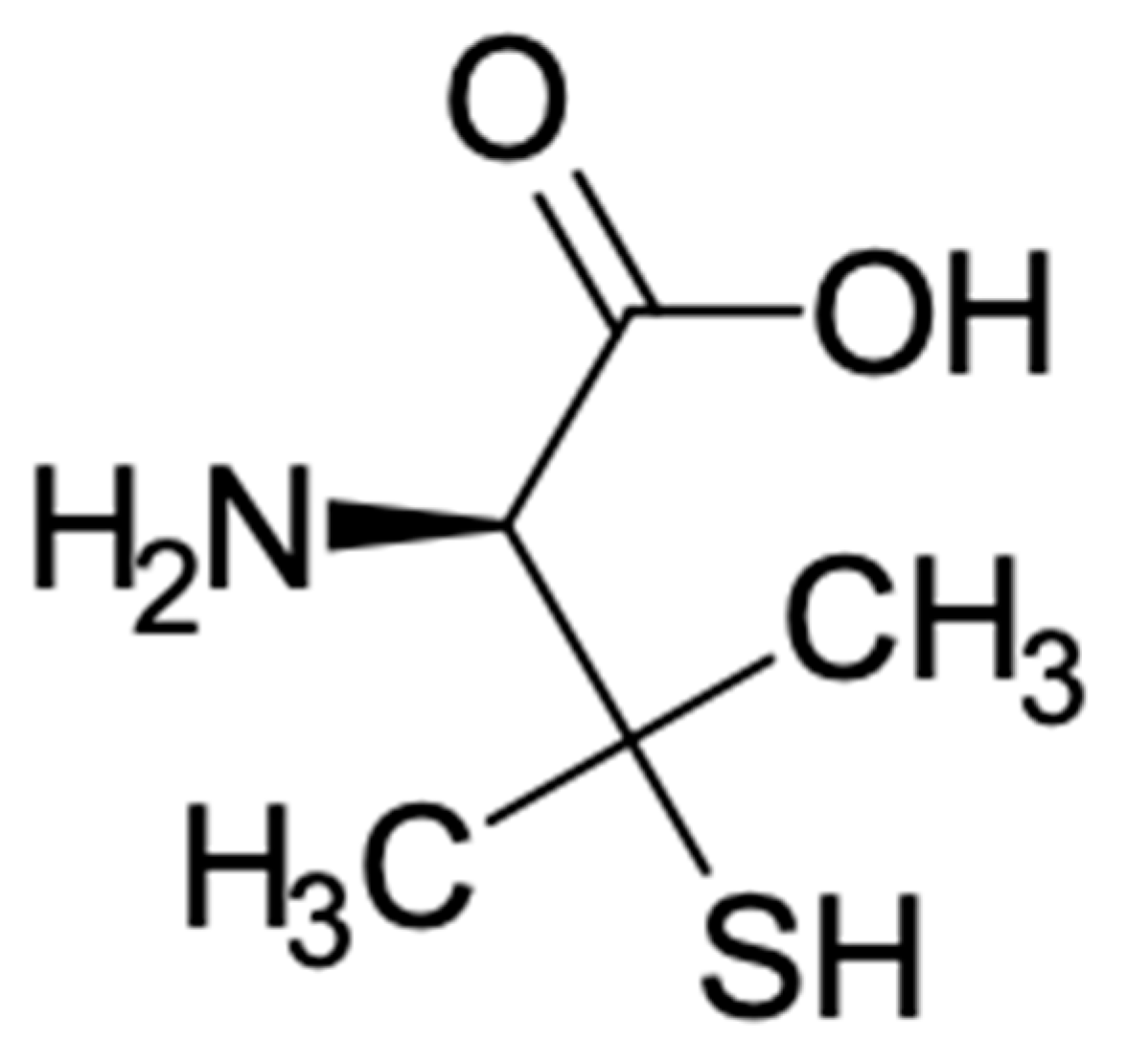
| US FDA Approved | References | Off-Label Uses | References |
|---|---|---|---|
| Rheumatoid arthritis | [33] | Lead poisoning | [8] |
| Wilson disease | [32] | Retinopathy of prematurity | [42,43] |
| Primary biliary cirrhosis | [41] | ||
| Keloids | [54] | ||
| Hemophilic synovitis | [55] | ||
| Lipoid proteinosis | [35] | ||
| Amyloidosis | [45] | ||
| Primary sclerosing cholangitis | [56] | ||
| Chronic active hepatitis | [25] | ||
| Alcohol detoxification | [25] | ||
| Keratosis follicularis | [57] | ||
| Systemic sclerosis (SSc) | [27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pugliese, M.; Biondi, V.; Gugliandolo, E.; Licata, P.; Peritore, A.F.; Crupi, R.; Passantino, A. D-Penicillamine: The State of the Art in Humans and in Dogs from a Pharmacological and Regulatory Perspective. Antibiotics 2021, 10, 648. https://doi.org/10.3390/antibiotics10060648
Pugliese M, Biondi V, Gugliandolo E, Licata P, Peritore AF, Crupi R, Passantino A. D-Penicillamine: The State of the Art in Humans and in Dogs from a Pharmacological and Regulatory Perspective. Antibiotics. 2021; 10(6):648. https://doi.org/10.3390/antibiotics10060648
Chicago/Turabian StylePugliese, Michela, Vito Biondi, Enrico Gugliandolo, Patrizia Licata, Alessio Filippo Peritore, Rosalia Crupi, and Annamaria Passantino. 2021. "D-Penicillamine: The State of the Art in Humans and in Dogs from a Pharmacological and Regulatory Perspective" Antibiotics 10, no. 6: 648. https://doi.org/10.3390/antibiotics10060648
APA StylePugliese, M., Biondi, V., Gugliandolo, E., Licata, P., Peritore, A. F., Crupi, R., & Passantino, A. (2021). D-Penicillamine: The State of the Art in Humans and in Dogs from a Pharmacological and Regulatory Perspective. Antibiotics, 10(6), 648. https://doi.org/10.3390/antibiotics10060648










