Carbon Quantum Dots Derived from Different Carbon Sources for Antibacterial Applications
Abstract
1. Introduction
2. Methods for Synthesizing Carbon Quantum Dots
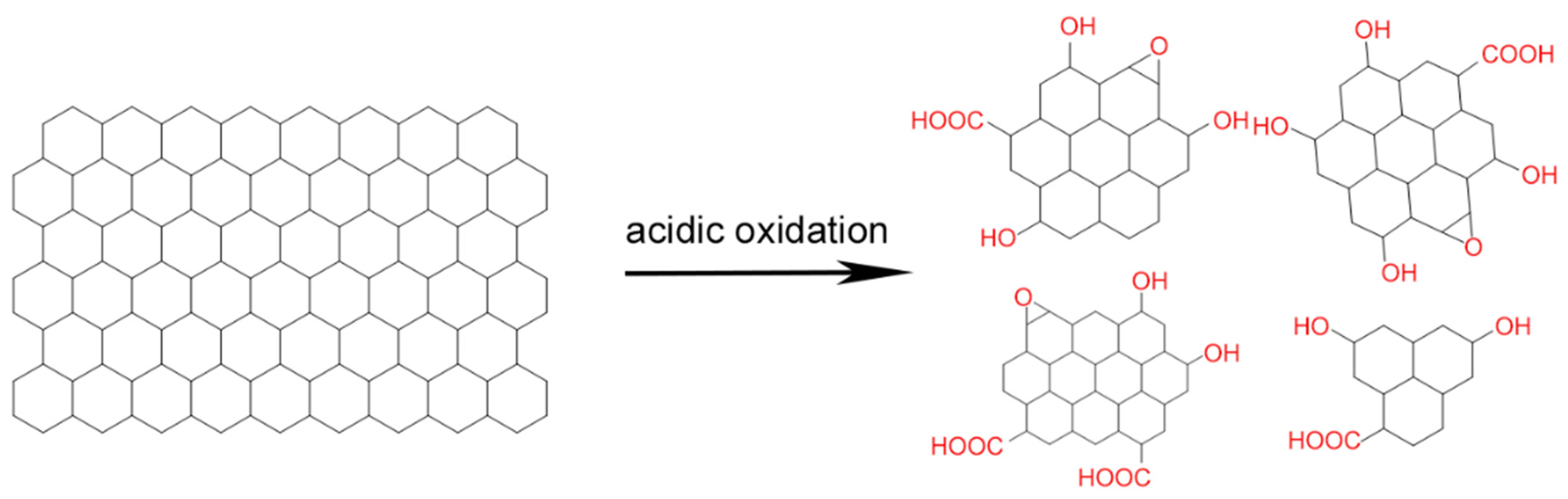
2.1. Acidic Oxidation
2.2. Pyrolysis
2.3. Hydrothermal Synthesis
2.4. Microwave-Assisted Synthesis
2.5. Laser Irradiation
2.6. Electrochemical Synthesis
2.7. Nanoreactor-Assisted Synthesis
3. Physico-Chemical and Functional Properties of Carbon Quantum Dots
3.1. Carbon Quantum Dots Derived from Organic Carbon Sources
3.2. Carbon Quantum Dots Derived from Inorganic Carbon Sources
3.3. Carbon Dots Derived from Natural Carbon Sources
3.4. Surface Modification of Carbon Quantum Dots to Enhance their Functionality
4. Antibacterial Activities of Carbon Quantum Dots
4.1. Bacterial Killing by Carbon Quantum Dots
4.2. Carbon Quantum Dots as a Biofilm Dispersant
4.3. Carbon Quantum Dots and Induction of Resistance
4.4. Mechanisms of Antibacterial Activity of Carbon Quantum Dots
4.5. Gram-Positive vs. Gram-Negative Strains
4.6. Synergistic Use of Carbon Quantum Dots Combined with Antibiotics or Photosensitizers
4.7. Use of Carbon Quantum Dots in In Vivo Studies
5. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Monack, D.M.; Mueller, A.; Falkow, S. Persistent Bacterial Infections: The Interface of the Pathogen and the Host Immune System. Nat. Rev. Microbiol. 2004, 2, 747–765. [Google Scholar] [CrossRef] [PubMed]
- Gilberg, K.; Laouri, M.; Wade, S.; Isonaka, S. Analysis of Medication Use Patterns: Apparent Overuse of Antibiotics and Underuse of Prescription Drugs for Asthma, Depression, and CHF. J. Manag. Care Pharm. 2003, 9, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.J.; Thottathil, S.E.; Newman, T.B. Antibiotics Overuse in Animal Agriculture: A Call to Action for Health Care Providers. Am. J. Public Health 2015, 105, 2409–2410. [Google Scholar] [CrossRef]
- Giedraitiene, A.; Vitkauskiene, A.; Naginiene, R.; Pavilonis, A. Antibiotic Resistance Mechanisms of Clinically Important Bacteria. Medicina 2011, 47, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Dugassa, J.; Shukuri, N. Review on Antibiotic Resistance and Its Mechanism of Development. J. Health Med. Nurs. 2017, 1, 1–17. [Google Scholar]
- Yu, T.; Jiang, G.; Gao, R.; Chen, G.; Ren, Y.; Liu, J.; Van der Mei, H.C.; Busscher, H.J. Circumventing Antimicrobial-Resistance and Preventing its Development in Novel, Bacterial Infection-Control Strategies. Expert Opin. Drug Deliv. 2020, 17, 1151–1164. [Google Scholar] [CrossRef]
- Sun, H.; Wu, L.; Wei, W.; Qu, X. Recent Advances in Graphene Quantum Dots for Sensing. Mater. Today 2013, 16, 433–442. [Google Scholar] [CrossRef]
- Yan, Y.; Gong, J.; Chen, J.; Zeng, Z.; Huang, W.; Pu, K.; Liu, J.; Chen, P. Recent Advances on Graphene Quantum Dots: From Chemistry and Physics to Applications. Adv. Mater. 2019, 31, e1808283. [Google Scholar] [CrossRef]
- Miao, X.; Qu, D.; Yang, D.; Nie, B.; Zhao, Y.; Fan, H.; Sun, Z. Synthesis of Carbon Dots with Multiple Color Emission by Controlled Graphitization and Surface Functionalization. Adv. Mater. 2018, 30, 1704740. [Google Scholar] [CrossRef]
- Kwon, W.; Do, S.; Kim, J.-H.; Seok Jeong, M.; Rhee, S.-W. Control of Photoluminescence of Carbon Nanodots via Surface Functionalization Using Para-Substituted Anilines. Sci. Rep. 2015, 5, 12604. [Google Scholar] [CrossRef]
- Tao, S.; Song, Y.; Zhu, S.; Shao, J.; Yang, B. A New Type of Polymer Carbon Dots with High Quantum Yield: From Synthesis to Investigation on Fluorescence Mechanism. Polymer 2017, 116, 472–478. [Google Scholar] [CrossRef]
- Li, H.; He, X.; Kang, Z.; Huang, H.; Liu, Y.; Liu, J.; Lian, S.; Tsang, C.H.A.; Yang, X.; Lee, S.T. Water-Soluble Fluorescent Carbon Quantum Dots and Photocatalyst Design. Angew. Chem. Int. Ed. 2010, 49, 4430–4434. [Google Scholar] [CrossRef] [PubMed]
- Shamsipur, M.; Barati, A.; Taherpour, A.A.; Jamshidi, M. Resolving the Multiple Emission Centers in Carbon Dots: From Fluorophore Molecular States to Aromatic Domain States and Carbon-Core States. J. Phys. Chem. Lett. 2018, 9, 4189–4198. [Google Scholar] [CrossRef]
- Molaei, M.J. Carbon Quantum Dots and Their Biomedical and Therapeutic Applications: A Review. RSC Adv. 2019, 9, 6460–6481. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, P.; Zhai, X.; Tian, F.; Li, W.; Yang, J.; Liu, Y.; Wang, H.; Wang, W.; Liu, W. Nano-Carrier for Gene Delivery and Bioimaging Based on Carbon Dots with PEI-Passivation Enhanced Fluorescence. Biomaterials 2012, 33, 3604–3613. [Google Scholar] [CrossRef]
- Feng, T.; Ai, X.; An, G.; Yang, P.; Zhao, Y. Charge-Convertible Carbon Dots for Imaging-Guided Drug Delivery with Enhanced In Vivo Cancer Therapeutic Efficiency. ACS Nano 2016, 10, 4410–4420. [Google Scholar] [CrossRef]
- Li, J.; Yang, S.; Deng, Y.; Chai, P.; Yang, Y.; He, X.; Xie, X.; Kang, Z.; Ding, G.; Zhou, H.; et al. Emancipating Target-Functionalized Carbon Dots from Autophagy Vesicles for a Novel Visualized Tumor Therapy. Adv. Funct. Mater. 2018, 28, 1800881. [Google Scholar] [CrossRef]
- Wang, C.; Xu, Z.; Zhang, C. Polyethyleneimine-Functionalized Fluorescent Carbon Dots: Water Stability, pH Sensing, and Cellular Imaging. ChemNanoMat 2015, 1, 122–127. [Google Scholar] [CrossRef]
- Li, Y.J.; Harroun, S.G.; Su, Y.C.; Huang, C.F.; Unnikrishnan, B.; Lin, H.J.; Lin, C.H.; Huang, C.C. Synthesis of Self-Assembled Spermidine-Carbon Quantum Dots Effective against Multidrug-Resistant Bacteria. Adv. Healthc. Mater. 2016, 5, 2545–2554. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Liu, Y.; Lee, S.-T. Carbon Dots for Bioimaging and Biosensing Applications. In Carbon-Based Nanosensor Technology; Springer Series on Chemical Sensors and Biosensors (Methods and Applications); Springer: Berlin, Germany, 2017; Volume 17, pp. 201–231. [Google Scholar]
- Bing, W.; Sun, H.; Yan, Z.; Ren, J.; Qu, X. Programmed Bacteria Death Induced by Carbon Dots with Different Surface Charge. Small 2016, 12, 4713–4718. [Google Scholar] [CrossRef]
- Hou, J.; Tian, Z.; Xie, H.; Tian, Q.; Ai, S. A Fluorescence Resonance Energy Transfer Sensor Based on Quaternized Carbon Dots and Ellman’s Test for Ultrasensitive Detection of Dichlorvos. Sens. Actuators B Chem. 2016, 232, 477–483. [Google Scholar] [CrossRef]
- Lin, F.; Li, C.; Chen, Z. Bacteria-Derived Carbon Dots Inhibit Biofilm Formation of Escherichia coli without Affecting Cell Growth. Front. Microbiol. 2018, 9, 259. [Google Scholar] [CrossRef]
- Al Awak, M.M.; Wang, P.; Wang, S.; Tang, Y.; Sun, Y.P.; Yang, L. Correlation of Carbon Dots’ Light-Activated Antimicrobial Activities and Fluorescence Quantum Yield. RSC Adv. 2017, 7, 30177–30184. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; Xiang, C.; Lin, J.; Peng, Z.; Huang, K.; Yan, Z.; Cook, N.P.; Samuel, E.L.G.; Hwang, C.-C.; Ruan, G.; et al. Coal as an Abundant Source of Graphene Quantum Dots. Nat. Commun. 2013, 4, 2943. [Google Scholar] [CrossRef] [PubMed]
- Chua, C.K.; Sofer, Z.; Šimek, P.; Jankovský, O.; Klímová, K.; Bakardjieva, S.; Hrdličková Kučková, Š.; Pumera, M. Synthesis of Strongly Fluorescent Graphene Quantum Dots by Cage-Opening Buckminsterfullerene. ACS Nano 2015, 9, 2548–2555. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Lin, J.; Chen, Y.; Fu, F.; Chi, Y.; Chen, G. Graphene Quantum Dots, Graphene Oxide, Carbon Quantum Dots and Graphite Nanocrystals in Coals. Nanoscale 2014, 6, 7410–7415. [Google Scholar] [CrossRef]
- Hu, C.; Yu, C.; Li, M.; Wang, X.; Yang, J.; Zhao, Z.; Eychmüller, A.; Sun, Y.-P.; Qiu, J. Chemically Tailoring Coal to Fluorescent Carbon Dots with Tuned Size and Their Capacity for Cu(II) Detection. Small 2014, 10, 4926–4933. [Google Scholar] [CrossRef]
- Meziani, M.J.; Dong, X.; Zhu, L.; Jones, L.P.; Lecroy, G.E.; Yang, F.; Wang, S.; Wang, P.; Zhao, Y.; Yang, L.; et al. Visible-Light-Activated Bactericidal Functions of Carbon “Quantum” Dots. ACS Appl. Mater. Interfaces 2016, 8, 10761–10766. [Google Scholar] [CrossRef]
- Abu Rabe, D.I.; Al Awak, M.M.; Yang, F.; Okonjo, P.A.; Dong, X.; Teisl, L.R.; Wang, P.; Tang, Y.; Pan, N.; Sun, Y.P.; et al. The Dominant Role of Surface Functionalization in Carbon Dots’ Photo-Activated Antibacterial Activity. Int. J. Nanomed. 2019, 14, 2655–2665. [Google Scholar] [CrossRef]
- Kuo, W.S.; Chang, C.Y.; Chen, H.H.; Hsu, C.L.L.; Wang, J.Y.; Kao, H.F.; Chou, L.C.S.; Chen, Y.C.; Chen, S.J.; Chang, W.T.; et al. Two-Photon Photoexcited Photodynamic Therapy and Contrast Agent with Antimicrobial Graphene Quantum Dots. ACS Appl. Mater. Interfaces 2016, 8, 30467–30474. [Google Scholar] [CrossRef]
- Kuo, W.S.; Chen, H.H.; Chen, S.Y.; Chang, C.Y.; Chen, P.C.; Hou, Y.I.; Shao, Y.T.; Kao, H.F.; Lilian Hsu, C.L.; Chen, Y.C.; et al. Graphene Quantum Dots with Nitrogen-Doped Content Dependence for Highly Efficient Dual-Modality Photodynamic Antimicrobial Therapy and Bioimaging. Biomaterials 2017, 120, 185–194. [Google Scholar] [CrossRef]
- Kuo, W.S.; Shao, Y.T.; Huang, K.S.; Chou, T.M.; Yang, C.H. Antimicrobial Amino-Functionalized Nitrogen-Doped Graphene Quantum Dots for Eliminating Multidrug-Resistant Species in Dual-Modality Photodynamic Therapy and Bioimaging under Two-Photon Excitation. ACS Appl. Mater. Interfaces 2018, 10, 14438–14446. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Bond, A.E.; Pan, N.; Coleman, M.; Tang, Y.; Sun, Y.P.; Yang, L. Synergistic Photoactivated Antimicrobial Effects of Carbon Dots Combined with Dye Photosensitizers. Int. J. Nanomed. 2018, 13, 8025–8035. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Gao, W.; Gupta, B.K.; Liu, Z.; Romero-Aburto, R.; Ge, L.; Song, L.; Alemany, L.B.; Zhan, X.; Gao, G.; et al. Graphene Quantum Dots Derived from Carbon Fibers. Nano Lett. 2012, 12, 844–849. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, X.; Ruan, H.; Yin, K.; Li, H. Production of Yellow-Emitting Carbon Quantum Dots from Fullerene Carbon Soot. Sci. China Mater. 2017, 60, 141–150. [Google Scholar] [CrossRef]
- Jian, H.J.; Wu, R.S.; Lin, T.Y.; Li, Y.J.; Lin, H.J.; Harroun, S.G.; Lai, J.Y.; Huang, C.C. Super-Cationic Carbon Quantum Dots Synthesized from Spermidine as an Eye Drop Formulation for Topical Treatment of Bacterial Keratitis. ACS Nano 2017, 11, 6703–6716. [Google Scholar] [CrossRef]
- Jian, H.J.; Yu, J.; Li, Y.J.; Unnikrishnan, B.; Huang, Y.F.; Luo, L.J.; Hui-Kang Ma, D.; Harroun, S.G.; Chang, H.T.; Lin, H.J.; et al. Highly Adhesive Carbon Quantum Dots from Biogenic Amines for Prevention of Biofilm Formation. Chem. Eng. J. 2020, 386. [Google Scholar] [CrossRef]
- Li, P.; Liu, S.; Cao, W.; Zhang, G.; Yang, X.; Gong, X.; Xing, X. Low-Toxicity Carbon Quantum Dots Derived from Gentamicin Sulfate to Combat Antibiotic Resistance and Eradicate Mature Biofilms. Chem. Commun. 2020, 56, 2316–2319. [Google Scholar] [CrossRef]
- Zhou, J.; Sheng, Z.; Han, H.; Zou, M.; Li, C. Facile Synthesis of Fluorescent Carbon Dots Using Watermelon Peel as a Carbon Source. Mater. Lett. 2012, 66, 222–224. [Google Scholar] [CrossRef]
- Hou, J.; Wang, W.; Zhou, T.; Wang, B.; Li, H.; Ding, L. Synthesis and Formation Mechanistic Investigation of Nitrogen-Doped Carbon Dots with High Quantum Yields and Yellowish-Green Fluorescence. Nanoscale 2016, 8, 11185–11193. [Google Scholar] [CrossRef]
- Lee, N.E.; Lee, S.Y.; Lim, H.S.; Yoo, S.H.; Cho, S.O. A Novel Route to High-Quality Graphene Quantum Dots by Hydrogen-Assisted Pyrolysis of Silicon Carbide. Nanomaterials 2020, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Teng, X.; Ma, C.; Ge, C.; Yan, M.; Yang, J.; Zhang, Y.; Morais, P.C.; Bi, H. Green Synthesis of Nitrogen-Doped Carbon Dots from Konjac Flour with “off–on” Fluorescence by Fe3+ and l-Lysine for Bioimaging. J. Mater. Chem. B 2014, 2, 4631. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, J.; Zhang, Y.; Xiao, Y.; Shi, Y.; Chen, Y.; Ding, L.; Xu, W. Influence of Group Modification at the Edges of Carbon Quantum Dots on Fluorescent Emission. Nanoscale Res. Lett. 2019, 14, 241. [Google Scholar] [CrossRef]
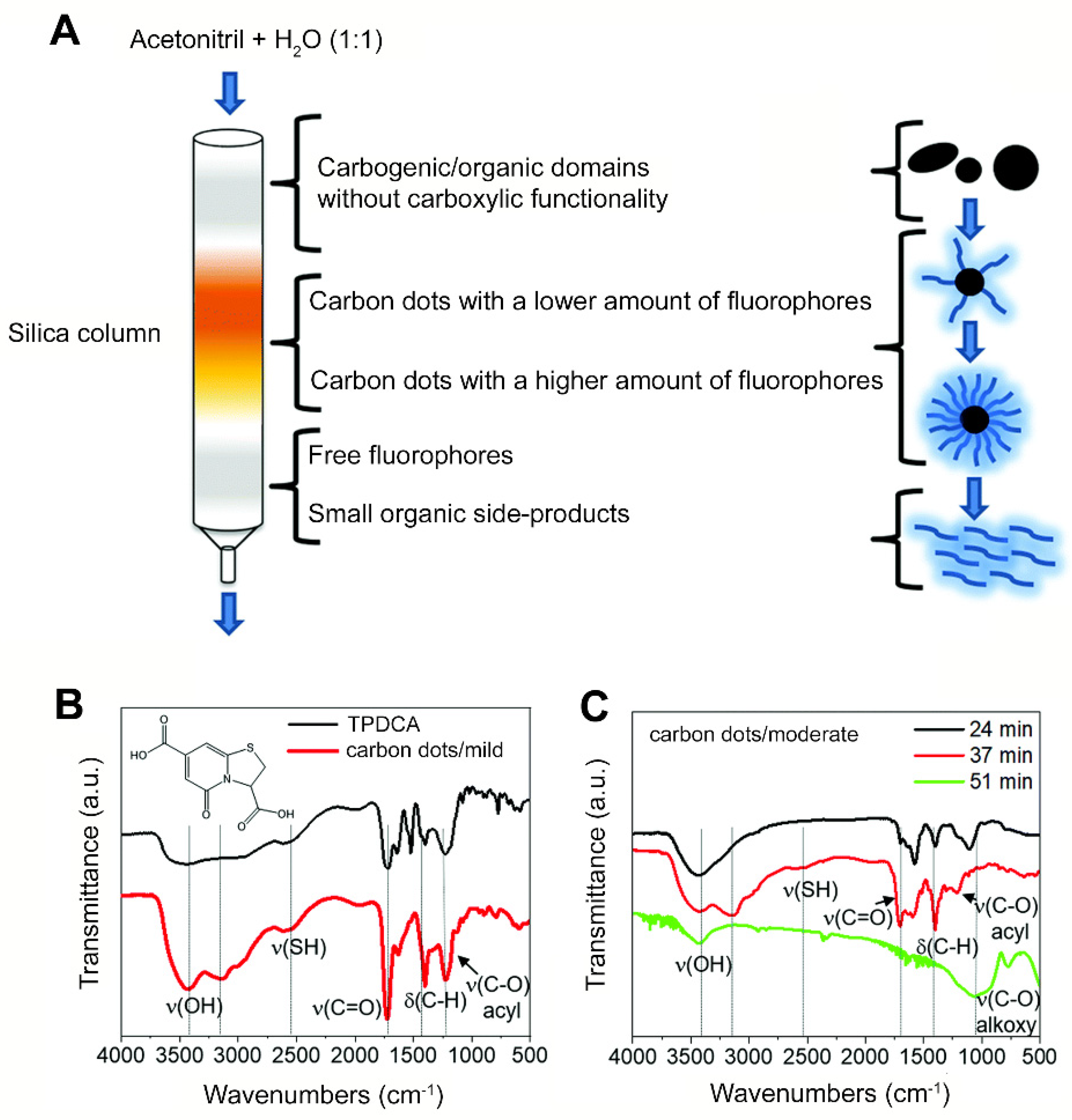
- Hinterberger, V.; Damm, C.; Haines, P.; Guldi, D.M.; Peukert, W. Purification and Structural Elucidation of Carbon Dots by Column Chromatography. Nanoscale 2019, 11, 8464–8474. [Google Scholar] [CrossRef] [PubMed]
- Travlou, N.A.; Giannakoudakis, D.A.; Algarra, M.; Labella, A.M.; Rodríguez-Castellón, E.; Bandosz, T.J. S- and N-Doped Carbon Quantum Dots: Surface Chemistry Dependent Antibacterial Activity. Carbon 2018, 135, 104–111. [Google Scholar] [CrossRef]
- Wang, H.; Song, Z.; Gu, J.; Li, S.; Wu, Y.; Han, H. Nitrogen-Doped Carbon Quantum Dots for Preventing Biofilm Formation and Eradicating Drug-Resistant Bacteria Infection. ACS Biomater. Sci. Eng. 2019, 5, 4739–4749. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.; Yang, T.; Liu, H.; Li, Y.F.; Huang, C.Z. An Active Structure Preservation Method for Developing Functional Graphitic Carbon Dots as an Effective Antibacterial Agent and a Sensitive pH and Al(III) Nanosensor. Nanoscale 2017, 9, 17334–17341. [Google Scholar] [CrossRef]
- Liu, J.; Lu, S.; Tang, Q.; Zhang, K.; Yu, W.; Sun, H.; Yang, B. One-Step Hydrothermal Synthesis of Photoluminescent Carbon Nanodots with Selective Antibacterial Activity against Porphyromonas gingivalis. Nanoscale 2017, 9, 7135–7142. [Google Scholar] [CrossRef]
- Huang, H.; Lv, J.-J.; Zhou, D.-L.; Bao, N.; Xu, Y.; Wang, A.-J.; Feng, J.-J. One-Pot Green Synthesis of Nitrogen-Doped Carbon Nanoparticles as Fluorescent Probes for Mercury Ions. RSC Adv. 2013, 3, 21691. [Google Scholar] [CrossRef]
- Xu, Q.; Pu, P.; Zhao, J.; Dong, C.; Gao, C.; Chen, Y.; Chen, J.; Liu, Y.; Zhou, H. Preparation of Highly Photoluminescent Sulfur-Doped Carbon Dots for Fe(III) Detection. J. Mater. Chem. A 2015, 3, 542–546. [Google Scholar] [CrossRef]
- Zhu, X.; Zuo, X.; Hu, R.; Xiao, X.; Liang, Y.; Nan, J. Hydrothermal Synthesis of Two Photoluminescent Nitrogen-Doped Graphene Quantum Dots Emitted Green and Khaki Luminescence. Mater. Chem. Phys. 2014, 147, 963–967. [Google Scholar] [CrossRef]
- Sahu, S.; Behera, B.; Maiti, T.K.; Mohapatra, S. Simple One-Step Synthesis of Highly Luminescent Carbon Dots from Orange Juice: Application as Excellent Bio-Imaging Agents. Chem. Commun. 2012, 48, 8835. [Google Scholar] [CrossRef]
- Sun, H.; Gao, N.; Dong, K.; Ren, J.; Qu, X. Graphene Quantum Dots-Band-Aids Used for Wound Disinfection. ACS Nano 2014, 8, 6202–6210. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, X.; Li, Y.; Wang, Z.; Yang, F.; Yang, X. Microwave Synthesis of Fluorescent Carbon Nanoparticles with Electrochemiluminescence Properties. Chem. Commun. 2009, 34, 5118–5120. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xiao, N.; Gong, N.; Wang, H.; Shi, X.; Gu, W.; Ye, L. One-Step Microwave-Assisted Polyol Synthesis of Green Luminescent Carbon Dots as Optical Nanoprobes. Carbon 2014, 68, 258–264. [Google Scholar] [CrossRef]
- Li, L.-L.; Ji, J.; Fei, R.; Wang, C.-Z.; Lu, Q.; Zhang, J.-R.; Jiang, L.-P.; Zhu, J.-J. A Facile Microwave Avenue to Electrochemiluminescent Two-Color Graphene Quantum Dots. Adv. Funct. Mater. 2012, 22, 2971–2979. [Google Scholar] [CrossRef]
- Kholikov, K.; Ilhom, S.; Sajjad, M.; Smith, M.E.; Monroe, J.D.; San, O.; Er, A.O. Improved Singlet Oxygen Generation and Antimicrobial Activity of Sulphur-Doped Graphene Quantum Dots Coupled with Methylene Blue for Photodynamic Therapy Applications. Photodiagnosis Photodyn. Ther. 2018, 24, 7–14. [Google Scholar] [CrossRef]
- Hu, S.-L.; Niu, K.-Y.; Sun, J.; Yang, J.; Zhao, N.-Q.; Du, X.-W. One-Step Synthesis of Fluorescent Carbon Nanoparticles by Laser Irradiation. J. Mater. Chem. 2009, 19, 484–488. [Google Scholar] [CrossRef]
- Yu, H.; Li, X.; Zeng, X.; Lu, Y. Preparation of Carbon Dots by Non-Focusing Pulsed Laser Irradiation in Toluene. Chem. Commun. 2016, 52, 819–822. [Google Scholar] [CrossRef]
- Li, H.; Huang, J.; Song, Y.; Zhang, M.; Wang, H.; Lu, F.; Huang, H.; Liu, Y.; Dai, X.; Gu, Z.; et al. Degradable Carbon Dots with Broad-Spectrum Antibacterial Activity. ACS Appl. Mater. Interfaces 2018, 10, 26936–26946. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Booker, C.; Li, R.; Zhou, X.; Sham, T.-K.; Sun, X.; Ding, Z. An Electrochemical Avenue to Blue Luminescent Nanocrystals from Multiwalled Carbon Nanotubes (MWCNTs). J. Am. Chem. Soc. 2007, 129, 744–745. [Google Scholar] [CrossRef]
- Bao, L.; Zhang, Z.-L.; Tian, Z.-Q.; Zhang, L.; Liu, C.; Lin, Y.; Qi, B.; Pang, D.-W. Electrochemical Tuning of Luminescent Carbon Nanodots: From Preparation to Luminescence Mechanism. Adv. Mater. 2011, 23, 5801–5806. [Google Scholar] [CrossRef]
- Ming, H.; Ma, Z.; Liu, Y.; Pan, K.; Yu, H.; Wang, F.; Kang, Z. Large Scale Electrochemical Synthesis of High Quality Carbon Nanodots and their Photocatalytic Property. Dalt. Trans. 2012, 41, 9526. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Chi, Y.; Dong, Y.; Lin, J.; Wang, B. Electrochemiluminescence of Water-Soluble Carbon Nanocrystals Released Electrochemically from Graphite. J. Am. Chem. Soc. 2009, 131, 4564–4565. [Google Scholar] [CrossRef]
- Deng, J.; Lu, Q.; Mi, N.; Li, H.; Liu, M.; Xu, M.; Tan, L.; Xie, Q.; Zhang, Y.; Yao, S. Electrochemical Synthesis of Carbon Nanodots Directly from Alcohols. Chem. A Eur. J. 2014, 20, 4993–4999. [Google Scholar] [CrossRef]
- Zong, J.; Zhu, Y.; Yang, X.; Shen, J.; Li, C. Synthesis of Photoluminescent Carbogenic Dots Using Mesoporous Silica Spheres as Nanoreactors. Chem. Commun. 2011, 47, 764–766. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-W.; Hsiao, Y.-H.; Peng, Y.-K.; Chou, P.-T. Facile Synthesis of Highly Emissive Carbon Dots from Pyrolysis of Glycerol; Gram Scale Production of Carbon Dots/MSiO2 for Cell Imaging and Drug Release. J. Mater. Chem. 2012, 22, 14403. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, D.; Han, S.; Hu, P.; Liu, R. Bottom-up Fabrication of Photoluminescent Carbon Dots with Uniform Morphology via a Soft–Hard Template Approach. Chem. Commun. 2013, 49, 4920. [Google Scholar] [CrossRef] [PubMed]
- Mikhraliieva, A.; Zaitsev, V.; Xing, Y.; Coelho-Júnior, H.; Sommer, R.L. Excitation-Independent Blue-Emitting Carbon Dots from Mesoporous Aminosilica Nanoreactor for Bioanalytical Application. ACS Appl. Nano Mater. 2020, 3, 3652–3664. [Google Scholar] [CrossRef]
- Lin, F.; Bao, Y.-W.; Wu, F.-G. Carbon Dots for Sensing and Killing Microorganisms. J. Carbon Res. C 2019, 5, 33. [Google Scholar] [CrossRef]
- Anand, A.; Unnikrishnan, B.; Wei, S.C.; Chou, C.P.; Zhang, L.Z.; Huang, C.C. Graphene Oxide and Carbon Dots as Broad-Spectrum Antimicrobial Agents-a Minireview. Nanoscale Horiz. 2019, 4, 117–137. [Google Scholar] [CrossRef]
- Sun, Y.-P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-Sized Carbon Dots for Bright and Colorful Photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef]
- Ran, H.H.; Cheng, X.; Bao, Y.W.; Hua, X.W.; Gao, G.; Zhang, X.; Jiang, Y.W.; Zhu, Y.X.; Wu, F.G. Multifunctional Quaternized Carbon Dots with Enhanced Biofilm Penetration and Eradication Efficiencies. J. Mater. Chem. B 2019, 7, 5104–5114. [Google Scholar] [CrossRef]
- Wu, Y.; Van der Mei, H.C.; Busscher, H.J.; Ren, Y. Enhanced Bacterial Killing by Vancomycin in Staphylococcal Biofilms Disrupted by Novel, DMMA-Modified Carbon Dots Depends on EPS Production. Colloids Surf. B Biointerfaces 2020, 193, 111114. [Google Scholar] [CrossRef]
- Stanković, N.K.; Bodik, M.; Šiffalovič, P.; Kotlar, M.; Mičušik, M.; Špitalsky, Z.; Danko, M.; Milivojević, D.D.; Kleinova, A.; Kubat, P.; et al. Antibacterial and Antibiofouling Properties of Light Triggered Fluorescent Hydrophobic Carbon Quantum Dots Langmuir-Blodgett Thin Films. ACS Sustain. Chem. Eng. 2018, 6, 4154–4163. [Google Scholar] [CrossRef]
- Wu, Z.L.; Gao, M.X.; Wang, T.T.; Wan, X.Y.; Zheng, L.L.; Huang, C.Z. A General Quantitative pH Sensor Developed with Dicyandiamide N-Doped High Quantum Yield Graphene Quantum Dots. Nanoscale 2014, 6, 3868–3874. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, M.; Ma, Y.; Wang, B.; Shao, M.; Huang, H.; Liu, Y.; Kang, Z. Selective Inactivation of Gram-Negative Bacteria by Carbon Dots Derived from Natural Biomass: Artemisia Argyi Leaves. J. Mater. Chem. B 2020, 8, 2666–2672. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Lu, F.; Li, H.; Wang, H.; Zhang, M.; Liu, Y.; Kang, Z. Degradable Carbon Dots from Cigarette Smoking with Broad-Spectrum Antimicrobial Activities against Drug-Resistant Bacteria. ACS Appl. BioMater. 2018, 1, 1871–1879. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Zhang, Y. One-Step Green Synthesized Fluorescent Carbon Nanodots from Bamboo Leaves for Copper(II) Ion Detection. Sens. Actuators B Chem. 2014, 196, 647–652. [Google Scholar] [CrossRef]
- Wei, J.; Shen, J.; Zhang, X.; Guo, S.; Pan, J.; Hou, X.; Zhang, H.; Wang, L.; Feng, B. Simple One-Step Synthesis of Water-Soluble Fluorescent Carbon Dots Derived from Paper Ash. RSC Adv. 2013, 3, 13119. [Google Scholar] [CrossRef]
- Yang, X.; Zhuo, Y.; Zhu, S.; Luo, Y.; Feng, Y.; Dou, Y. Novel and Green Synthesis of High-Fluorescent Carbon Dots Originated from Honey for Sensing and Imaging. Biosens. Bioelectron. 2014, 60, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Lu, W.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Microwave-Assisted Rapid Green Synthesis of Photoluminescent Carbon Nanodots from Flour and Their Applications for Sensitive and Selective Detection of Mercury(II) Ions. Sens. Actuators B Chem. 2013, 184, 156–162. [Google Scholar] [CrossRef]
- Otis, G.; Bhattacharya, S.; Malka, O.; Kolusheva, S.; Bolel, P.; Porgador, A.; Jelinek, R. Selective Labeling and Growth Inhibition of Pseudomonas aeruginosa by Aminoguanidine Carbon Dots. ACS Infect. Dis. 2019, 5, 292–302. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Niu, J.; Chen, Y. Mechanism of Photogenerated Reactive Oxygen Species and Correlation with the Antibacterial Properties of Engineered Metal-Oxide Nanoparticles. ACS Nano 2021, 6, 5164–5173. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 217037. [Google Scholar] [CrossRef]
- Schmitt, F.-J.; Renger, G.; Friedrich, T.; Kreslavski, V.D.; Zharmukhamedov, S.K.; Los, D.A.; Kuznetsov, V.V.; Allakhverdiev, S.I. Reactive Oxygen Species: Re-evaluation of Generation, Monitoring and Role in Stress-Signaling in Phototrophic Organisms. Biochim. Biophys. Acta 2014, 1837, 835–848. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Wang, H.; Wu, Y.; Gu, J.; Li, S.; Han, H. Fabrication of Bis-Quaternary Ammonium Salt as an Efficient Bactericidal Weapon Against Escherichia coli and Staphylococcus aureus. ACS Omega 2018, 3, 14517–14525. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, X.; Ma, Y.H.; Gao, G.; Chen, X.; Jia, H.R.; Li, Y.H.; Chen, Z.; Wu, F.G. Carbon Dot-Based Platform for Simultaneous Bacterial Distinguishment and Antibacterial Applications. ACS Appl. Mater. Interfaces 2016, 8, 32170–32181. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, X.; Yu, L.; Wu, L.; Hao, X.; Liu, Q.; Lin, L.; Huang, Z.; Weng, S.; Liu, A.; et al. Quaternized Carbon Quantum Dots with Broad-Spectrum Antibacterial Activity for Treatment of Wounds Infected with Mixed Bacteria. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Zhao, C.; Wu, L.; Wang, X.; Weng, S.; Ruan, Z.; Liu, Q.; Lin, L.; Lin, X. Quaternary Ammonium Carbon Quantum Dots as an Antimicrobial Agent against Gram-Positive Bacteria for the Treatment of MRSA-Infected Pneumonia in Mice. Carbon 2020, 163, 70–84. [Google Scholar] [CrossRef]
- Hao, X.; Huang, L.; Zhao, C.; Chen, S.; Lin, W.; Lin, Y.; Zhang, L.; Sun, A.; Miao, C.; Lin, X.; et al. Antibacterial Activity of Positively Charged Carbon Quantum Dots without Detectable Resistance for Wound Healing with Mixed Bacteria Infection. Mater. Sci. Eng. C 2021, 123, 111971. [Google Scholar] [CrossRef]
- Dong, X.; Al Awak, M.; Tomlinson, N.; Tang, Y.; Sun, Y.P.; Yang, L. Antibacterial Effects of Carbon Dots in Combination with Other Antimicrobial Reagents. PLoS ONE 2017, 12, e0185324. [Google Scholar] [CrossRef]
- Wang, Y.; Kadiyala, U.; Qu, Z.; Elvati, P.; Altheim, C.; Kotov, N.A.; Violi, A.; Vanepps, J.S. Anti-Biofilm Activity of Graphene Quantum Dots via Self-Assembly with Bacterial Amyloid Proteins. ACS Nano 2019, 13, 4278–4289. [Google Scholar] [CrossRef] [PubMed]
- Davies, D. Understanding Biofilm Resistance to Antibacterial Agents. Nat. Rev. Drug Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An Emergent Form of Bacterial Life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Levy, S.B.; Bonnie, M. Antibacterial Resistance Worldwide: Causes, Challenges and Responses. Nat. Med. 2004, 10, S122–S129. [Google Scholar] [CrossRef] [PubMed]
- Charles, P.G.P.; Grayson, M.L. The Dearth of New Antibiotic Development: Why We Should Be Worried and What We Can Do about It. Med. J. Aust. 2004, 181, 549–553. [Google Scholar] [CrossRef]
- Vatansever, F.; De Melo, W.C.M.A.; Avci, P.; Vecchio, D.; Sadasivam, M.; Gupta, A.; Chandran, R.; Karimi, M.; Parizotto, N.A.; Yin, R.; et al. Antimicrobial Strategies Centered Around Reactive Oxygen Species-Bactericidal Antibiotics, Photodynamic Therapy, and Beyond. FEMS Microbiol. Rev. 2013, 37, 955–989. [Google Scholar] [CrossRef] [PubMed]
- Salleh, A.; Fauzi, M.B. The In Vivo, In Vitro and In Ovo Evaluation of Quantum Dots in Wound Healing: A Review. Polymers 2021, 13, 191. [Google Scholar] [CrossRef]
- Wang, K.; Gao, Z.; Gao, G.; Wo, Y.; Wang, Y.; Shen, G.; Cui, D. Systematic Safety Evaluation on Photoluminescent Carbon Dots. Nanoscale Res. Lett. 2013, 8, 122. [Google Scholar] [CrossRef]
- Nguyen, K.C.; Zhang, Y.; Todd, J.; Kittle, K.; Patry, D.; Caldwell, D.; Lalande, M.; Smith, S.; Parks, D.; Navarro, M.; et al. Biodistribution and Systemic Effects in Mice Following Intravenous Administration of Cadmium Telluride Quantum Dot Nanoparticles. Chem. Res. Toxicol. 2019, 32, 1491–1503. [Google Scholar] [CrossRef] [PubMed]
- Painuly, D.; Bhatt, A.; Krishnan, V.K. Mercaptoethanol Capped CdSe Quantum Dots and CdSe/ZnS Core/Shell: Synthesis, Characterization and Cytotoxicity Evaluation. J. Biomed. Nanotechnol. 2013, 9, 257–266. [Google Scholar] [CrossRef] [PubMed]
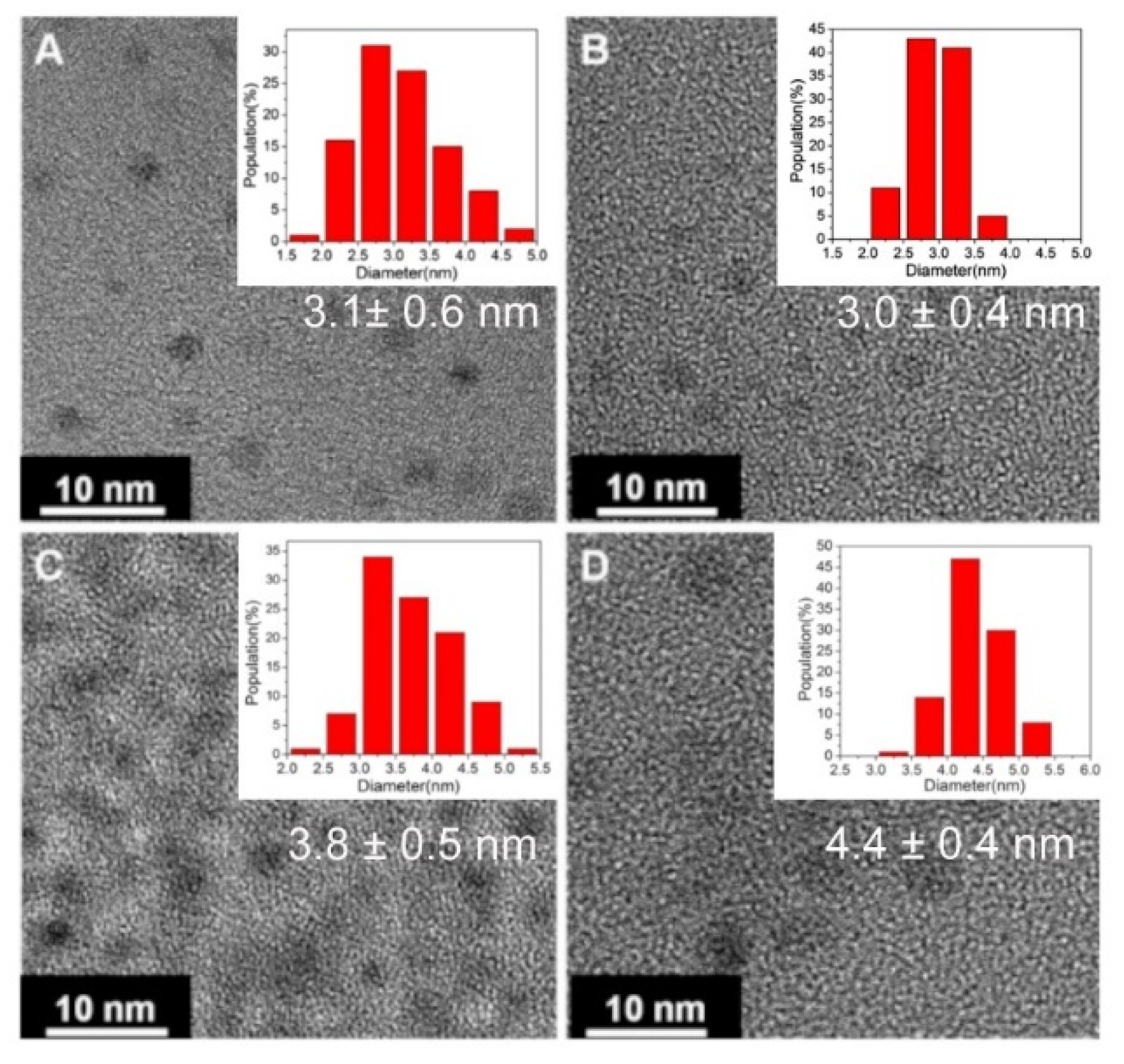
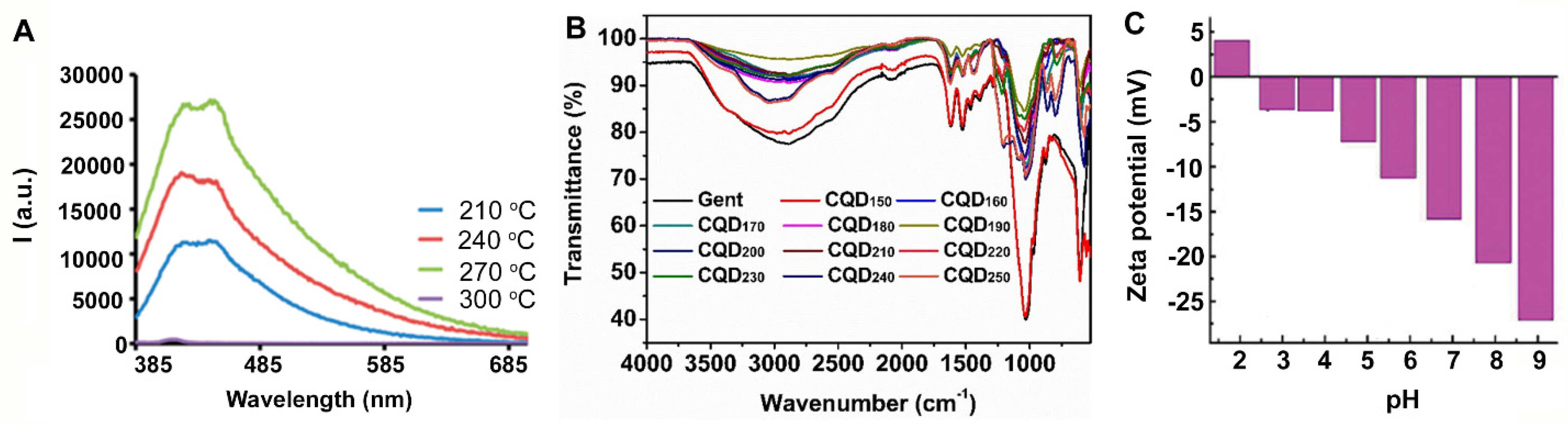
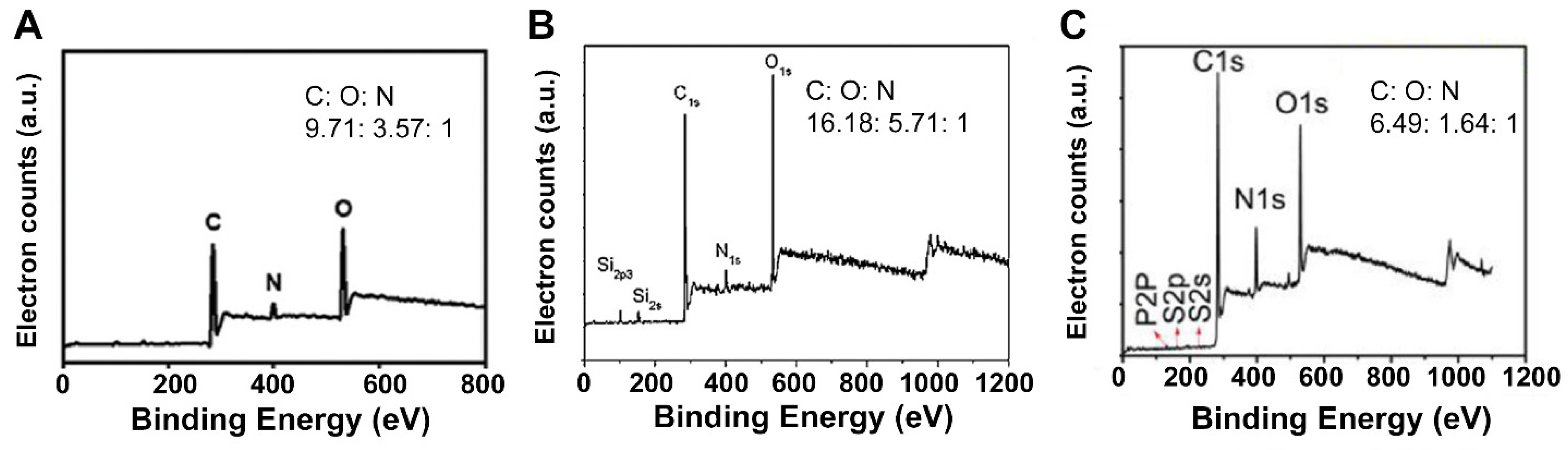
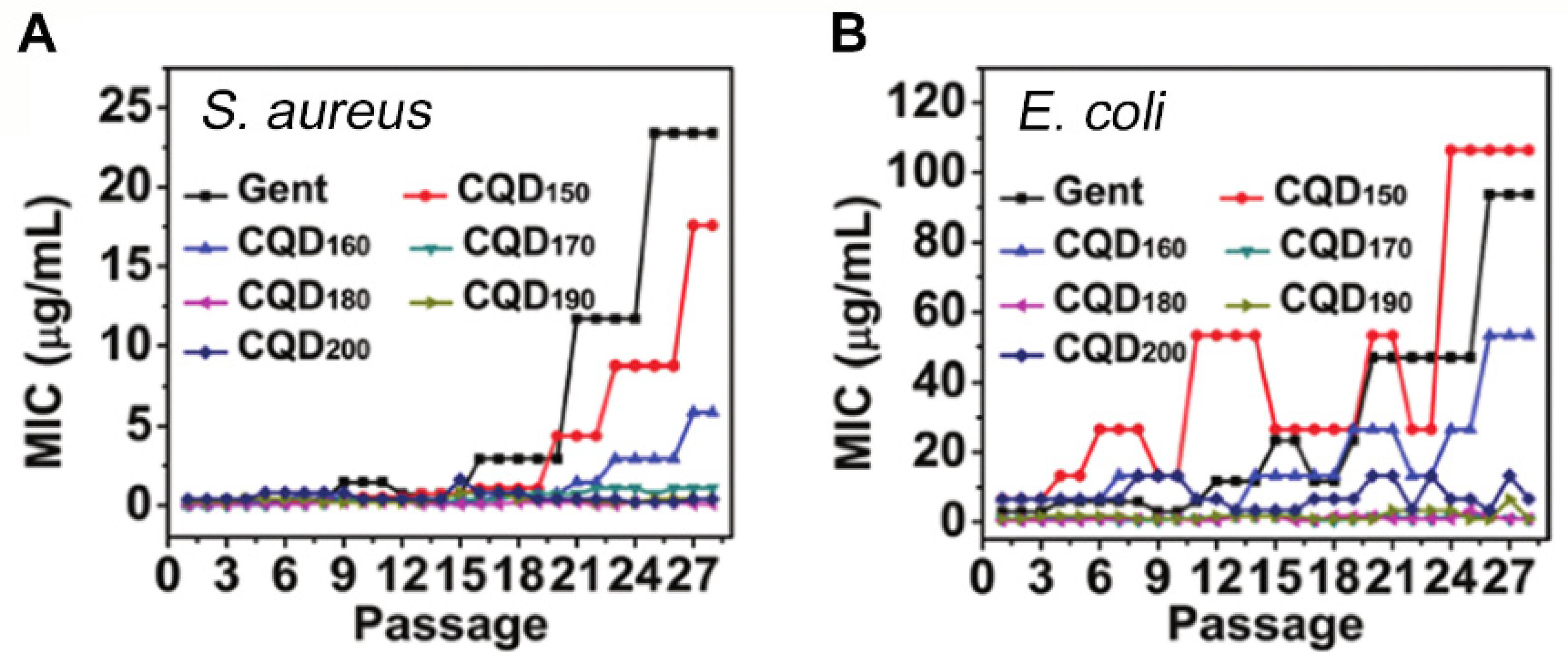
| Method | Schematic Synthesis | Advantages | Disadvantages | Reference |
|---|---|---|---|---|
| Acidic oxidation |  | Large scale synthesis | Poor size control, risk of burning or explosion, mainly inorganic carbon sources | [21,24,25,26,27,28,29,30,31,32,33,34,35,36] |
| Pyrolysis |  | Avoids use of strong acids or alkalis, cost effective, suitable for widely different carbon sources | Poor size control | [19,37,38,39,40,41,42,43,44] |
| Hydrothermal synthesis |  | One step procedure, avoids use of strong acids or alkalis, cost effective, suitable for many carbon sources | Poor size control | [22,23,45,46,47,48,49,50,51,52,53] |
| Microwave-assisted synthesis |  | Short reaction time, suitable for many carbon sources | Poor size control | [21,54,55,56,57] |
| Laser irradiation |  | Short reaction time | Poor size control | [58,59,60] |
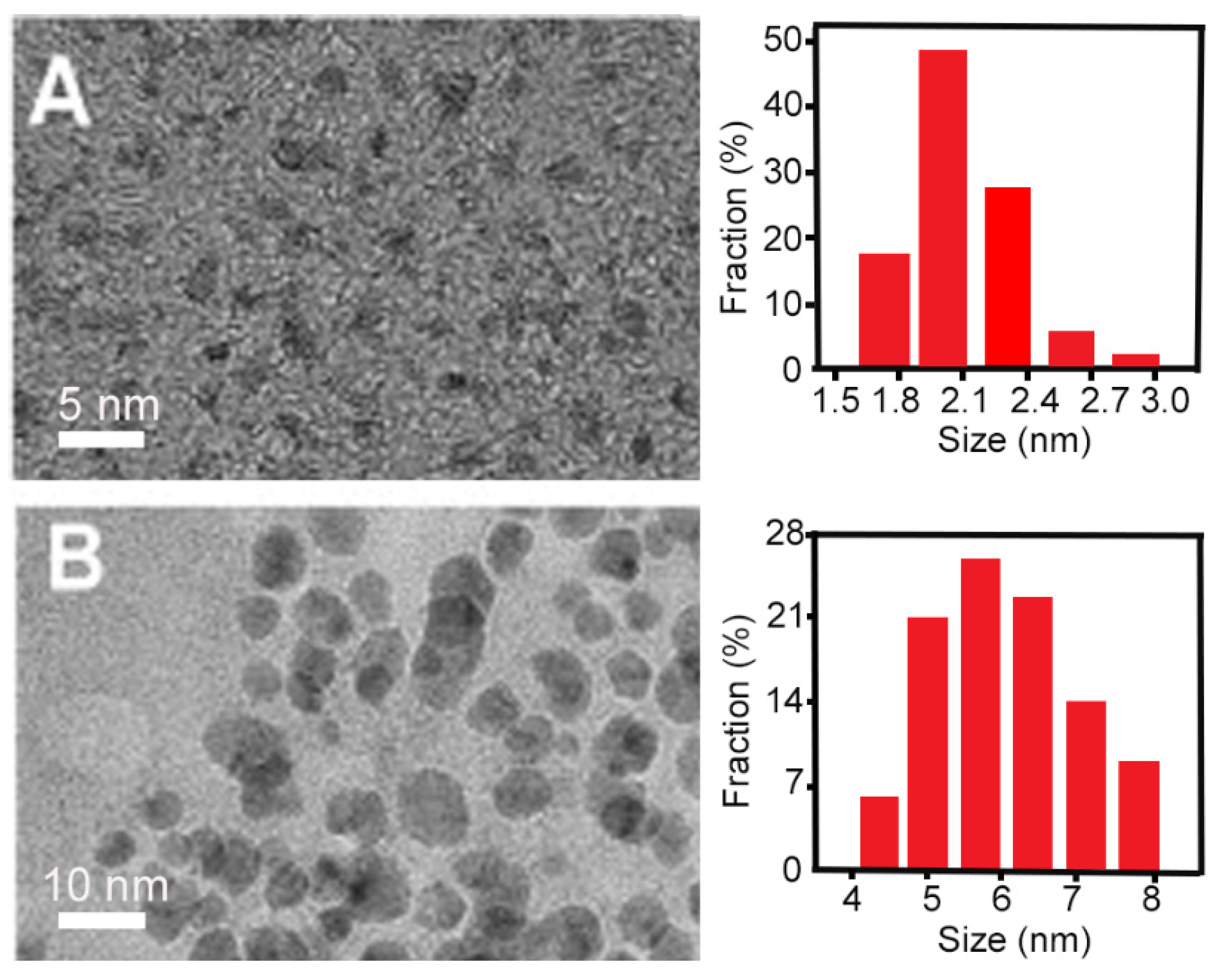
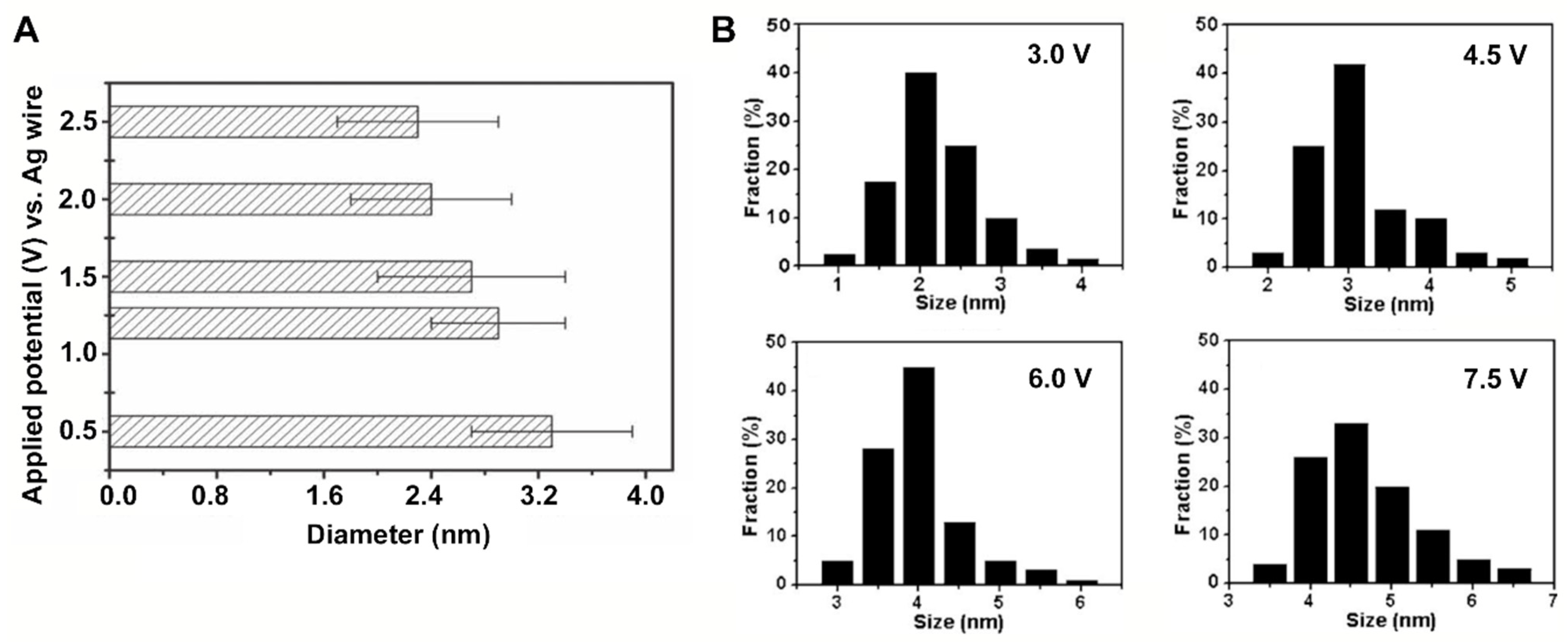
| Electrochemical synthesis | 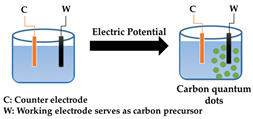 | Good size control | Mainly inorganic sources, few available small molecule precursors | [12,61,62,63,64,65,66] |
| Nanoreactor-assisted synthesis |  | Good size control | Time consuming, nanoreactor preparation is difficult, only liquid precursors | [67,68,69,70] |
| Carbon Source | Synthetic Method | Antibacterial Activity | Bacterial Strains Used | MIC * (µg/mL) | Ref. |
|---|---|---|---|---|---|
| From Organic Reagents | |||||
| Polyamine, polyamine combined with ammonium, dopamine | Pyrolysis, microwave-assisted synthesis | Bacterial killing through cell wall damage; ROS generation | Gram-positive Staphylococcus aureus, Bacillus subtilis, Salmonella enterica, methicillin-resistant S. aureus (MRSA) | 0.9–8 | [19,37,38] |
| Gram-negative Escherichia coli, Pseudomonas aeruginosa | 0.9–8 | ||||
| Bis-quaternary ammonium salt | Hydrothermal method | Bacterial killing through cell wall damage; ROS generation; biofilm growth inhibition; biofilm dispersal through electrostatic interactions | Gram-positive MRSA, S. aureus | 2–4 | [47] |
| Gram-negative E. coli, ampicillin-resistant E. coli (AREC) | 8 | ||||
| Dimethyloctadecyl- [3-(trimethoxysilyl)propyl]ammonium chloride | Hydrothermal method | Biofilm dispersal through electrostatic and hydrophobic interaction with Gram-positive bacteria | Gram-positive S. aureus | No MIC reported | [74] |
| Gram-negative E. coli | No activity | ||||
| 3-[2-(2- aminoethylamino)ethylamino]propyl-trimethoxysilane, glycerol, quaternary ammonium compound lauryl betaine | Pyrolysis | Bacterial killing through cell wall damage | Gram-positive S. aureus, Micrococcus luteus, B. subtilis | 8 – 12 | [89] |
| Gram-negative E. coli, P. aeruginosa, Proteusbacillus vulgaris | >200 | ||||
| Dimethyldiallyl ammonium chloride, glucose | Pyrolysis | Acted on ribosomal proteins in Gram-positive bacteria and downregulated metabolization-related proteins of Gram-negative bacteria | Gram-positive S. aureus, MRSA, Staphylococcus epidermidis, Enterococcus faecalis | 12.5–25 | [90] |
| Gram-negative E. coli, P. aeruginosa | 25–50 | ||||
| Diallyldimethylammonium chloride, 2,3-epoxypropyltrimethylammonium chloride | Pyrolysis | Affected protein translation, posttranslational modification and protein turnover | Gram-positive S. aureus, MRSA, S. epidermidis, Listera monocytogenes, E. faecalis | 5 – 20 | [91] |
| Gram-negative E. coli, Serratia marcescens, Salmonella paratyphi-β | No activity | ||||
| Citric acid, l-glutathion, polyethene polyamine | Pyrolysis | Bacterial killing through cell wall damage; ROS generation | Gram-positive S. aureus, MRSA, L. monocytogenes, E. faecalis | 15–60 | [92] |
| Gram-negative E. coli, P. aeruginosa, S. marcescens, Drug-resistant P. aeruginosa, Drug-resistant E. coli | 120–480 | ||||
| Citric acid combined with aminoguanidine | Hydrothermal method | Bacterial killing through cell wall damage; biofilm growth inhibition | Gram-positive S. aureus, B. cereus | No activity | [84] |
| Gram-negative E. coli, Salmonella enteritidis, Salmonella typhimurium, P. aeruginosa | 0.5–1 (P. aeruginosa), >1000 (other strains) | ||||
| Citric acid combined with branched polyethyleneimine, 2,3-dimethylmaleic anhydride | Hydrothermal method | Biofilm dispersal through electrostatic and hydrophobic interaction with Gram-positive bacteria | Gram-positive S. epidermidis | No MIC reported | [75] |
| Gentamicin sulfate | Pyrolysis | Biofilm dispersal; bacterial killing through cell wall damage; ROS generation and maintenance of antibiotic features | Gram-positive S. aureus | 0.002 (at pH 5.5) | [39] |
| Gram-negative E. coli | 0.203 (at pH 5.5) | ||||
| Ciprofloxacin hydrochloride | Hydrothermal method | Bacterial killing through maintenance of antibiotic features | Gram-positive S. aureus | 1.0 | [48] |
| Gram-negative E. coli | 0.025 | ||||
| Metronidazole | Hydrothermal method | Bacterial killing through maintenance of antibiotic features | Gram-positive S. mutans | No activity | [49] |
| Gram-negative E. coli, Porphyromonas gingivalis | No MIC reported | ||||
| Vitamin C | Electrochemical method | Bacterial killing through cell wall damage | Gram-positive S. aureus, Bacillus sp. WL-6, B. Subtilis | No MIC reported | [61] |
| Gram-negative E. coli, AREC | No MIC reported | ||||
| Poly-oxyethylene, -oxypropylene, -oxyethylene Pluronic 68 | Pyrolysis | Bacteria killing through ROS production upon blue light irradiation | Gram-positive S. aureus, B. cereus | No MIC reported | [76] |
| Gram-negative P. aeruginosa | No MIC reported | ||||
| From Inorganic Carbon Sources | |||||
| Carbon nanopowder, 2,2′-(ethylenedioxy) bis(ethylamine) | Acidic oxidation | Bacterial killing through ROS production upon visible light irradiation | Gram-positive B. subtilis | 64 | [24,29,30,34,93] |
| Gram-negative E. coli | 64 | ||||
| Graphite | Acidic oxidation | Bacterial killing through ROS generation under laser irradiation | Gram-positive MRSA, S. aureus | No MIC reported | [31,32,33] |
| Gram-negative E. coli | No MIC reported | ||||
| Carbon fibers | Acidic oxidation | Biofilm dispersal through interference with the self-assembly of amyloid peptides | Gram-positive S. aureus | No MIC reported | [94] |
| From Natural Carbon Sources | |||||
| Lactobacillus plantarum | Hydrothermal methods | Biofilm growth inhibition | Gram-negative E. coli | No MIC reported | [23] |
| Artemisia argyi leaves | Smoking | Bacterial killing by cell wall damage through cell wall-related enzyme inhibition | Gram-positive S. aureus, B. Subtilis | No activity | [78] |
| Gram-negative E. coli, P. aeruginosa, P. vulgaris | No MIC reported | ||||
| Cigarettes | Smoking | Bacterial killing through destruction of DNA double helix structure | Gram-positive S. aureus, AREC, B. subtilis | No MIC reported | [79] |
| Gram-negative E. coli, kanamycin-resistant E. coli, P. vulgaris, P. aeruginosa | No MIC reported | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Li, C.; van der Mei, H.C.; Busscher, H.J.; Ren, Y. Carbon Quantum Dots Derived from Different Carbon Sources for Antibacterial Applications. Antibiotics 2021, 10, 623. https://doi.org/10.3390/antibiotics10060623
Wu Y, Li C, van der Mei HC, Busscher HJ, Ren Y. Carbon Quantum Dots Derived from Different Carbon Sources for Antibacterial Applications. Antibiotics. 2021; 10(6):623. https://doi.org/10.3390/antibiotics10060623
Chicago/Turabian StyleWu, Yanyan, Cong Li, Henny C. van der Mei, Henk J. Busscher, and Yijin Ren. 2021. "Carbon Quantum Dots Derived from Different Carbon Sources for Antibacterial Applications" Antibiotics 10, no. 6: 623. https://doi.org/10.3390/antibiotics10060623
APA StyleWu, Y., Li, C., van der Mei, H. C., Busscher, H. J., & Ren, Y. (2021). Carbon Quantum Dots Derived from Different Carbon Sources for Antibacterial Applications. Antibiotics, 10(6), 623. https://doi.org/10.3390/antibiotics10060623






