Rescue Therapies for H. pylori Infection in Italy
Abstract
1. Introduction
2. Results
2.1. Descriptive Analysis
2.2. Eradication Rates
3. Discussion
4. Materials and Methods
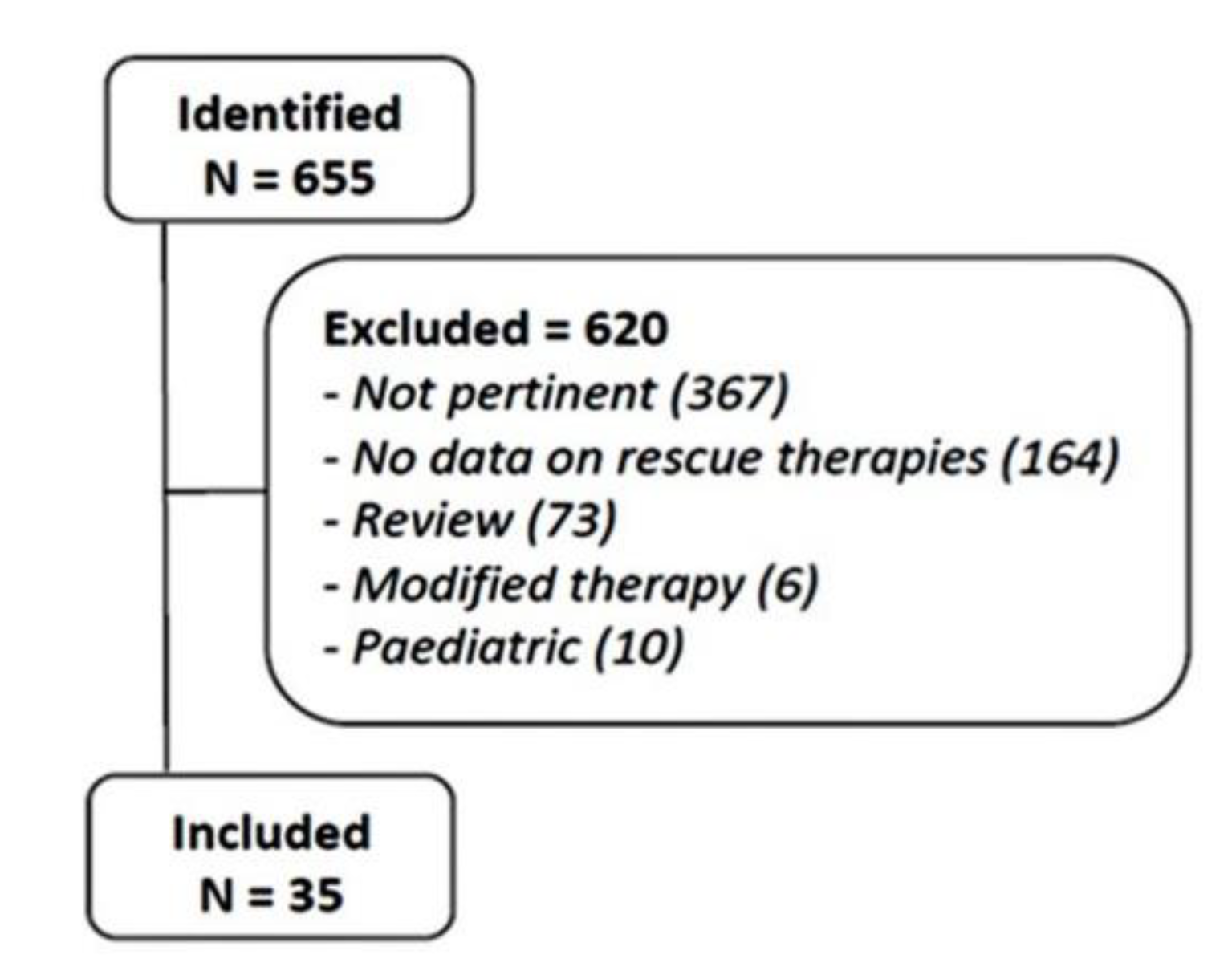
4.1. Literature Review
4.2. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leja, M.; Grinberga-Derica, I.; Bilgilier, C.; Steininger, C. Review: Epidemiology of Helicobacter pylori infection. Helicobacter 2019, 24 (Suppl. 1), e12635. [Google Scholar] [CrossRef] [PubMed]
- Zagari, R.M.; Romano, M.; Ojetti, V.; Stockbrugger, R.; Gullini, S.; Annibale, B.; Farinati, F.; Ierardi, E.; Maconi, G.; Rugge, M.; et al. Guidelines for the management of Helicobacter pylori infection in Italy: The III Working Group Consensus Report. Dig. Liver Dis. 2015, 47, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Abrignani, M.G.; Gatta, L.; Gabrielli, D.; Milazzo, G.; de Francesco, V.; de Luca, L.; Francese, M.; Imazio, M.; Riccio, E.; Rossini, R.; et al. Gastroprotection in patients on antiplatelet and/or anticoagulant therapy: A position paper of National Association of Hospital Cardiologists (ANMCO) and the Italian Association of Hospital Gastroenterologists and Endoscopists (AIGO). Eur. J. Intern. Med. 2021, 85, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Pezeshki, S.M.S.; Saki, N.; Ghandali, M.V.; Ekrami, A.; Avarvand, A.Y. Effect of Helicobacter pylori eradication on patients with ITP: A meta-analysis of studies conducted in the Middle East. Blood Res. 2021, 56, 38–43. [Google Scholar] [CrossRef]
- Elli, L.; Norsa, L.; Zullo, A.; Carroccio, A.; Girelli, C.; Oliva, S.; Romano, C.; Leandro, G.; Bellini, M.; Marmo, R.; et al. Diagnosis of chronic anaemia in gastrointestinal disorders: A guideline by the Italian Association of Hospital Gastroenterologists and Endoscopists (AIGO) and the Italian Society of Paediatric Gastroenterology Hepatology and Nutrition (SIGENP). Dig. Liver Dis. 2019, 51, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Gravina, A.G.; Priadko, K.; Ciamarra, P.; Granata, L.; Facchiano, A.; Miranda, A.; Dallio, M.; Federico, A.; Romano, M. Extra-Gastric Manifestations of Helicobacter pylori Infection. J. Clin. Med. 2020, 9, 3887. [Google Scholar] [CrossRef] [PubMed]
- Zullo, A.; de Francesco, V.; Hassan, C. Predicting Helicobacter pylori eradication: How to teach an old dog new tricks! J. Clin. Gastroenterol. 2012, 46, 259–261. [Google Scholar] [CrossRef]
- Saracino, I.M.; Fiorini, G.; Zullo, A.; Pavoni, M.; Saccomanno, L.; Vaira, D. Trends in Primary Antibiotic Resistance in H. pylori Strains Isolated in Italy between 2009 and 2019. Antibiotics 2020, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, V.; Bellesia, A.; Ridola, L.; Manta, R.; Zullo, A. First-line therapies for Helicobacter pylori eradication: A critical reappraisal of updated guidelines. Ann. Gastroenterol. 2017, 30, 373–379. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef]
- Zullo, A.; Hassan, C.; Lorenzetti, R.; Winn, S.; Morini, S. A clinical practice viewpoint: To culture or not to culture Helicobacter pylori? Dig. Liver Dis. 2003, 35, 357–361. [Google Scholar] [CrossRef]
- De Francesco, V.; Zullo, A.; Fiorini, G.; Saracino, I.M.; Pavoni, M.; Vaira, D. Role of MIC levels of resistance to clarithromycin and metronidazole in Helicobacter pylori eradication. J. Antimicrob. Chemother. 2019, 74, 772–774. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, N.; Sánchez-Delgado, J.; Baylina, M.; Puig, I.; López-Góngora, S.; Suarez, D.; Calvet, X. Systematic review, meta-analysis, and meta-regression: Successful second-line treatment for Helicobacter pylori. Helicobacter 2018, 23, e12488. [Google Scholar] [CrossRef] [PubMed]
- Saracino, I.M.; Pavoni, M.; Zullo, A.; Fiorini, G.; Saccomanno, L.; Lazzarotto, T.; Antonelli, G.; Cavallo, R.; Borghi, C.; Vaira, D. Rifabutin-based triple therapy or bismuth-based quadruple regimen as rescue therapies for Helicobacter pylori infection. Eur. J. Intern. Med. 2020, 81, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Saracino, I.M.; Pavoni, M.; Zullo, A.; Fiorini, G.; Saccomanno, L.; Lazzarotto, T.; Antonelli, G.; Cavallo, R.; Borghi, C.; Vaira, D. Rescue Therapies for Helicobacter pylori infection in foreign patients treated in Italy. J. Clin. Gastroenterol. 2020. [Google Scholar] [CrossRef]
- Saracino, I.M.; Pavoni, M.; Zullo, A.; Fiorini, G.; Saccomanno, L.; Lazzarotto, T.; Cavallo, R.; Antonelli, G.; Vaira, D. Antibiotic resistance and therapy outcome in H. pylori eradication failure patients. Antibiotics 2020, 9, 121. [Google Scholar]
- Mascellino, M.T.; Oliva, A.; Miele, M.C.; de Angelis, M.; Bruno, G.; Severi, C. Secondary antibiotic resistance, correlation between genotypic and phenotypic methods and treatment in Helicobacter pylori infected patients: A retrospective study. Antibiotics 2020, 9, 549. [Google Scholar] [CrossRef]
- Ribaldone, D.G.; Fagoonee, S.; Astegiano, M.; Durazzo, M.; Morgando, A.; Sprujevnik, T.; Giordanino, C.; Baronio, M.; de Angelis, C.; Saracco, G.M.; et al. Rifabutin-based rescue therapy for Helicobacter pylori eradication: A long-term prospective study in a large cohort of difficult-to-treat patients. J. Clin. Med. 2019, 8, 199. [Google Scholar] [CrossRef]
- Tursi, A.; Franceschi, M.; Allegretta, L.; Savarino, E.; de Bastiani, R.; Elisei, W.; Baldassarre, G.; Ferronato, A.; Scida, S.; Miraglia, C.; et al. Effectiveness and safety of Pylera® in patients infected by Helicobacter pylori: A multicenter, retrospective, real-life study. Dig. Dis. 2018, 36, 264–268. [Google Scholar] [CrossRef]
- Fiorini, G.; Zullo, A.; Vakil, N.; Saracino, I.M.; Ricci, C.; Castelli, V.; Gatta, L.; Vaira, D. Rifabutin triple therapy is effective in patients with multidrug-resistant strains of Helicobacter pylori. J. Clin. Gastroenterol. 2018, 52, 137–140. [Google Scholar] [CrossRef]
- Di Ciaula, A.; Scaccianoce, G.; Venerito, M.; Zullo, A.; Bonfrate, L.; Rokkas, T.; Portincasa, P. Eradication rates in Italian subjects heterogeneously managed for Helicobacter pylori infection. Time to abandon empiric treatments in Southern Europe. J. Gastrointest. Liver Dis. 2017, 26, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Zagari, R.M.; Romiti, A.; Ierardi, E.; Gravina, A.G.; Panarese, A.; Grande, G.; Savarino, E.; Maconi, G.; Stasi, E.; Eusebi, L.H.; et al. The “three-in-one” formulation of bismuth quadruple therapy for Helicobacter pylori eradication with or without probiotics supplementation: Efficacy and safety in daily clinical practice. Helicobacter 2018, 23, e12502. [Google Scholar] [CrossRef] [PubMed]
- Fiorini, G.; Saracino, I.M.; Zullo, A.; Gatta, L.; Pavoni, M.; Vaira, D. Rescue therapy with bismuth quadruple regimen in patients with Helicobacter pylori-resistant strains. Helicobacter 2017, 22, e12448. [Google Scholar] [CrossRef] [PubMed]
- Zullo, A.; de Francesco, V.; Bellesia, A.; Vassallo, R.; D’Angelo, A.; Scaccianoce, G.; Sacco, R.; Bresci, G.; Eramo, A.; Tanzilli, A.; et al. Bismuth-based quadruple therapy following H. pylori eradication failures: A multicenter study in clinical practice. J. Gastrointest. Liver Dis. 2014, 26, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Tursi, A.; di Mario, F.; Franceschi, M.; de Bastiani, R.; Elisei, W.; Baldassarre, G.; Ferronato, A.; Grillo, S.; Landi, S.; Zamparella, M.; et al. New bismuth-containing quadruple therapy in patients infected with Helicobacter pylori: A first Italian experience in clinical practice. Helicobacter 2017, 22, e12371. [Google Scholar] [CrossRef]
- De Francesco, V.; Ridola, L.; Hassan, C.; Bellesia, A.; Alvaro, D.; Vaira, D.; Zullo, A. Two-week Triple Therapy with either Standard or high-dose esomeprazole for first-line H. pylori eradication. J. Gastrointest. Liver Dis. 2016, 25, 147–150. [Google Scholar] [CrossRef]
- Ciccaglione, A.F.; Tavani, R.; Grossi, L.; Cellini, L.; Manzoli, L.; Marzio, L. Rifabutin containing triple therapy and rifabutin with bismuth containing quadruple therapy for third-line treatment of Helicobacter pylori infection: Two pilot studies. Helicobacter 2016, 21, 375–381. [Google Scholar] [CrossRef]
- Ierardi, E.; Giangaspero, A.; Losurdo, G.; Giorgio, F.; Amoruso, A.; de Francesco, V.; Di Leo, A.; Principi, M. Quadruple rescue therapy after first and second-line failure for Helicobacter pylori treatment: Comparison between two tetracycline-based regimens. J. Gastrointest. Liver Dis. 2014, 23, 367–370. [Google Scholar] [CrossRef]
- Zullo, A.; Ridola, L.; Efrati, C.; Giorgio, F.; Nicolini, G.; Cannaviello, C.; Alvaro, D.; Hassan, C.; Gatta, L.; Francesco, V.D. First- and second-line Helicobacter pylori eradication with modified sequential therapy and modified levofloxacin-amoxicillin-based triple therapy. Ann. Gastroenterol. 2014, 27, 357–361. [Google Scholar]
- Zullo, A.; Scaccianoce, G.; de Francesco, V.; Ruggiero, V.; D’Ambrosio, P.; Castorani, L.; Bonfrate, L.; Vannella, L.; Hassan, C.; Portincasa, P. Concomitant, sequential, and hybrid therapy for H. pylori eradication: A pilot study. Clin. Res. Hepatol. Gastroenterol. 2013, 37, 647–650. [Google Scholar] [CrossRef]
- Fiorini, G.; Vakil, N.; Zullo, A.; Saracino, I.M.; Castelli, V.; Ricci, C.; Zaccaro, C.; Gatta, L.; Vaira, D. Culture-based selection therapy for patients who did not respond to previous treatment for Helicobacter pylori infection. Clin. Gastroenterol. Hepatol. 2013, 101, 507–510. [Google Scholar] [CrossRef]
- Federico, A.; Nardone, G.; Gravina, A.G.; Iovene, M.R.; Miranda, A.; Compare, D.; Pilloni, P.A.; Rocco, A.; Ricciardiello, L.; Marmo, R.; et al. Efficacy of 5-day levofloxacin-containing concomitant therapy in eradication of Helicobacter pylori infection. Gastroenterology 2012, 143, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Sereni, G.; Azzolini, F.; Camellini, L.; Formisano, D.; Decembrino, F.; Iori, V.; Tioli, C.; Cavina, M.; Di Mario, F.; Bedogni, G.; et al. Efficacy of a therapeutic strategy for eradication of Helicobacter pylori infection. World J. Gastronterol. 2012, 18, 4542–4548. [Google Scholar] [CrossRef] [PubMed]
- D’Elios, M.M.; Silvestri, E.; Emmi, G.; Barnini, T.; Prisco, D. Helicobacter pylori: Usefulness of an empirical fourth-line rifabutin-based regimen. Expert Rev. Gastroenterol. Hepatol. 2012, 6, 437–439. [Google Scholar] [CrossRef]
- Ojetti, V.; Bruno, G.; Ainora, M.E.; Gigante, G.; Rizzo, G.; Roccarina, D.; Gasbarrini, A. Impact of Lactobacillus reuteri supplementation on anti-Helicobacter pylori levofloxacin-based second-line therapy. Gastroenterol. Res. Pract. 2012, 2012, 740381. [Google Scholar] [CrossRef]
- Manfredi, M.; Bizzarri, B.; de’Angelis, G.L. Helicobacter pylori infection: Sequential therapy followed by levofloxacin-containing triple therapy provides a good cumulative eradication rate. Helicobacter 2012, 17, 246–253. [Google Scholar] [CrossRef]
- Urgesi, R.; Pelecca, G.; Cianci, R.; Masini, A.; Zampaletta, C.; Riccioni, M.E.; Faggiani, R. Helicobacter pylori infection: Is sequential therapy superior to standard triple therapy? A single-centre Italian study in treatment-naive and non-treatment-naive patients. Can. J. Gastroenterol. 2011, 25, 315–318. [Google Scholar] [CrossRef][Green Version]
- Zullo, A.; de Francesco, V.; Manes, G.; Scaccianoce, G.; Cristofari, F.; Hassan, C. Second-line and rescue therapies for Helicobacter pylori eradication in clinical practice. J. Gastrointestin. Liver Dis. 2010, 19, 131–134. [Google Scholar]
- Zullo, A.; Hassan, C.; Cristofari, F.; Iegri, C.; de Francesco, V. How to manage Helicobacter pylori after sequential therapy failure? J. Clin. Gastroenterol. 2010, 44, 459–460. [Google Scholar] [CrossRef] [PubMed]
- Pontone, S.; Standoli, M.; Angelini, R.; Pontone, P. Efficacy of H. pylori eradication with a sequential regimen followed by rescue therapy in clinical practice. Dig. Liver Dis. 2010, 42, 541–543. [Google Scholar] [CrossRef]
- Di Caro, S.; Franceschi, F.; Mariani, A.; Thompson, F.; Raimondo, D.; Masci, E.; Testoni, A.; La Rocca, E.; Gasbarrini, A. Second-line levofloxacin-based triple schemes for Helicobacter pylori eradication. Dig. Liver Dis. 2019, 41, 480–485. [Google Scholar] [CrossRef]
- Perna, F.; Zullo, A.; Ricci, C.; Hassan, C.; Morini, S.; Vaira, D. Levofloxacin-based triple therapy for Helicobacter pylori re-treatment: Role of bacterial resistance. Dig. Liver Dis. 2007, 39, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Tursi, A.; Elisei, W.; Brandimarte, G.; Giorgetti, G.M.; Modeo, M.E.; Aiello, F. Effect of lactoferrin supplementation on the effectiveness and tolerability of a 7-day quadruple therapy after failure of a first attempt to cure Helicobacter pylori infection. Med. Sci. Monit. 2007, 13, CR187–CR190. [Google Scholar] [PubMed]
- Marzio, L.; Coraggio, D.; Capodicasa, S.; Grossi, L.; Cappello, G. Role of the preliminary susceptibility testing for initial and after failed therapy of Helicobacter pylori infection with levofloxacin, amoxicillin, and esomeprazole. Helicobacter 2006, 11, 237–242. [Google Scholar] [CrossRef]
- Giannini, E.G.; Bilardi, C.; Dulbecco, P.; Mamone, M.; Santi, M.L.; Testa, R.; Mansi, C.; Savarino, V. A study of 4- and 7-day triple therapy with rabeprazole, high-dose levofloxacin and tinidazole rescue treatment for Helicobacter pylori eradication. Aliment. Pharmacol. Ther. 2006, 23, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Zullo, A.; de Francesco, V.; Hassan, C.; Panella, C.; Morini, S.; Ierardi, E. Second-line treatment for Helicobacter pylori eradication after sequential therapy failure: A pilot study. Therapy 2006, 3, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Gatta, L.; Zullo, A.; Perna, F.; Ricci, C.; de Francesco, V.; Tampieri, A.; Bernabucci, V.; Cavina, M.; Hassan, C.; Ierardi, E.; et al. A 10-day levofloxacin-based triple therapy in patients who have failed two eradication courses. Aliment. Pharmacol. Ther. 2005, 22, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Toracchio, S.; Capodicasa, S.; Soraja, D.B.; Cellini, L.; Marzio, L. Rifabutin based triple therapy for eradication of H. pylori primary and secondary resistant to tinidazole and clarithromycin. Dig. Liver Dis. 2005, 37, 33–38. [Google Scholar] [CrossRef]
- Hu, Y.; Zhu, Y.; Lu, N.H. Novel and Effective Therapeutic Regimens for Helicobacter pylori in an Era of Increasing Antibiotic Resistance. Front. Cell. Infect. Microbiol. 2017, 7, 168. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. WHO Pathogens Priority List Working Group. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet. Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Gisbert, J.P. Empirical or susceptibility-guided treatment for Helicobacter pylori infection? A comprehensive review. Ther. Adv. Gastroenterol. 2020, 13, 1–16. [Google Scholar] [CrossRef]
- Gisbert, J.P. Optimization strategies aimed to increase the efficacy of Helicobacter pylori eradication therapies with quinolones. Molecules 2020, 25, 5084. [Google Scholar] [CrossRef]
- Moellering, R. A cause for worldwide concern. N. Engl. J. Med. 2010, 363, 2377–2379. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, V.; Zullo, A.; Manta, R.; Gatta, L.; Fiorini, G.; Saracino, I.M.; Vaira, D. Helicobacter pylori eradication following first-line treatment failure in Europe: What, how and when chose among different standard regimens? A systematic review. Eur. J. Gastroenterol. Hepatol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Zullo, A. The current role of dual therapy for treatment of Helicobacter pylori: Back to the future? Eur. J. Gastroenterol. Hepatol. 2020, 32, 555–556. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, Y.; Fan, L.; Zhu, Y.J.; Wang, T.Y.; Wang, X.W.; Chen, D.F.; Lan, C.H. Eradication efficacy of modified dual therapy compared with bismuth-containing quadruple therapy as a first-line treatment of Helicobacter pylori. Am. J. Gastroenterol. 2019, 114, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.P.; Zhang, D.; Zhang, T.; Wang, J.X.; Han, S.X.; Graham, D.Y.; Lu, H. PPI-amoxicillin dual therapy for Helicobacter pylori infection: An update based on a systematic review and meta-analysis. Helicobacter 2020, 25, e12692. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Zhang, Y.; Wang, T.Y.; Zhao, J.T.; Zhao, Z.; Zhu, J.R.; Lan, C.H. High dose PPI-amoxicillin dual therapy for the treatment of Helicobacter pylori infection: A systematic review with meta-analysis. Ther. Adv. Gastroenterol. 2020, 13, 1–12. [Google Scholar] [CrossRef]
| Therapy Regimen | Second-Line % (95% CI) | Third-Line % (95% CI) | ≥Fourth-Line % (95% CI) |
|---|---|---|---|
| Levofloxacin (N) | 1273 | 151 | 63 |
| ITT | 78.7 (76.5–81) | 84.7 (79–90.5) | 74.6 (63.8–85.3) |
| PP | 81.6 (79.3–84) | 88.2 (83–93.5) | 77 (66.5–87.6) |
| Pylera–quadruple (N) | 873 | 222 | 164 |
| ITT | 90.6 (88.6–92.5) | 88.2 (84–92.5) | 77.4 (71–83.8) |
| PP | 94 (92.2–95.7) | 91.4 (86.5–96.2) | 81.9 (75.1–88.7) |
| Bismuth–quadruple (N) | 154 | 27 | - |
| ITT | 92.8 (88.7–96.9) | 51.8 (33–70.6) | - |
| PP | 98.6 (95.9–100) | 58.3 (38.6–78) | - |
| Rifabutin (N) | 1009 | 428 | 301 |
| ITT | 65.9 (62.9–68.8) | 77.3 (73.3–81.3) | 66.4 (61.1–71.7) |
| PP | 76.4 (73.6–79.2) | 82.1 (78.3–85.8) | 73.3 (67.8–78.2) |
| Sequential (N) | 118 | 29 | 18 |
| ITT | 89.8 (84.3–95.2) | 79.3 (64.5–94) | 77.2 (51.5–92.9) |
| PP | 95.5 (91.6–99.3) | 85.1 (71.7–98.5) | 81.2 (62.1–100) |
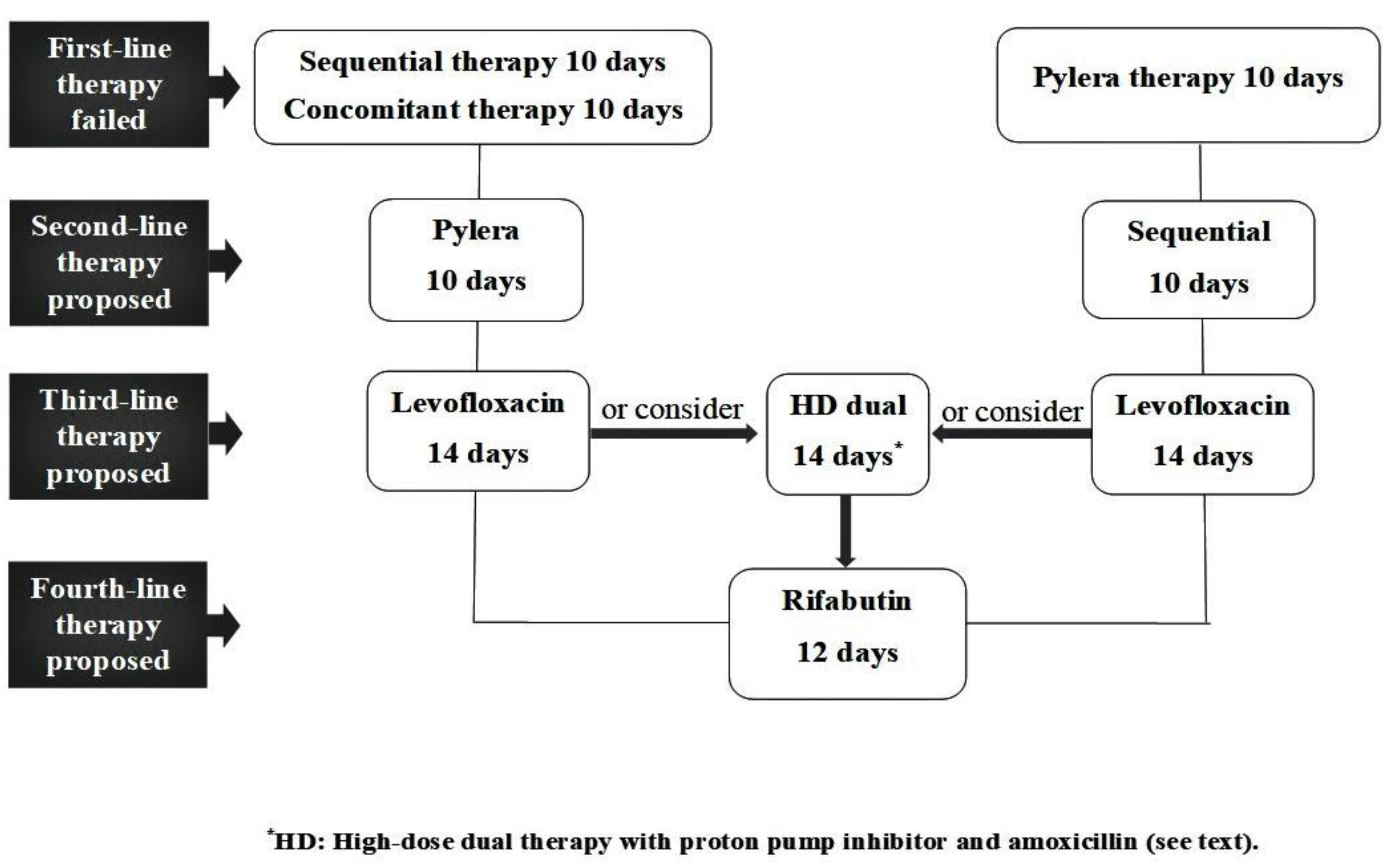
| Therapy | Schedule | Duration |
|---|---|---|
| Sequential | Esomeprazole 40 mg and amoxycillin 1 g, all b.i.d for 5 days followed by: Esomeprazole 40 mg, clarithromycin 500 mg, and tinidazole 500 mg, all b.i.d for 5 days | 10 days |
| Pylera® | 3 tablets q.i.d. and omeprazole 20 mg b.i.d | 10 days |
| Levofloxacin | Esomeprazole 40 mg, levofloxacin 250 mg, and amoxycillin 1 g, all b.i.d | 14 days |
| High-dose dual | Esomeprazole 40 mg and amoxycillin 1 g, all t.i.d | 14 days |
| Rifabutin | Esomeprazole 40 mg and amoxycillin 1 g b.i.d plus rifabutin 150 mg q.d | 12 days |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Francesco, V.; Zullo, A.; Gatta, L.; Manta, R.; Pavoni, M.; Saracino, I.M.; Fiorini, G.; Vaira, D. Rescue Therapies for H. pylori Infection in Italy. Antibiotics 2021, 10, 525. https://doi.org/10.3390/antibiotics10050525
De Francesco V, Zullo A, Gatta L, Manta R, Pavoni M, Saracino IM, Fiorini G, Vaira D. Rescue Therapies for H. pylori Infection in Italy. Antibiotics. 2021; 10(5):525. https://doi.org/10.3390/antibiotics10050525
Chicago/Turabian StyleDe Francesco, Vincenzo, Angelo Zullo, Luigi Gatta, Raffaele Manta, Matteo Pavoni, Ilaria Maria Saracino, Giulia Fiorini, and Dino Vaira. 2021. "Rescue Therapies for H. pylori Infection in Italy" Antibiotics 10, no. 5: 525. https://doi.org/10.3390/antibiotics10050525
APA StyleDe Francesco, V., Zullo, A., Gatta, L., Manta, R., Pavoni, M., Saracino, I. M., Fiorini, G., & Vaira, D. (2021). Rescue Therapies for H. pylori Infection in Italy. Antibiotics, 10(5), 525. https://doi.org/10.3390/antibiotics10050525








