Evaluation of the MeroRisk Calculator, A User-Friendly Tool to Predict the Risk of Meropenem Target Non-Attainment in Critically Ill Patients
Abstract
1. Introduction
2. Results
2.1. Data and Patients
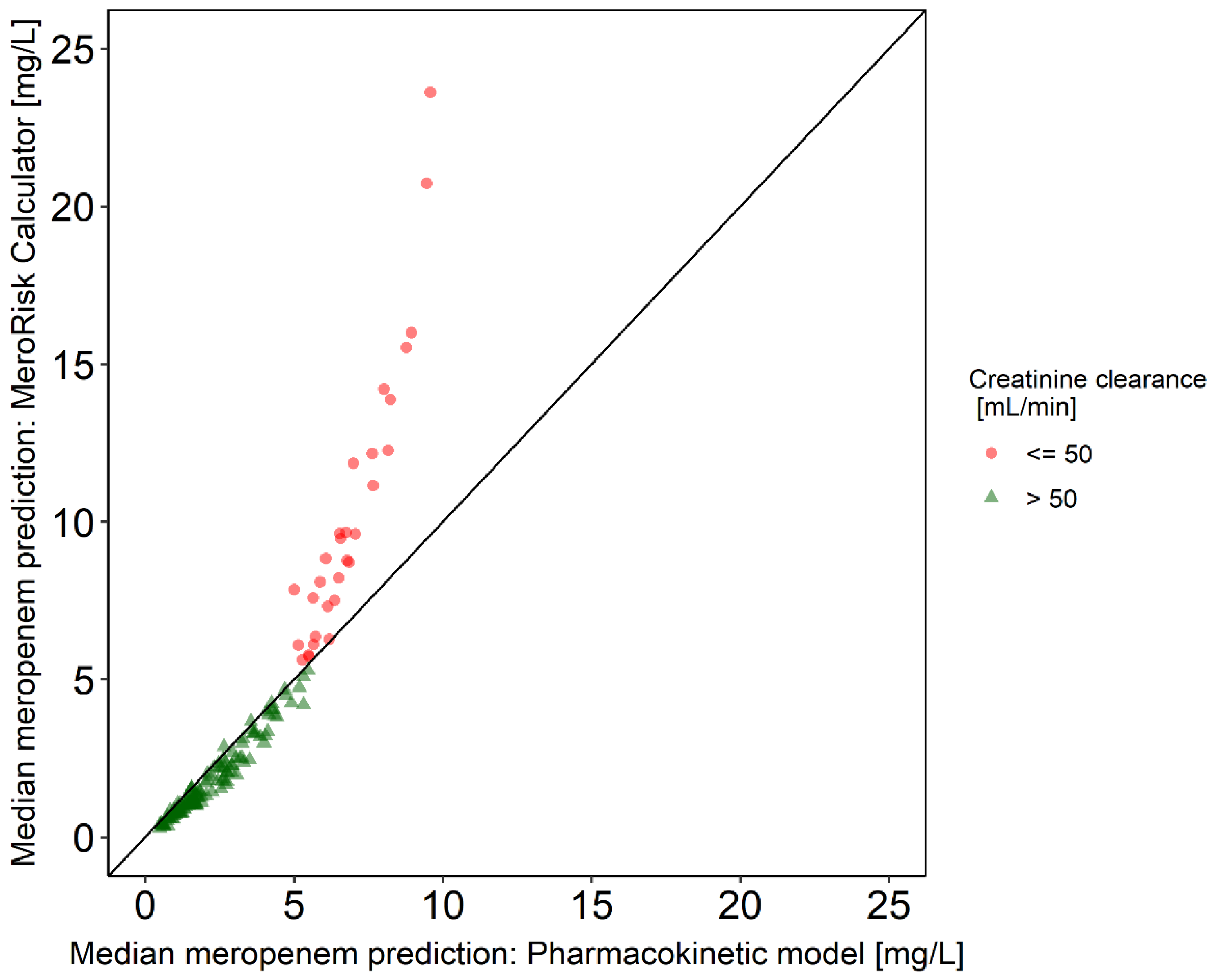
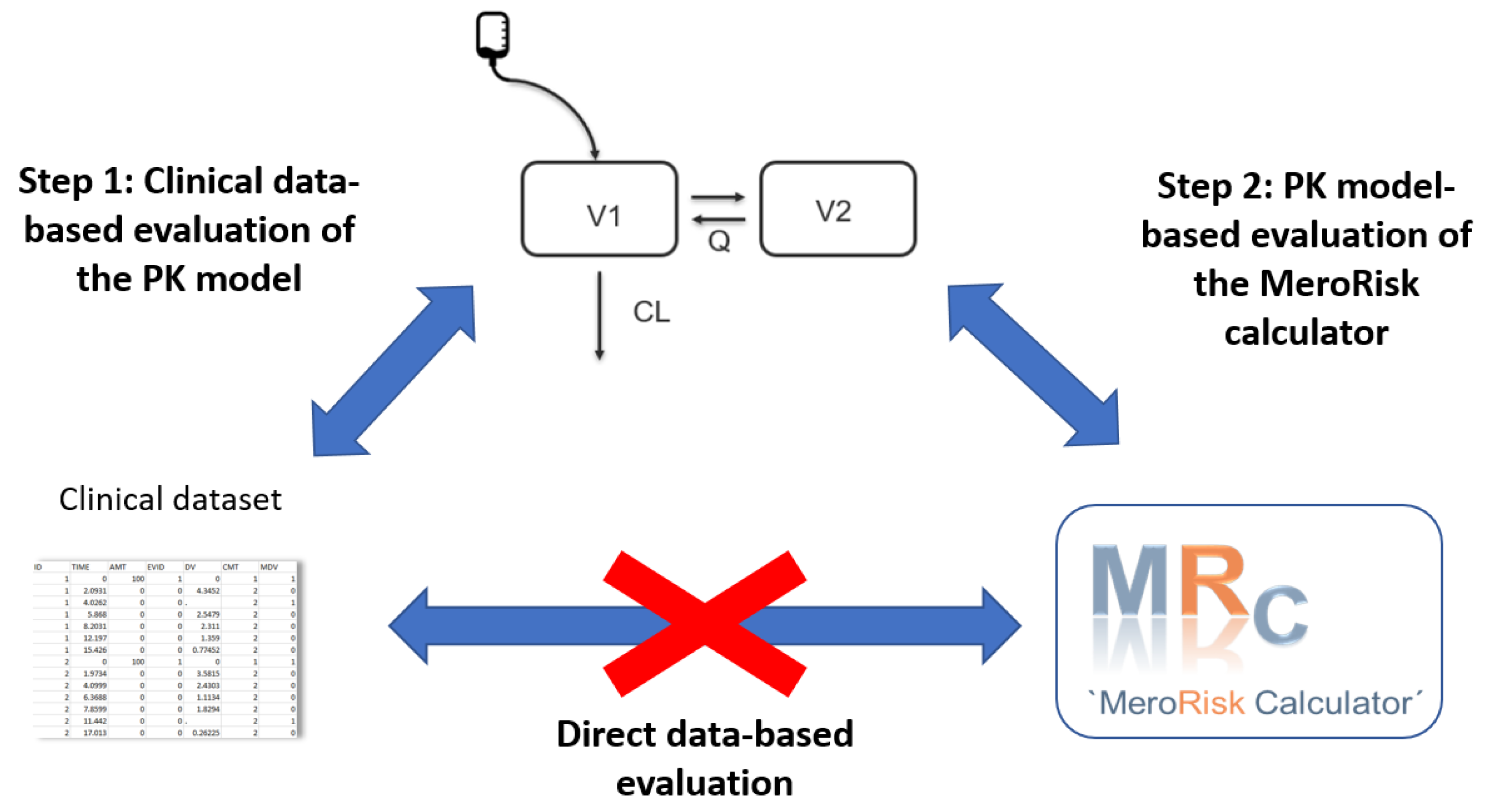
2.2. Evaluation Step 1: Evaluation of the Potential of the Selected Meropenem Population Pharmacokinetic (PK) Model to Predict the Clinical Routine Dataset
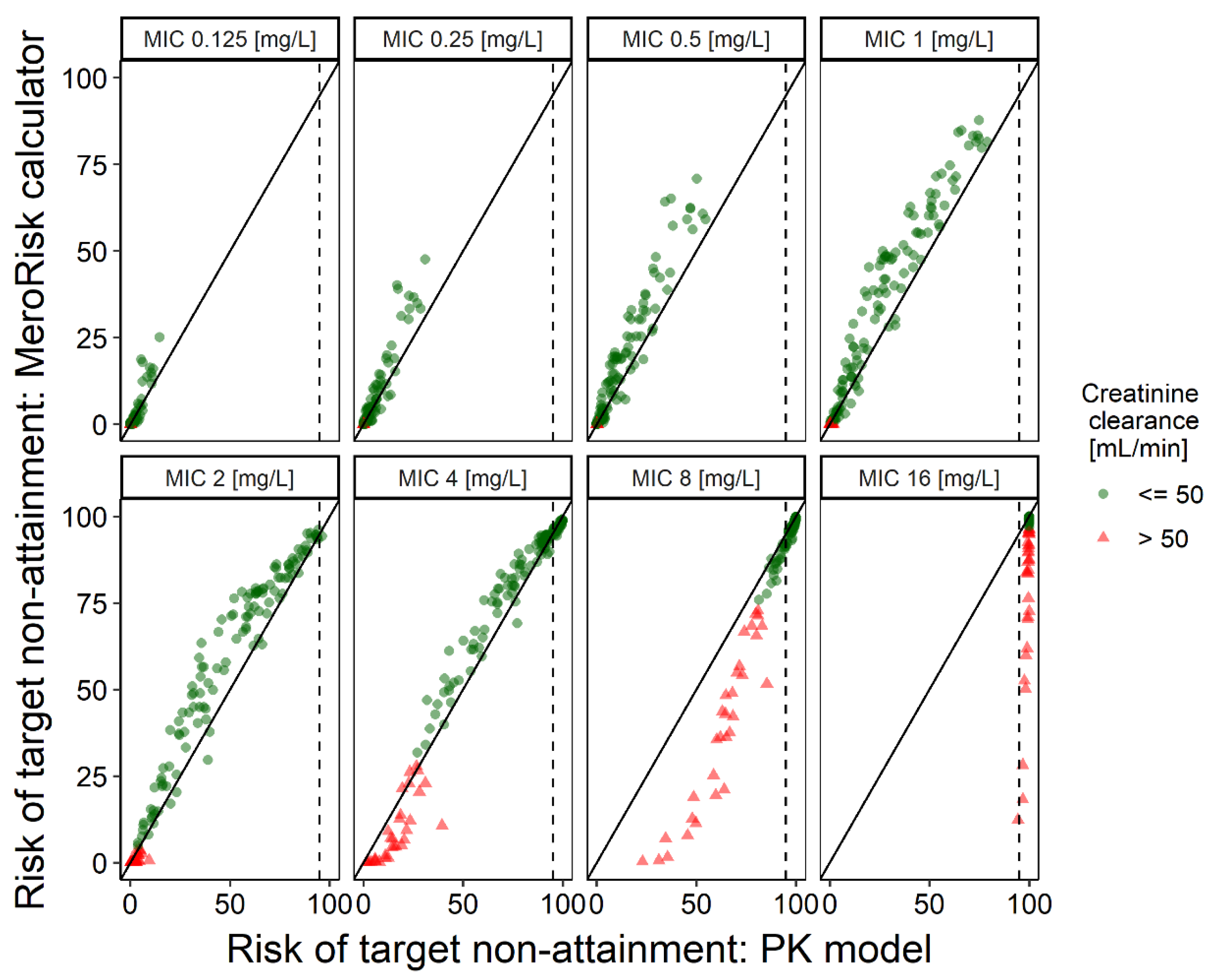
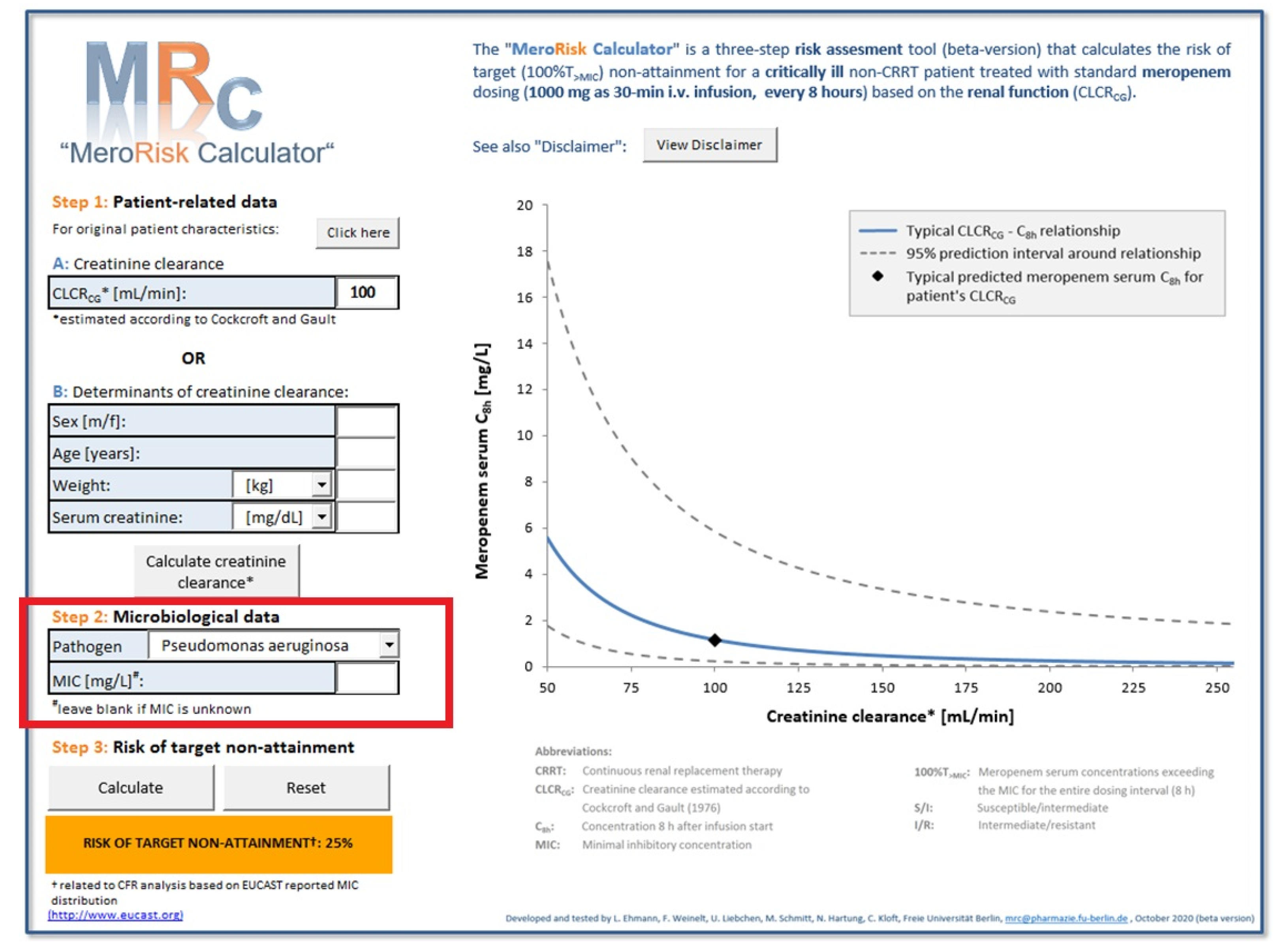
2.3. Evaluation Step 2: Evaluation of the MeroRisk-Calculator Based on PK Model Predictions of Meropenem Concentrations 8 h after Dosing
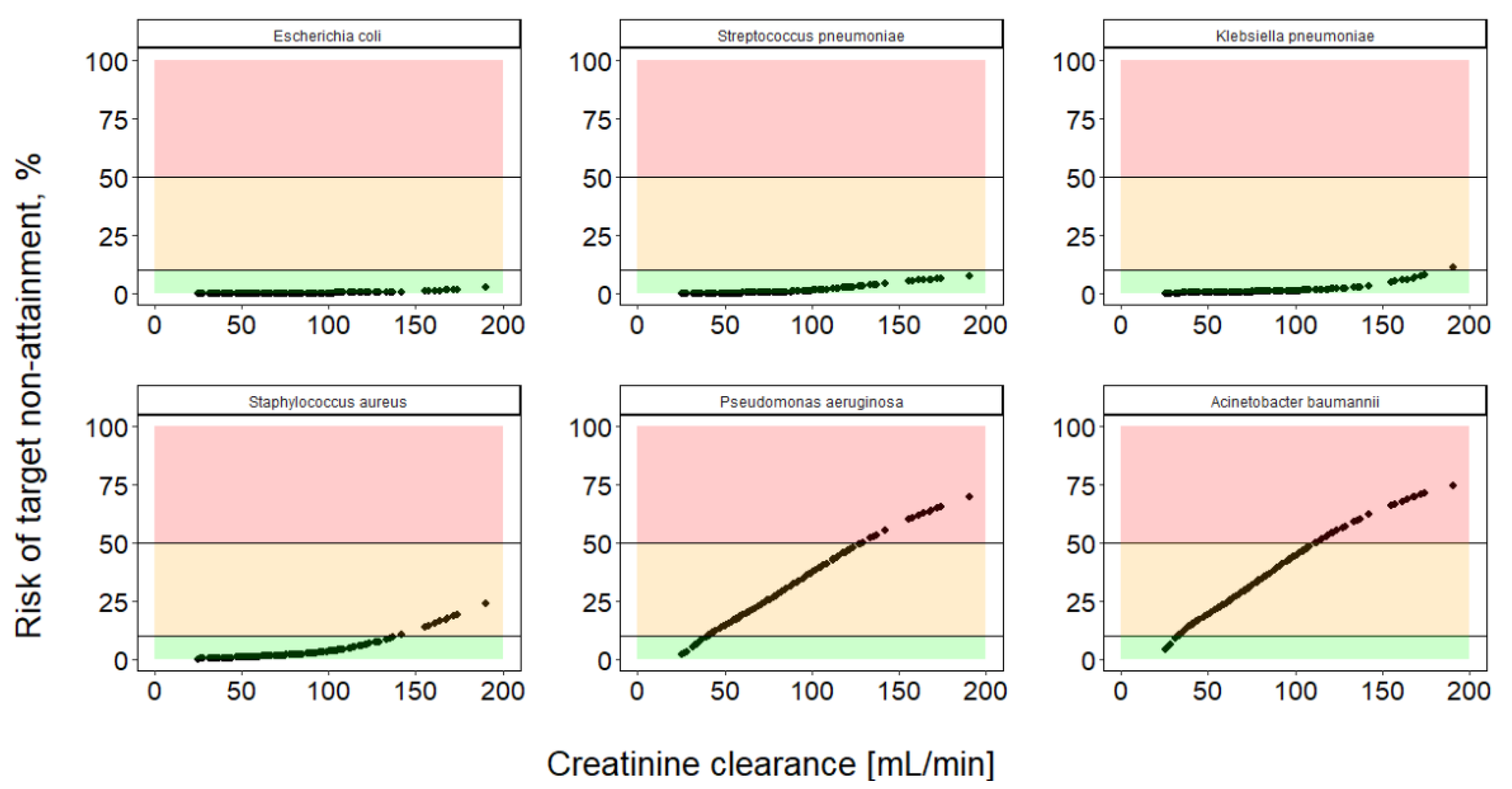
2.4. Extending Risk Predictions to Include General Pathogen Sensitivity Data
3. Discussion
4. Materials and Methods
4.1. Evaluation Strategy for the MeroRisk-Calculator
- Step 1: Evaluation of the potential of the selected meropenem population pharmacokinetic (PK) model to predict the clinical routine dataset.
- Step 2: Evaluation of the MeroRisk-Calculator based on PK model predictions of meropenem concentrations 8 h after dosing.
4.2. Clinical Data and Patients
4.3. Evaluation Step 1: Evaluation of the Potential of the Selected Meropenem Population Pharmacokinetic (PK) Model to Predict the Clinical Routine Dataset
4.4. Evaluation Step 2: Evaluation of the MeroRisk-Calculator Based on PK Model Predictions of Meropenem Concentrations 8 h after Dosing
4.5. Integration of Risk Assessment Based on Pathogen-Specific MIC Distribution
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Engel, C.; Brunkhorst, F.M.; Bone, H.-G.; Brunkhorst, R.; Gerlach, H.; Grond, S.; Gruendling, M.; Huhle, G.; Jaschinski, U.; John, S.; et al. Epidemiology of Sepsis in Germany: Results from a National Prospective Multicenter Study. Intensiv. Care Med. 2007, 33, 606–618. [Google Scholar] [CrossRef]
- Vincent, J.-L.; Rello, J.; Marshall, J.; Silva, E.; Anzueto, A.; Martin, C.D.; Moreno, R.; Lipman, J.; Gomersall, C.; Sakr, Y.; et al. International Study of the Prevalence and Outcomes of Infection in Intensive Care Units. JAMA 2009, 302, 2323–2329. [Google Scholar] [CrossRef]
- Cecconi, M.; Evans, L.; Levy, M.; Rhodes, A. Sepsis and Septic Shock. Lancet 2018, 392, 75–87. [Google Scholar] [CrossRef]
- Kumar, A.; Roberts, D.; Wood, K.E.; Light, B.; Parrillo, J.E.; Sharma, S.; Suppes, R.; Feinstein, D.; Zanotti, S.; Taiberg, L.; et al. Duration of Hypotension before Initiation of Effective Antimicrobial Therapy Is the Critical Determinant of Survival in Human Septic Shock. Crit. Care Med. 2006, 34, 1589–1596. [Google Scholar] [CrossRef]
- Ferrer, R.; Martin-Loeches, I.; Phillips, G.; Osborn, T.M.; Townsend, S.; Dellinger, R.P.; Artigas, A.; Schorr, C.; Levy, M.M. Empiric Antibiotic Treatment Reduces Mortality in Severe Sepsis and Septic Shock from the First Hour: Results from a Guideline-Based Performance Improvement Program. Crit. Care Med. 2014, 42, 1749–1755. [Google Scholar] [CrossRef]
- Kim, R.Y.; Ng, A.M.; Persaud, A.K.; Furmanek, S.P.; Kothari, Y.N.; Price, J.D.; Wiemken, T.L.; Saad, M.A.; Guardiola, J.J.; Cavallazzi, R.S. Antibiotic Timing and Outcomes in Sepsis. Am. J. Med. Sci. 2018, 355, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves-Pereira, J.; Póvoa, P. Antibiotics in Critically Ill Patients: A Systematic Review of the Pharmacokinetics of Beta-Lactams. Crit. Care 2011, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Udy, A.A.; Roberts, J.A.; Lipman, J. Clinical Implications of Antibiotic Pharmacokinetic Principles in the Critically Ill. Intensiv. Care Med. 2013, 39, 2070–2082. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Paul, S.K.; Akova, M.; Bassetti, M.; Waele, J.J.D.; Dimopoulos, G.; Kaukonen, K.-M.; Koulenti, D.; Martin, C.; Montravers, P.; et al. DALI: Defining Antibiotic Levels in Intensive Care Unit Patients: Are Current β-Lactam Antibiotic Doses Sufficient for Critically Ill Patients? Clin. Infect. Dis. 2014, 58, 1072–1083. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.H.; Lipman, J.; Roberts, J.A. Identifying “at-Risk” Patients for Sub-Optimal Beta-Lactam Exposure in Critically Ill Patients with Severe Infections. Crit. Care 2017, 21, 283. [Google Scholar] [CrossRef]
- Ehmann, L.; Zoller, M.; Minichmayr, I.K.; Scharf, C.; Maier, B.; Schmitt, M.V.; Hartung, N.; Huisinga, W.; Vogeser, M.; Frey, L.; et al. Role of Renal Function in Risk Assessment of Target Non-Attainment after Standard Dosing of Meropenem in Critically Ill Patients: A Prospective Observational Study. Crit. Care 2017, 21, 263. [Google Scholar] [CrossRef] [PubMed]
- Scharf, C.; Paal, M.; Schroeder, I.; Vogeser, M.; Draenert, R.; Irlbeck, M.; Zoller, M.; Liebchen, U. Therapeutic Drug Monitoring of Meropenem and Piperacillin in Critical Illness—Experience and Recommendations from One Year in Routine Clinical Practice. Antibiotics 2020, 9, 131. [Google Scholar] [CrossRef]
- Wu, C.-C.; Tai, C.H.; Liao, W.-Y.; Wang, C.-C.; Kuo, C.-H.; Lin, S.-W.; Ku, S.-C. Augmented Renal Clearance Is Associated with Inadequate Antibiotic Pharmacokinetic/Pharmacodynamic Target in Asian ICU Population: A Prospective Observational Study. Infect. Drug Resist. 2019, 12, 2531–2541. [Google Scholar] [CrossRef] [PubMed]
- Keough, L.A.; Krauss, A.; Hudson, J.Q. Inadequate Antibiotic Dosing in Patients Receiving Sustained Low Efficiency Dialysis. Int. J. Clin. Pharm. 2018, 40, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Udy, A.A.; Roberts, J.A.; Boots, R.J.; Paterson, D.L.; Lipman, J. Augmented Renal Clearance: Implications for Antibacterial Dosing in the Critically Ill. Clin. Pharmacokinet. 2010, 49, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ehmann, L.; Zoller, M.; Minichmayr, I.K.; Scharf, C.; Huisinga, W.; Zander, J.; Kloft, C. Development of a Dosing Algorithm for Meropenem in Critically Ill Patients Based on a Population Pharmacokinetic/Pharmacodynamic Analysis. Int. J. Antimicrob. Agents 2019, 54, 309–317. [Google Scholar] [CrossRef]
- Baldwin, C.M.; Lyseng-Williamson, K.A.; Keam, S.J. Meropenem: A Review of Its Use in the Treatment of Serious Bacterial Infections. Drugs 2008, 68, 803–838. [Google Scholar] [CrossRef]
- Craig, W.A. Interrelationship between Pharmacokinetics and Pharmacodynamics in Determining Dosage Regimens for Broad-Spectrum Cephalosporins. Diagn. Microbiol. Infect. Dis. 1995, 22, 89–96. [Google Scholar] [CrossRef]
- Liebchen, U.; Paal, M.; Scharf, C.; Schroeder, I.; Grabein, B.; Zander, J.; Siebers, C.; Zoller, M. The ONTAI Study—A Survey on Antimicrobial Dosing and the Practice of Therapeutic Drug Monitoring in German Intensive Care Units. J. Crit. Care 2020, 60, 260–266. [Google Scholar] [CrossRef]
- German S2k Guideline Parenteral Antibiotics. Available online: https://www.awmf.org/uploads/tx_szleitlinien/S82-006l_S2k_Parenterale_Antibiotika_2018-1.pdf (accessed on 18 November 2020).
- Roberts, J.A.; Kumar, A.; Lipman, J. Right Dose, Right Now: Customized Drug Dosing in the Critically Ill. Crit. Care Med. 2017, 45, 331–336. [Google Scholar] [CrossRef]
- Charmillon, A.; Novy, E.; Agrinier, N.; Leone, M.; Kimmoun, A.; Levy, B.; Demoré, B.; Dellamonica, J.; Pulcini, C. The Antibioperf Study: A Nationwide Cross-Sectional Survey about Practices for β-Lactam Administration and Therapeutic Drug Monitoring among Critically Ill Patients in France. Clin. Microbiol. Infect. 2016, 22, 625–631. [Google Scholar] [CrossRef]
- Cockcroft, D.W.; Gault, M.H. Prediction of Creatinine Clearance from Serum Creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef]
- Sherwin, C.M.T.; Kiang, T.K.L.; Spigarelli, M.G.; Ensom, M.H.H. Fundamentals of Population Pharmacokinetic Modelling. Clin. Pharm. 2012, 18. [Google Scholar] [CrossRef]
- McBride, G. A Proposal of Strength-of-Agreement Criteria for Lins Concordance Correlation Coefficient; National Institute of Water & Atmospheric Research Ltd.: Hamilton, New Zealand, 2020. [Google Scholar]
- Mouton, J.W.; Dudley, M.N.; Cars, O.; Derendorf, H.; Drusano, G.L. Standardization of Pharmacokinetic/Pharmacodynamic (PK/PD) Terminology for Anti-Infective Drugs: An Update. J. Antimicrob. Chemother. 2005, 55, 601–607. [Google Scholar] [CrossRef]
- Neely, M.; Philippe, M.; Rushing, T.; Fu, X.; van Guilder, M.; Bayard, D.; Schumitzky, A.; Bleyzac, N.; Goutelle, S. Accurately Achieving Target Busulfan Exposure in Children and Adolescents with Very Limited Sampling and the Best Dose Software. Therapeutic Drug Monit. 2016, 38, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Heil, E.L.; Nicolau, D.P.; Farkas, A.; Roberts, J.A.; Thom, K.A. Pharmacodynamic Target Attainment for Cefepime, Meropenem, and Piperacillin-Tazobactam Using a Pharmacokinetic/Pharmacodynamic-Based Dosing Calculator in Critically Ill Patients. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef]
- Mould, D.; D’Haens, G.; Upton, R. Clinical Decision Support Tools: The Evolution of a Revolution. Clin. Pharmacol. Ther. 2016, 99, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Abdul-Aziz, M.H.; Lipman, J.; Mouton, J.W.; Vinks, A.A.; Felton, T.W.; Hope, W.W.; Farkas, A.; Neely, M.N.; Schentag, J.J.; et al. Individualised Antibiotic Dosing for Patients Who Are Critically Ill: Challenges and Potential Solutions. Lancet Infect. Dis. 2014, 14, 498–509. [Google Scholar] [CrossRef]
- Dhaese, S.A.M.; Farkas, A.; Colin, P.; Lipman, J.; Stove, V.; Verstraete, A.G.; Roberts, J.A.; De Waele, J.J. Population Pharmacokinetics and Evaluation of the Predictive Performance of Pharmacokinetic Models in Critically Ill Patients Receiving Continuous Infusion Meropenem: A Comparison of Eight Pharmacokinetic Models. J. Antimicrob. Chemother. 2019, 74, 432–441. [Google Scholar] [CrossRef]
- Heine, R.; Keizer, R.J.; Steeg, K.; Smolders, E.J.; Luin, M.; Derijks, H.J.; Jager, C.P.C.; Frenzel, T.; Brüggemann, R. Prospective Validation of a Model-informed Precision Dosing Tool for Vancomycin in Intensive Care Patients. Br. J. Clin. Pharmacol. 2020. [Google Scholar] [CrossRef]
- Mattioli, F.; Fucile, C.; Del Bono, V.; Marini, V.; Parisini, A.; Molin, A.; Zuccoli, M.L.; Milano, G.; Danesi, R.; Marchese, A.; et al. Population Pharmacokinetics and Probability of Target Attainment of Meropenem in Critically Ill Patients. Eur. J. Clin. Pharmacol. 2016, 72, 839–848. [Google Scholar] [CrossRef]
- Burger, R.; Guidi, M.; Calpini, V.; Lamoth, F.; Decosterd, L.; Robatel, C.; Buclin, T.; Csajka, C.; Marchetti, O. Effect of Renal Clearance and Continuous Renal Replacement Therapy on Appropriateness of Recommended Meropenem Dosing Regimens in Critically Ill Patients with Susceptible Life-Threatening Infections. J. Antimicrob. Chemother. 2018, 73, 3413–3422. [Google Scholar] [CrossRef] [PubMed]
- Singbartl, K.; Kellum, J.A. AKI in the ICU: Definition, Epidemiology, Risk Stratification, and Outcomes. Kidney Int. 2012, 81, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.S.K.; Chung, K.C.; Gill, M.A. Pharmacokinetics of Meropenem in Animals, Healthy Volunteers, and Patients. Clin. Infect. Dis. 1997, 24, S249–S255. [Google Scholar] [CrossRef] [PubMed]
- Paal, M.; Zoller, M.; Schuster, C.; Vogeser, M.; Schütze, G. Simultaneous Quantification of Cefepime, Meropenem, Ciprofloxacin, Moxifloxacin, Linezolid and Piperacillin in Human Serum Using an Isotope-Dilution HPLC–MS/MS Method. J. Pharm. Biomed. Anal. 2018, 152, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.I. A Concordance Correlation Coefficient to Evaluate Reproducibility. Biometrics 1989, 45, 255–268. [Google Scholar] [CrossRef]
| Patient Characteristic | |
|---|---|
| Categorical | n (%) |
| No. of patients No. of male patients No. of meropenem samples | 155 101 (65.2) 891 |
| No. of meropenem samples collected during extracorporeal membrane oxygenation | 64 (7.18) |
| Continuous (unit) | Median (5th–95th percentile) |
| Meropenem concentration (mg/L) | 9.05 (1.09–36.5) |
| Age (years) | 57.0 (33.7–79.0) |
| Weight (kg) | 73.0 (50.0–97.3) |
| Creatinine clearance # (mL/min) | 86.4 (35.4–161) |
| Serum albumin concentration (g/dL) | 2.5 (2.3–3.2) |
| MIC (mg/L) | Lin’s Concordance Correlation Coefficient (95% CI) | |
|---|---|---|
| All Patients | CLCRCG > 50 mL/min | |
| 0.125 | 0.791 (0.746–0.830) * | 0.999 (0.998–0.999) **** |
| 0.25 | 0.845 (0.811–0.872) * | 0.997 (0.996–0.998) **** |
| 0.5 | 0.894 (0.869–0.914) * | 0.992 (0.991–0.994) **** |
| 1 | 0.921 (0.899–0.938) * | 0.930 (0.910–0.946) ** |
| 2 | 0.957 (0.942–0.967) ** | 0.919 (0.893–0.938) * |
| 4 | 0.979 (0.972–0.984) *** | 0.954 (0.938–0.967) ** |
| 8 | 0.857 (0.834–0.877) * | 0.978 (0.970–0.984) *** |
| 16 | 0.087 (0.077–0.097) * | 0.945 (0.925–0.960) ** |
| 0.125–16 | 0.983 (0.981–0.984) *** | 0.990 (0.988–0.991) *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liebchen, U.; Klose, M.; Paal, M.; Vogeser, M.; Zoller, M.; Schroeder, I.; Schmitt, L.; Huisinga, W.; Michelet, R.; Zander, J.; et al. Evaluation of the MeroRisk Calculator, A User-Friendly Tool to Predict the Risk of Meropenem Target Non-Attainment in Critically Ill Patients. Antibiotics 2021, 10, 468. https://doi.org/10.3390/antibiotics10040468
Liebchen U, Klose M, Paal M, Vogeser M, Zoller M, Schroeder I, Schmitt L, Huisinga W, Michelet R, Zander J, et al. Evaluation of the MeroRisk Calculator, A User-Friendly Tool to Predict the Risk of Meropenem Target Non-Attainment in Critically Ill Patients. Antibiotics. 2021; 10(4):468. https://doi.org/10.3390/antibiotics10040468
Chicago/Turabian StyleLiebchen, Uwe, Marian Klose, Michael Paal, Michael Vogeser, Michael Zoller, Ines Schroeder, Lisa Schmitt, Wilhelm Huisinga, Robin Michelet, Johannes Zander, and et al. 2021. "Evaluation of the MeroRisk Calculator, A User-Friendly Tool to Predict the Risk of Meropenem Target Non-Attainment in Critically Ill Patients" Antibiotics 10, no. 4: 468. https://doi.org/10.3390/antibiotics10040468
APA StyleLiebchen, U., Klose, M., Paal, M., Vogeser, M., Zoller, M., Schroeder, I., Schmitt, L., Huisinga, W., Michelet, R., Zander, J., Scharf, C., Weinelt, F. A., & Kloft, C. (2021). Evaluation of the MeroRisk Calculator, A User-Friendly Tool to Predict the Risk of Meropenem Target Non-Attainment in Critically Ill Patients. Antibiotics, 10(4), 468. https://doi.org/10.3390/antibiotics10040468







