Molecular Epidemiology of Extensively Drug-Resistant mcr Encoded Colistin-Resistant Bacterial Strains Co-Expressing Multifarious β-Lactamases
Abstract
1. Introduction
2. Results
2.1. Demographic Characteristics of Patients Infected with Col-R Strains
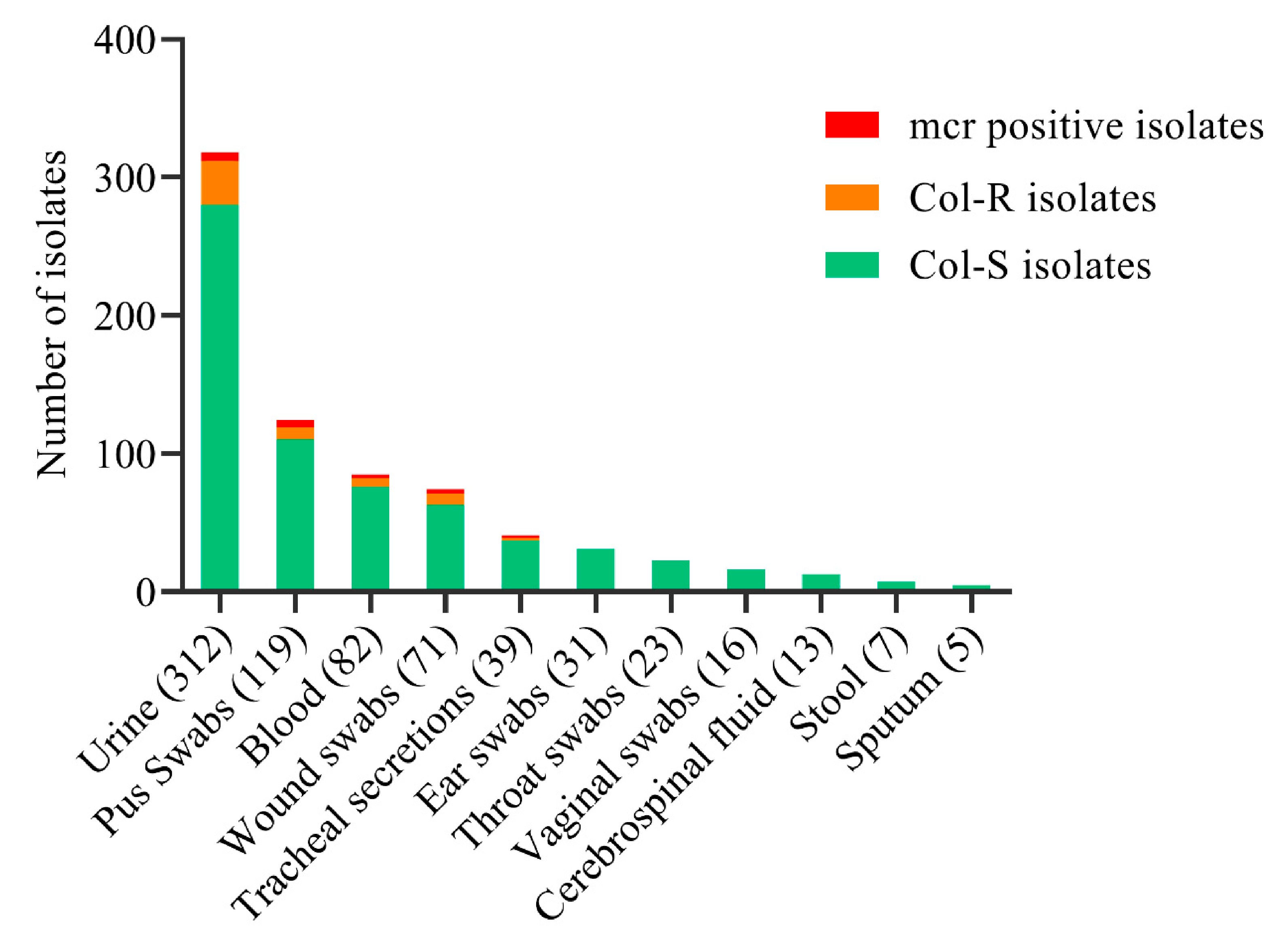
2.2. Distribution of Col-R and Col-S Bacterial Strains
2.3. Molecular Detection of mcr and β-Lactam Drug-Resistant Gene Variants
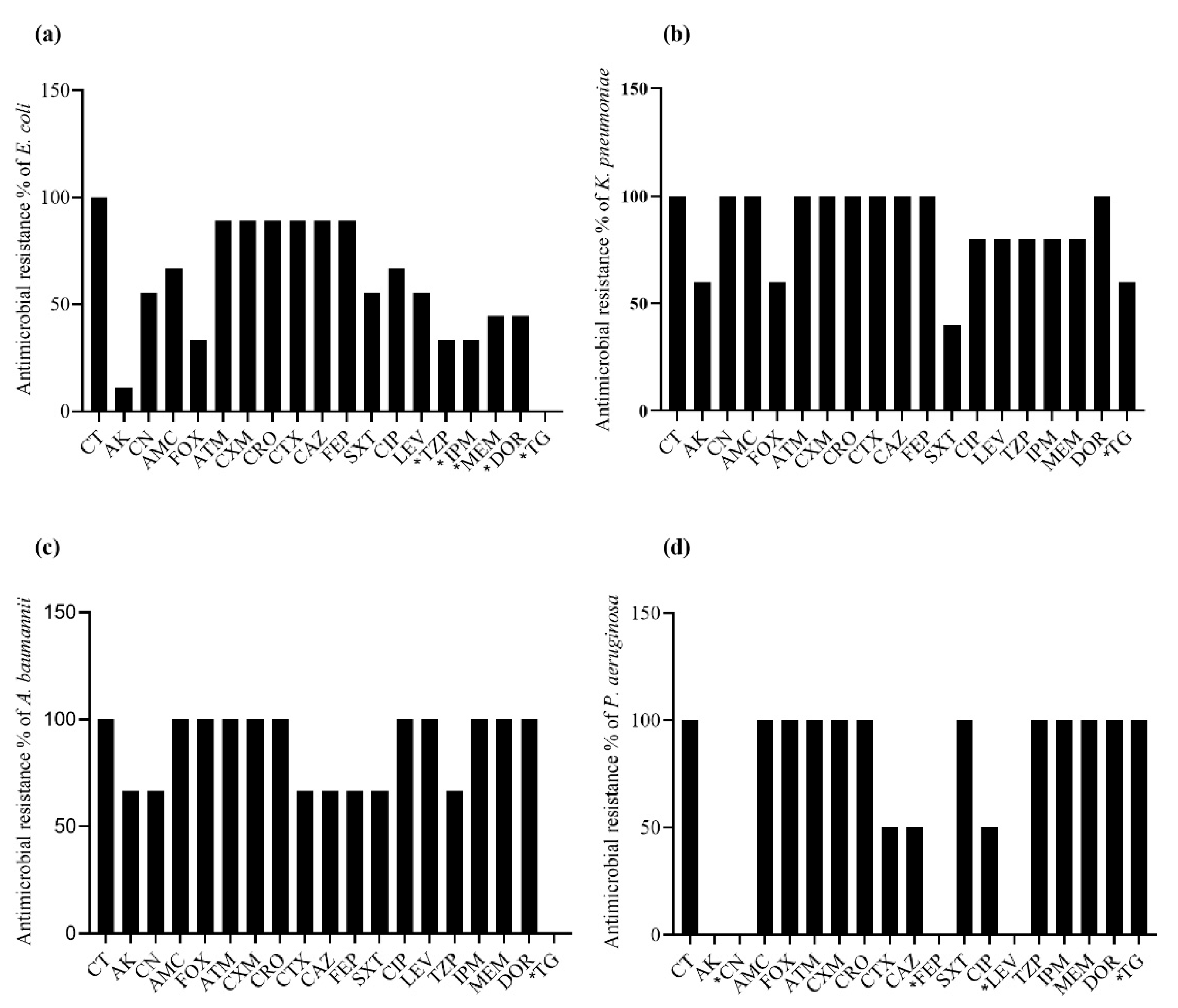
2.4. Antibacterial Resistance Spectrum in mcr-Positive Bacteria
2.5. MIC50 and MIC90 in mcr Gene-Harboring Bacterial Strains
3. Discussion
4. Materials and Methods
4.1. Study Design and Ethics Approval
4.2. Specimen Collection and Processing
4.3. Bacterial Growth and Characterization
4.4. Resistance Profile and MIC Determination
4.5. Screening of ESBLs, AmpC, and Carbapenemases
4.6. Molecular Characterization of mcr Genes
4.7. Molecular Characterization ESBLs, AmpC, Carbapenemases, and Integrons
4.8. Quality Control (QC) Analysis
4.9. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.Y.; Wang, Y.; Walsh, T.R.; Yi, L.X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- MacNair, C.R.; Stokes, J.M.; Carfrae, L.A.; Fiebig-Comyn, A.A.; Coombes, B.K.; Mulvey, M.R.; Brown, E.D. Overcoming mcr-1 mediated colistin resistance with colistin in combination with other antibiotics. Nat. Commun. 2018, 9, 458. [Google Scholar] [CrossRef] [PubMed]
- Temkin, E.; Adler, A.; Lerner, A.; Carmeli, Y. Carbapenem-resistant Enterobacteriaceae: Biology, epidemiology, and management. Ann. N. Y. Acad. Sci. 2014, 1323, 22–42. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Johnson, A.P. Transferable resistance to colistin: A new but old threat. J. Antimicrob. Chemother. 2016, 71, 2066–2070. [Google Scholar] [CrossRef]
- Olaitan, A.O.; Morand, S.; Rolain, J.M. Mechanisms of polymyxin resistance: Acquired and intrinsic resistance in bacteria. Front. Microbiol. 2014, 5, 643. [Google Scholar] [CrossRef]
- Ling, Z.; Yin, W.; Shen, Z.; Wang, Y.; Shen, J.; Walsh, T.R. Epidemiology of mobile colistin resistance genes mcr-1 to mcr-9. J. Antimicrob. Chemother. 2020. [Google Scholar] [CrossRef]
- Malhotra-Kumar, S.; Xavier, B.B.; Das, A.J.; Lammens, C.; Butaye, P.; Goossens, H. Colistin resistance gene mcr-1 harboured on a multidrug resistant plasmid. Lancet Infect. Dis. 2016, 16, 283–284. [Google Scholar] [CrossRef]
- Javed, H.; Saleem, S.; Zafar, A.; Ghafoor, A.; Shahzad, A.B.; Ejaz, H.; Junaid, K.; Jahan, S. Emergence of plasmid-mediated mcr genes from Gram-negative bacteria at the human-animal interface. Gut Pathog. 2020, 12, 54. [Google Scholar] [CrossRef]
- Ejaz, H. Dissemination of SHV, TEM and CTX-M Genotypes in Pseudomonas aeruginosa: A Pre-eminent Reason for Therapeutic Failure in Pediatrics. Ann. Clin. Lab. Sci. 2020, 50, 797–805. [Google Scholar]
- Manchanda, V.; Singh, N.P. Occurrence and detection of AmpC beta-lactamases among Gram-negative clinical isolates using a modified three-dimensional test at Guru Tegh Bahadur Hospital, Delhi, India. J. Antimicrob. Chemother. 2003, 51, 415–418. [Google Scholar] [CrossRef]
- van Duin, D.; Doi, Y. Outbreak of Colistin-Resistant, Carbapenemase-Producing Klebsiella pneumoniae: Are We at the End of the Road? J. Clin. Microbiol. 2015, 53, 3116–3117. [Google Scholar] [CrossRef]
- Li, X.; Mu, X.; Zhang, P.; Zhao, D.; Ji, J.; Quan, J.; Zhu, Y.; Yu, Y. Detection and characterization of a clinical Escherichia coli ST3204 strain coproducing NDM-16 and MCR-1. Infect. Drug Resist. 2018, 11, 1189–1195. [Google Scholar] [CrossRef]
- Ejaz, H.; Alzahrani, B.; Hamad, M.F.S.; Abosalif, K.O.A.; Junaid, K.; Abdalla, A.E.; Elamir, M.Y.M.; Aljaber, N.J.; Hamam, S.S.M.; Younas, S. Molecular Analysis of the Antibiotic Resistant NDM-1 Gene in Clinical Isolates of Enterobacteriaceae. Clin. Lab. 2020, 66. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, G.B.; Zhang, R.; Shen, Y.; Tyrrell, J.M.; Huang, X.; Zhou, H.; Lei, L.; Li, H.Y.; Doi, Y.; et al. Prevalence, risk factors, outcomes, and molecular epidemiology of mcr-1-positive Enterobacteriaceae in patients and healthy adults from China: An epidemiological and clinical study. Lancet Infect. Dis. 2017, 17, 390–399. [Google Scholar] [CrossRef]
- Saavedra, S.Y.; Diaz, L.; Wiesner, M.; Correa, A.; Arévalo, S.A.; Reyes, J.; Hidalgo, A.M.; de la Cadena, E.; Perenguez, M.; Montaño, L.A.; et al. Genomic and Molecular Characterization of Clinical Isolates of Enterobacteriaceae Harboring mcr-1 in Colombia, 2002 to 2016. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Bayram, Y.; Parlak, M.; Aypak, C.; Bayram, I. Three-year review of bacteriological profile and antibiogram of burn wound isolates in Van, Turkey. Int. J. Med. Sci. 2013, 10, 19–23. [Google Scholar] [CrossRef]
- Gill, M.M.; Usman, J.; Kaleem, F.; Hassan, A.; Khalid, A.; Anjum, R.; Fahim, Q. Frequency and antibiogram of multi-drug resistant Pseudomonas aeruginosa. J. Coll. Physicians Surg. Pak. 2011, 21, 531–534. [Google Scholar]
- Hameed, F.; Khan, M.A.; Muhammad, H.; Sarwar, T.; Bilal, H.; Rehman, T.U. Plasmid-mediated mcr-1 gene in Acinetobacter baumannii and Pseudomonas aeruginosa: First report from Pakistan. Rev. Soc. Bras. Med. Trop. 2019, 52, e20190237. [Google Scholar] [CrossRef]
- Aghapour, Z.; Hasani, A.; Aghazadeh, M.; Rezaee, M.A.; Ganbarov, K.; Pourlak, T.; Gholizadeh, P.; Asgharzadeh, M.; Tanomand, A.; Kafil, H. Genes involved in colistin resistance of gram-negative isolates in the northwest of Iran. Gene Rep. 2019, 14, 81–86. [Google Scholar] [CrossRef]
- Wise, M.G.; Estabrook, M.A.; Sahm, D.F.; Stone, G.G.; Kazmierczak, K.M. Prevalence of mcr-type genes among colistin-resistant Enterobacteriaceae collected in 2014-2016 as part of the INFORM global surveillance program. PLoS ONE 2018, 13, e0195281. [Google Scholar] [CrossRef]
- Elbediwi, M.; Li, Y.; Paudyal, N.; Pan, H.; Li, X.; Xie, S.; Rajkovic, A.; Feng, Y.; Fang, W.; Rankin, S.C.; et al. Global Burden of Colistin-Resistant Bacteria: Mobilized Colistin Resistance Genes Study (1980–2018). Microorganisms 2019, 7, 461. [Google Scholar] [CrossRef]
- Wang, R.; van Dorp, L.; Shaw, L.P.; Bradley, P.; Wang, Q.; Wang, X.; Jin, L.; Zhang, Q.; Liu, Y.; Rieux, A.; et al. The global distribution and spread of the mobilized colistin resistance gene mcr-1. Nat. Commun. 2018, 9, 1179. [Google Scholar] [CrossRef]
- Yoon, E.J.; Hong, J.S.; Yang, J.W.; Lee, K.J.; Lee, H.; Jeong, S.H. Detection of mcr-1 Plasmids in Enterobacteriaceae Isolates from Human Specimens: Comparison With Those in Escherichia coli Isolates From Livestock in Korea. Ann. Lab. Med. 2018, 38, 555–562. [Google Scholar] [CrossRef]
- Faccone, D.; Rapoport, M.; Albornoz, E.; Celaya, F.; De Mendieta, J.; De Belder, D.; Lucero, C.; Gomez, S.; Danze, D.; Pasteran, F.; et al. Plasmidic resistance to colistin mediated by mcr-1 gene in Escherichia coli clinical isolates in Argentina: A retrospective study, 2012–2018. Rev. Panam. Salud Publica 2020, 44, e55. [Google Scholar] [CrossRef]
- Moosavian, M.; Emam, N. The first report of emerging mobilized colistin-resistance (mcr) genes and ERIC-PCR typing in Escherichia coli and Klebsiella pneumoniae clinical isolates in southwest Iran. Infect. Drug Resist. 2019, 12, 1001–1010. [Google Scholar] [CrossRef]
- Giske, C.G. Contemporary resistance trends and mechanisms for the old antibiotics colistin, temocillin, fosfomycin, mecillinam and nitrofurantoin. Clin. Microbiol. Infect. 2015, 21, 899–905. [Google Scholar] [CrossRef]
- Baraka, A.; Traglia, G.M.; Montaña, S.; Tolmasky, M.E.; Ramirez, M.S. An Acinetobacter non-baumannii Population Study: Antimicrobial Resistance Genes (ARGs). Antibiotics 2020, 10, 16. [Google Scholar] [CrossRef]
- Lin, D.L.; Traglia, G.M.; Baker, R.; Sherratt, D.J.; Ramirez, M.S.; Tolmasky, M.E. Functional Analysis of the Acinetobacter baumannii XerC and XerD Site-Specific Recombinases: Potential Role in Dissemination of Resistance Genes. Antibiotics 2020, 9, 405. [Google Scholar] [CrossRef]
- Thirapanmethee, K.; Srisiri, A.N.T.; Houngsaitong, J.; Montakantikul, P.; Khuntayaporn, P.; Chomnawang, M.T. Prevalence of OXA-Type β-Lactamase Genes among Carbapenem-Resistant Acinetobacter baumannii Clinical Isolates in Thailand. Antibiotics 2020, 9, 864. [Google Scholar] [CrossRef]
- El-Mokhtar, M.A.; Daef, E.; Mohamed Hussein, A.A.R.; Hashem, M.K.; Hassan, H.M. Emergence of Nosocomial Pneumonia Caused by Colistin-Resistant Escherichia coli in Patients Admitted to Chest Intensive Care Unit. Antibiotics 2021, 10, 226. [Google Scholar] [CrossRef]
- Reeves, C.M.; Magallon, J.; Rocha, K.; Tran, T.; Phan, K.; Vu, P.; Yi, Y.; Oakley-Havens, C.L.; Cedano, J.; Jimenez, V.; et al. Aminoglycoside 6’-N-acetyltransferase Type Ib [AAC(6′)-Ib]-Mediated Aminoglycoside Resistance: Phenotypic Conversion to Susceptibility by Silver Ions. Antibiotics 2020, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Dong, H.; Xu, H.; Lv, J.; Zhang, J.; Jiang, X.; Du, Y.; Xiao, Y.; Li, L. Coexistence of MCR-1 and NDM-1 in Clinical Escherichia coli Isolates. Clin. Infect. Dis. 2016, 63, 1393–1395. [Google Scholar] [CrossRef] [PubMed]
- Mediavilla, J.R.; Patrawalla, A.; Chen, L.; Chavda, K.D.; Mathema, B.; Vinnard, C.; Dever, L.L.; Kreiswirth, B.N. Colistin- and Carbapenem-Resistant Escherichia coli Harboring mcr-1 and blaNDM-5, Causing a Complicated Urinary Tract Infection in a Patient from the United States. mBio 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Gillings, M.R. Integrons: Past, present, and future. Microbiol. Mol. Biol. Rev. 2014, 78, 257–277. [Google Scholar] [CrossRef]
- Association, W.M. WMA Declaration of Helsinki–Ethical Principles for Medical Research Involving Human Subjects. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed on 2 March 2021).
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; Clinical and Laboratory Standard Institute (CLSI): Wayne, PA, USA, 2020; Volume CLSI, Supplement M100. [Google Scholar]
- Amin, H.; Zafar, A.; Ejaz, H.; Jameel, N.U. Phenotypic characterization of ESBL producing Enterobacter cloacae among children. Pak. J. Med. Sci. 2013, 29, 144–147. [Google Scholar] [CrossRef]
- Ejaz, H.; Younas, S.; Abosalif, K.O.A.; Junaid, K.; Alzahrani, B.; Alsrhani, A.; Abdalla, A.E.; Ullah, M.I.; Qamar, M.U.; Hamam, S.S.M. Molecular analysis of blaSHV, blaTEM, and blaCTX-M in extended-spectrum β-lactamase producing Enterobacteriaceae recovered from fecal specimens of animals. PLoS ONE 2021, 16, e0245126. [Google Scholar] [CrossRef]
- Younas, S.; Ejaz, H.; Zafar, A.; Ejaz, A.; Saleem, R.; Javed, H. AmpC beta-lactamases in Klebsiella pneumoniae: An emerging threat to the paediatric patients. J. Pak. Med. Assoc. 2018, 68, 893–897. [Google Scholar]
- Javed, H.; Ejaz, H.; Zafar, A.; Rathore, A.W. Metallo-beta-lactamase producing Escherichia coli and Klebsiella pneumoniae: A rising threat for hospitalized children. J. Pak. Med. Assoc. 2016, 66, 1068–1072. [Google Scholar]
- Qamar, M.U.; Ejaz, H.; Walsh, T.R.; Shah, A.A.; Al Farraj, D.A.; Alkufeidy, R.M.; Alkubaisi, N.A.; Saleem, S.; Jahan, S. Clonal relatedness and plasmid profiling of extensively drug-resistant New Delhi metallo-β-lactamase-producing Klebsiella pneumoniae clinical isolates. Future Microbiol. 2021. [Google Scholar] [CrossRef]
- Rebelo, A.R.; Bortolaia, V.; Kjeldgaard, J.S.; Pedersen, S.K.; Leekitcharoenphon, P.; Hansen, I.M.; Guerra, B.; Malorny, B.; Borowiak, M.; Hammerl, J.A.; et al. Multiplex PCR for detection of plasmid-mediated colistin resistance determinants, mcr-1, mcr-2, mcr-3, mcr-4 and mcr-5 for surveillance purposes. Euro Surveill 2018, 23. [Google Scholar] [CrossRef]
- Lv, D.; Duan, R.; Fan, R.; Mu, H.; Liang, J.; Xiao, M.; He, Z.; Qin, S.; Yang, J.; Jing, H.; et al. bla(NDM) and mcr-1 to mcr-5 Gene Distribution Characteristics in Gut Specimens from Different Regions of China. Antibiotics 2021, 10, 233. [Google Scholar] [CrossRef]
- Mohd Khari, F.I.; Karunakaran, R.; Rosli, R.; Tee Tay, S. Genotypic and Phenotypic Detection of AmpC β-lactamases in Enterobacter spp. Isolated from a Teaching Hospital in Malaysia. PLoS ONE 2016, 11, e0150643. [Google Scholar] [CrossRef]
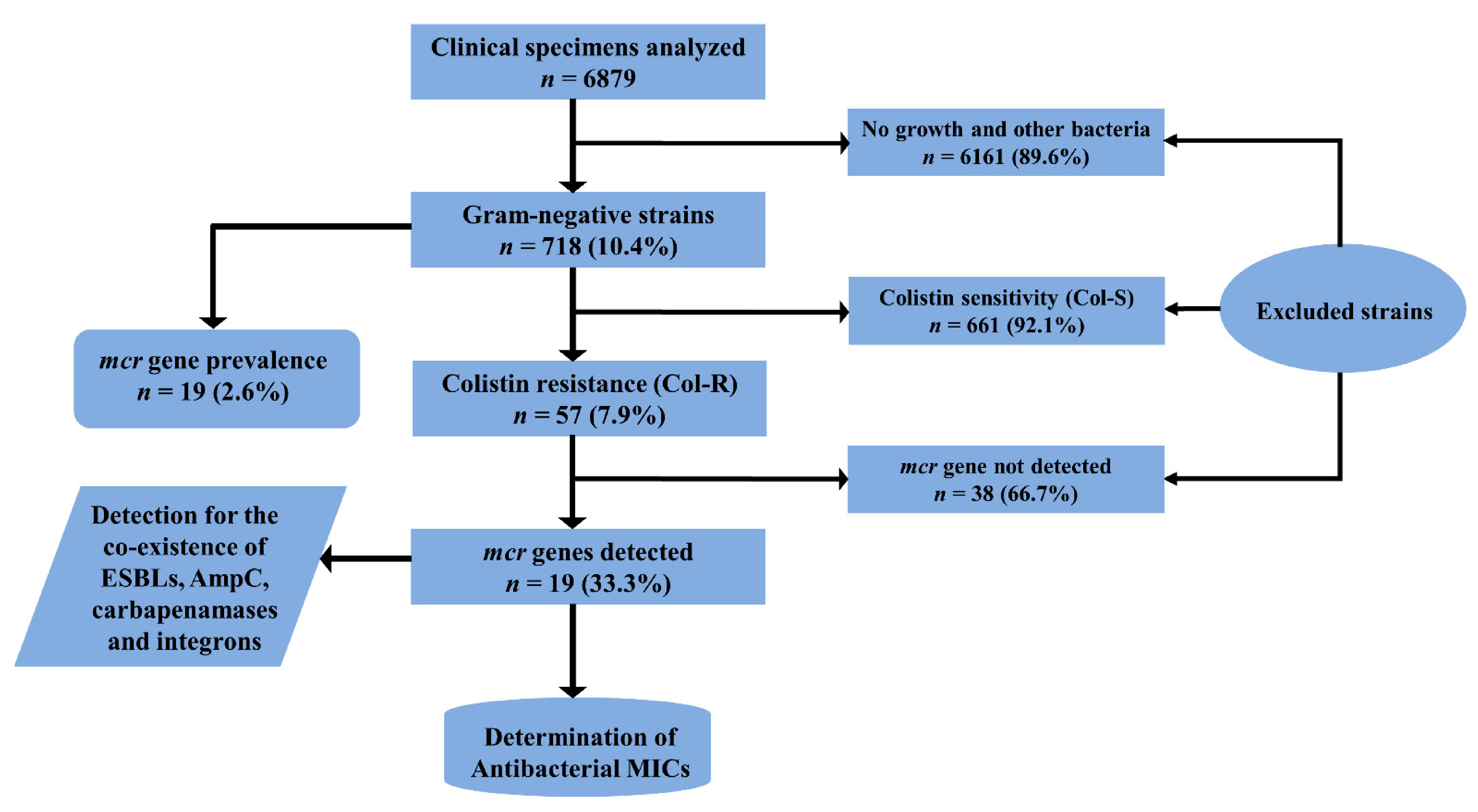
| Characteristic | Col-R (n = 57) n (%) | mcr Detected (n = 19) n (%) | p-Value |
|---|---|---|---|
| Sex | |||
| Male | 19 (33.3) | 7 (36.8) | 0.78 |
| Female | 38 (66.7) | 12 (63.2) | |
| Wards | |||
| Medical ward | 18 (31.6) | 5 (26.3) | 0.16 |
| ICU | 7 (12.3) | 5 (26.3) | 0.01 |
| Surgery | 6 (10.5) | 4 (21.1) | 0.01 |
| Nephrology | 16 (28.1) | 3 (15.8) | 0.74 |
| OPD | 9 (15.8) | 1 (5.3) | 0.71 |
| Orthopedic | 1 (1.8) | 1 (5.3) | 0.12 |
| Sources | |||
| Urine | 32 (56.1) | 6 (31.6) | 0.55 |
| Pus | 9 (15.8) | 5 (26.3) | 0.01 |
| Wound swab | 10 (17.5) | 4 (21.1) | 0.06 |
| Blood | 4 (7) | 2 (10.5) | 0.38 |
| Tracheal secretions | 2 (3.5) | 2 (10.5) | 0.03 |
| Organism | mcr Positive n (%) | mcr Negative n (%) | p-Value |
|---|---|---|---|
| Escherichia coli (n = 17) | 9 (52.9) | 8 (47.1) | 0.43 |
| Klebsiella pneumoniae (n = 9) | 5 (55.6) | 4 (44.4) | 0.78 |
| Acinetobacter baumannii (n = 4) | 3 (75) | 1 (25) | 0.49 |
| Pseudomonas aeruginosa (n = 2) | 2 (100) | 0 (0) | - |
| Proteus mirabilis (n = 25) | 0 (0) | 25 (100) | - |
| Strain | Source | Ward | Sex | Age (years) | Organism | Detected Genes |
|---|---|---|---|---|---|---|
| mcr ST-1 | Urine | Medical | Male | 48 | E. coli | CTX-M-1, mcr-1 |
| mcr ST-2 | Pus | OPD | Female | 30 | K. pneumoniae | CTX-M-1, NDM-1, mcr-1, Int-I |
| mcr ST-3 | Pus | Nephrology | Female | 60 | E. coli | CTX-M-15, mcr-1, Int-I |
| mcr ST-4 | Urine | ICU | Female | 53 | E. coli | CTX-M-1, mcr-1, Int-I |
| mcr ST-5 | Pus | Surgery | Female | 49 | K. pneumoniae | CTX-M-15, IMP, mcr-1, Int-I |
| mcr ST-6 | Urine | Medical | Male | 45 | E. coli | CMY-2, mcr-1, Int-I |
| mcr ST-7 | Urine | Nephrology | Female | 53 | E. coli | VIM, mcr-1, Int-I |
| mcr ST-8 | Pus | Orthopedic | Female | 30 | A. baumannii | NDM-5, mcr-1, Int-I |
| mcr ST-9 | Wound | Surgery | Male | 25 | P. aeruginosa | SHV-28, OXA-51, mcr-1, Int-I |
| mcr ST-10 | Tracheal secretions | ICU | Male | 71 | K. pneumoniae | CTX-M-1, NDM-1, mcr-2, Int-2 |
| mcr ST-11 | Wound | ICU | Female | 65 | E. coli | CTX-M-11, mcr-1, Int-I |
| mcr ST-12 | Wound | ICU | Female | 46 | A. baumannii | CTX-M-1, OXA-48, mcr-1, Int-I |
| mcr ST-13 | Blood | Nephrology | Male | 43 | E. coli | CMY-2, mcr-1 |
| mcr ST-14 | Blood | Medical | Female | 20 | K. pneumoniae | CTX-M-10, mcr-1, Int-I |
| mcr ST-15 | Urine | Medical | Female | 46 | E. coli | TEM-52, CTX-M-15, mcr-1, Int-I |
| mcr ST-16 | Wound | Surgery | Male | 43 | K. pneumoniae | CTX-M-1, mcr-1, Int-I |
| mcr ST-17 | Tracheal secretions | ICU | Female | 54 | A. baumannii | CTX-M-1, mcr-1, Int-I |
| mcr ST-18 | Urine | Medical | Male | 36 | P. aeruginosa | CTX-M-1, CMY-2, NDM-5, mcr-1, Int-2 |
| mcr ST-19 | Pus | Surgery | Female | 52 | E. coli | SHV-12, mcr-1, Int-I |
| Antibiotic | E. coli (n = 9) | K. pneumoniae (n = 5) | A. baumannii (n = 3) | P. aeruginosa (n = 2) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Breakpoints | MIC50 | MIC90 | Breakpoints | MIC50 | MIC90 | Breakpoints | MIC50 | MIC90 | Breakpoints | MIC50 | MIC90 | |
| Colistin | ≥4 | 12 | 24 | ≥4 | 12 | 32 | ≥4 | 8 | 12 | ≥4 | 32 | 64 |
| Amikacin | ≥64 | 2 | 64 | ≥64 | 64 | 64 | ≥64 | 128 | 128 | ≥64 | 2 | 2 |
| Gentamicin | ≥16 | 16 | 32 | ≥16 | 32 | 32 | ≥16 | 32 | 32 | ≥16 | 2 | 2 |
| Co-amoxiclav | ≥32/16 | 32/16 | 64/32 | ≥32/16 | 32/16 | 64/32 | ≥32/16 | ≥128/64 | ≥128/64 | ≥32/16 | ≥128/64 | ≥128/64 |
| Cefoxitin | ≥32 | 4 | ≥128 | ≥32 | 64 | 64 | ≥32 | ≥128 | ≥128 | ≥32 | ≥128 | ≥128 |
| Aztreonam | ≥16 | 64 | ≥128 | ≥16 | ≥128 | ≥128 | ≥32 | ≥128 | ≥128 | ≥32 | ≥128 | ≥128 |
| Cefuroxime | ≥32 | ≥128 | ≥128 | ≥32 | ≥128 | ≥128 | ≥32 | ≥128 | ≥128 | ≥32 | ≥128 | ≥128 |
| Ceftriaxone | ≥4 | 64 | ≥128 | ≥4 | ≥128 | ≥128 | ≥64 | ≥128 | ≥128 | ≥64 | ≥128 | ≥128 |
| Cefotaxime | ≥4 | 64 | ≥128 | ≥4 | ≥128 | ≥128 | ≥64 | ≥128 | ≥128 | ≥64 | ≥128 | ≥128 |
| Ceftazidime | ≥16 | 64 | ≥128 | ≥16 | ≥128 | ≥128 | ≥32 | ≥128 | ≥128 | ≥32 | 2 | 32 |
| Cefepime | ≥16 | 32 | 64 | ≥16 | ≥128 | ≥128 | ≥32 | ≥128 | ≥128 | ≥32 | 2 | 8 |
| Co-trimoxazole | ≥4/76 | 4/76 | 8/152 | ≥4/76 | 2/38 | 4/76 | ≥4/76 | 4/76 | 16/304 | ≥4/76 | 32/608 | 64/1216 |
| Ciprofloxacin | ≥1 | 16 | ≥64 | ≥1 | 32 | 32 | ≥4 | ≥64 | ≥64 | ≥2 | 0.25 | 4 |
| Levofloxacin | ≥2 | 8 | 32 | ≥2 | 32 | 32 | ≥8 | ≥64 | ≥64 | ≥4 | 0.5 | 0.5 |
| Piperacillin-tazobactam | ≥128/4 | 8/4 | 512/4 | ≥128/4 | 128/4 | 256/4 | ≥128/4 | 256/4 | 512/4 | ≥128/4 | 256/4 | 512/4 |
| Imipenem | ≥4 | 1 | 16 | ≥4 | 64 | 64 | ≥8 | ≥128 | ≥128 | ≥8 | 64 | ≥128 |
| Meropenem | ≥4 | 1 | 32 | ≥4 | 64 | 64 | ≥8 | ≥128 | ≥128 | ≥8 | ≥128 | ≥128 |
| Doripenem | ≥4 | 1 | 32 | ≥4 | 64 | 64 | ≥8 | ≥128 | ≥128 | ≥8 | ≥128 | ≥128 |
| Tigecycline | ≥2 | 0.5 | 1 | ≥2 | 8 | 16 | ≥8 | 1 | 1 | ≥8 | ≥128 | ≥128 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ejaz, H.; Younas, S.; Qamar, M.U.; Junaid, K.; Abdalla, A.E.; Abosalif, K.O.A.; Alameen, A.A.M.; Elamir, M.Y.M.; Ahmad, N.; Hamam, S.S.M.; et al. Molecular Epidemiology of Extensively Drug-Resistant mcr Encoded Colistin-Resistant Bacterial Strains Co-Expressing Multifarious β-Lactamases. Antibiotics 2021, 10, 467. https://doi.org/10.3390/antibiotics10040467
Ejaz H, Younas S, Qamar MU, Junaid K, Abdalla AE, Abosalif KOA, Alameen AAM, Elamir MYM, Ahmad N, Hamam SSM, et al. Molecular Epidemiology of Extensively Drug-Resistant mcr Encoded Colistin-Resistant Bacterial Strains Co-Expressing Multifarious β-Lactamases. Antibiotics. 2021; 10(4):467. https://doi.org/10.3390/antibiotics10040467
Chicago/Turabian StyleEjaz, Hasan, Sonia Younas, Muhammad Usman Qamar, Kashaf Junaid, Abualgasim Elgaili Abdalla, Khalid Omer Abdalla Abosalif, Ayman Ali Mohammed Alameen, Mohammed Yagoub Mohammed Elamir, Naveed Ahmad, Sanaa Samir Mohamed Hamam, and et al. 2021. "Molecular Epidemiology of Extensively Drug-Resistant mcr Encoded Colistin-Resistant Bacterial Strains Co-Expressing Multifarious β-Lactamases" Antibiotics 10, no. 4: 467. https://doi.org/10.3390/antibiotics10040467
APA StyleEjaz, H., Younas, S., Qamar, M. U., Junaid, K., Abdalla, A. E., Abosalif, K. O. A., Alameen, A. A. M., Elamir, M. Y. M., Ahmad, N., Hamam, S. S. M., Salem, E. H. M., & Bukhari, S. N. A. (2021). Molecular Epidemiology of Extensively Drug-Resistant mcr Encoded Colistin-Resistant Bacterial Strains Co-Expressing Multifarious β-Lactamases. Antibiotics, 10(4), 467. https://doi.org/10.3390/antibiotics10040467











