Performance Evaluation of the Newly Developed In Vitro Rapid Diagnostic Test for Detecting OXA-48-Like, KPC-, NDM-, VIM- and IMP-Type Carbapenemases: The RESIST-5 O.K.N.V.I. Multiplex Lateral Flow Assay
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Bacterial Identification
2.3. Conventional PCR Method
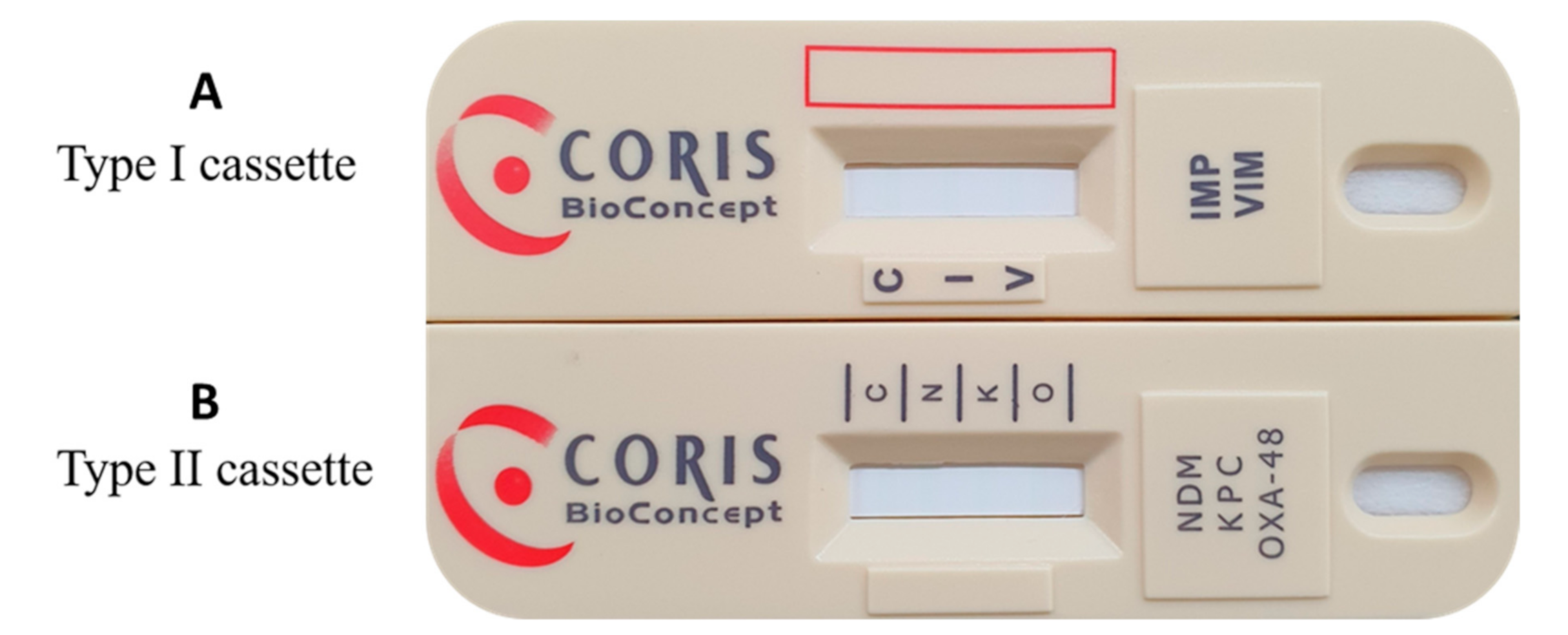
2.4. RESIST-5 O.K.N.V.I. Assay
3. Results
3.1. Identification of Single Carbapenemase Producers
3.2. Identification of Multiple Carbapenemase Producers
3.3. Identification of Non-Targeted Carbapenemase Producers and Non-CP CRE Isolates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bonomo, R.A.; Burd, E.M.; Conly, J.; Limbago, B.M.; Poirel, L.; Segre, J.A.; Westblade, L.F. Carbapenemase-Producing Organisms: A Global Scourge. Clin. Infect. Dis. 2018, 66, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Logan, L.K.; Weinstein, R.A. The Epidemiology of Carbapenem-Resistant Enterobacteriaceae: The Impact and Evolution of a Global Menace. J. Infect. Dis. 2017, 215, S28–S36. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.S.; Yoon, E.J.; Lee, H.; Jeong, S.H.; Lee, K. Clonal Dissemination of Pseudomonas aeruginosa Sequence Type 235 Isolates Carrying blaIMP-6 and Emergence of blaGES-24 and blaIMP-10 on Novel Genomic Islands PAGI-15 and -16 in South Korea. Antimicrob. Agents. Chemother. 2016, 60, 7216–7223. [Google Scholar] [CrossRef] [PubMed]
- van Duin, D.; Doi, Y. The global epidemiology of carbapenemase-producing Enterobacteriaceae. Virulence 2017, 8, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Gniadek, T.J.; Carroll, K.C.; Simner, P.J. Carbapenem-Resistant Non-Glucose-Fermenting Gram-Negative Bacilli: The Missing Piece to the Puzzle. J. Clin. Microbiol. 2016, 54, 1700–1710. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Garg, J.; Upadhyay, G.C.; Agarwal, A.; Bhattacharjee, A. Evaluation of the Rapidec Carba NP Test Kit for Detection of Carbapenemase-Producing Gram-Negative Bacteria. Antimicrob. Agents. Chemother. 2015, 59, 7870–7872. [Google Scholar] [CrossRef] [PubMed]
- Yoon, E.J.; Yang, J.W.; Kim, J.O.; Lee, H.; Lee, K.J.; Jeong, S.H. Carbapenemase-producing Enterobacteriaceae in South Korea: A report from the National Laboratory Surveillance System. Future Microbiol. 2018, 13, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Maurer, F.P.; Castelberg, C.; Quiblier, C.; Bloemberg, G.V.; Hombach, M. Evaluation of carbapenemase screening and confirmation tests with Enterobacteriaceae and development of a practical diagnostic algorithm. J. Clin. Microbiol. 2015, 53, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Sung, J.Y.; Yong, D.; Jeong, S.H.; Song, W.; Lee, K.; Chong, Y. Disk Carbapenemase Test for the Rapid Detection of KPC-, NDM-, and Other Metallo-β-Lactamase-Producing Gram-Negative Bacilli. Ann. Lab. Med. 2016, 36, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.S.; Kim, D.; Yoon, E.J.; Lee, H.; Jeong, S.H. Performance evaluation of the PANA RealTyper™ CRE Kit for detecting carbapenemase genes in Gram-negative bacilli. J. Glob. Antimicrob. Resist. 2019, 18, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Bogaerts, P.; Berger, A.S.; Evrard, S.; Huang, T.D. Comparison of two multiplex immunochromatographic assays for the rapid detection of major carbapenemases in Enterobacterales. J. Antimicrob. Chemother. 2020, 75, 1491–1494. [Google Scholar] [CrossRef] [PubMed]
- Boutal, H.; Vogel, A.; Bernabeu, S.; Devilliers, K.; Creton, E.; Cotellon, G.; Plaisance, M.; Oueslati, S.; Dortet, L.; Jousset, A.; et al. A multiplex lateral flow immunoassay for the rapid identification of NDM-, KPC-, IMP- and VIM-type and OXA-48-like carbapenemase-producing Enterobacteriaceae. J. Antimicrob. Chemother. 2018, 73, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Hrabák, J.; Chudáčková, E.; Papagiannitsis, C.C. Detection of carbapenemases in Enterobacteriaceae: A challenge for diagnostic microbiological laboratories. Clin. Microbiol. Infect. 2014, 20, 839–853. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Hong, S.G.; Yong, D.; Jeong, S.H.; Kim, H.S.; Kim, H.S.; Kim, J.S.; Bae, I.K. Combined use of the modified Hodge test and carbapenemase inhibition test for detection of carbapenemase-producing Enterobacteriaceae and metallo-β-lactamase-producing Pseudomonas spp. Ann. Lab. Med. 2015, 35, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Park, B.Y.; Mourad, D.; Hong, J.S.; Yoon, E.J.; Kim, D.; Lee, H.; Jeong, S.H. Performance Evaluation of the Newly Developed BD Phoenix NMIC-500 Panel Using Clinical Isolates of Gram-Negative Bacilli. Ann. Lab. Med. 2019, 39, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Traczewski, M.M.; Carretto, E.; Canton, R.; Moore, N.M.; Carba-R Study Team. Multicenter Evaluation of the Xpert Carba-R Assay for Detection of Carbapenemase Genes in Gram-Negative Isolates. J. Clin. Microbiol. 2018, 56. [Google Scholar] [CrossRef] [PubMed]
- van der Zwaluw, K.; de Haan, A.; Pluister, G.N.; Bootsma, H.J.; de Neeling, A.J.; Schouls, L.M. The carbapenem inactivation method (CIM), a simple and low-cost alternative for the Carba NP test to assess phenotypic carbapenemase activity in gram-negative rods. PLoS ONE 2015, 10, e0123690. [Google Scholar] [CrossRef] [PubMed]
- O.K.N.V.I. RESIST-5 [Package Insert], Gembloux(Belgium): Coris BioCencept Instruction for Use (IFU) IFU-58R11/TB/01. Available online: https://v3.globalcube.net/clients/beldico/content/medias/products/diagnostic/coris/ifu-coris-oknvi_resist-5_imp.pdf (accessed on 19 April 2020).
- Daikos, G.L.; Markogiannakis, A. Carbapenemase-producing Klebsiella pneumoniae: (when) might we still consider treating with carbapenems? Clin. Microbiol. Infect. 2011, 17, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, P.; Gniadkowski, M.; Giske, C.G.; Poirel, L.; Woodford, N.; Miriagou, V.; European Network on Carbapenemases. Identification and screening of carbapenemase-producing Enterobacteriaceae. Clin. Microbiol. Infect. 2012, 18, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Yoon, E.J.; Kim, J.O.; Yang, J.W.; Kim, H.S.; Lee, K.J.; Jeong, S.H.; Lee, H.; Lee, K. The blaOXA-23-associated transposons in the genome of Acinetobacter spp. represent an epidemiological situation of the species encountering carbapenems. J. Antimicrob. Chemother. 2017, 72, 2708–2714. [Google Scholar] [CrossRef] [PubMed]
| Carbapenemase (n) | Variants (n) | Species (n) |
|---|---|---|
| KPC (40) | KPC-2 (20) | K. pneumoniae (10), E. coli (6), Enterobacter species (2), K. oxytoca (1), S. marcescens (1) |
| KPC-3 (5) | K. pneumoniae (4), E. coli (1) | |
| KPC-4 (15) | K. pneumoniae (13), E. coli (1), Enterobacter species (1) | |
| GES (15) | GES-5 (14) | Enterobacter species (6), P. aeruginosa (6), K. pneumoniae (2), |
| GES-24 (1) | P. aeruginosa (1) | |
| IMP (45) | IMP-1 (4) | K. pneumoniae (3), Enterobacter species (1) |
| IMP-4 (3) | Enterobacter species (3) | |
| IMP-6 (37) | P. aeruginosa (37) | |
| IMP-10 (1) | P. aeruginosa (1) | |
| NDM (41) | NDM-1 (30) | Enterobacter species (9), E. coli (12), K. pneumoniae (4), C. freundii (3), K. oxytoca (1), C. amalonaticus (1) |
| NDM-5 (8) | E. coli (7), C. freundii (1) | |
| NDM-7 (2) | E. coli (2) | |
| NDM-9 (1) | E. coli (1) | |
| VIM (42) | VIM-1 (8) | K. pneumoniae (4), Enterobacter species (3), C. freundii (1) |
| VIM-2 (34) | Enterobacter species (23), K. pneumoniae (3), P. aeruginosa (3), S. marcescens (2), C. freundii (1), E. coli (1), K. oxytoca (1), | |
| OXA-48-like (40) | OXA-232 (40) | K. pneumoniae (40) |
| Co-producers (20) | KPC-2 and NDM-1 (6) | K. variicola (2), C. freundii (2), K. oxytoca (1), R. ornithinolytica (1), |
| KPC-3 and NDM-1 (3) | Raoultella planticola (3) | |
| KPC-4 and NDM-1 (1) | K. pneumoniae (1) | |
| KPC-4 and NDM-5 (1) | K. pneumoniae (1) | |
| NDM-1 and OXA-232 (2) | K. pneumoniae (2) | |
| NDM-1 and OXA-181 (3) | K. pneumoniae (3) | |
| NDM-5 and OXA-232 (1) | K. pneumoniae (1) | |
| GES-5, VIM-2 and OXA-48 (1) | C. freundii (1) | |
| IMP-1 and VIM-2 (1) | Enterobacter species (1) | |
| VIM-2 and NDM-1 (1) | E. coli (1) | |
| OXA-23 (15) | OXA-23 (15) | Acinetobacter baumannii (15) |
| Non-CP CRE (10) | K. pneumoniae (3), Enterobacter species (3), E. coli (2), K. oxytoca (1), C. freundii (1) |
| Number (%) of Clinical Isolates Tested by RESIST-5 | ||||||||
|---|---|---|---|---|---|---|---|---|
| KPC (n = 40) | NDM (n = 41) | VIM (n = 42) | IMP (n = 45) | OXA-48-Like (n = 40) | GES (n = 15) | OXA-23 (n = 15) | Non-CP CRE (n = 10) | |
| KPC | 40 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| NDM | 0 (0) | 40 (97.6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| VIM | 0 (0) | 0 (0) | 39 (92.9) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| IMP | 0 (0) | 0 (0) | 0 (0) | 45 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| OXA-48-like | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 40 (100) | 0 (0) | 0 (0) | 0 (0) |
| Negative | 0 (0) | 1 (2.4) | 3 (7.1) | 0 (0) | 0 (0) | 15 (100) | 15 (100) | 10 (100) |
| Carbapenemase Genes | Sensitivity, % (n/n) | Specificity, % (n/n) | Positive Predictive Value, % (n/n) | Negative Predictive Value, % (n/n) |
|---|---|---|---|---|
| blaKPC | 100 (51/51) | 100 (217/217) | 100 (51/51) | 100 (217/217) |
| blaIMP | 100 (46/46) | 100 (222/222) | 100 (46/46) | 100 (222/222) |
| blaNDM | 97.6 (59/60) | 100 (208/208) | 100(59/59) | 99.5 (208/209) |
| blaVIM | 93.3 (42/45) | 100 (223/223) | 100 (42/42) | 98.7 (223/226) |
| blaOXA-48-like | 100 (47/47) | 100 (221/221) | 100 (47/47) | 100 (221/221) |
| Total | 98.4 (245/249) | 100 (1091/1091) | 100 (245/245) | 99.6 (1091/1095) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, J.; Kang, D.; Kim, D. Performance Evaluation of the Newly Developed In Vitro Rapid Diagnostic Test for Detecting OXA-48-Like, KPC-, NDM-, VIM- and IMP-Type Carbapenemases: The RESIST-5 O.K.N.V.I. Multiplex Lateral Flow Assay. Antibiotics 2021, 10, 460. https://doi.org/10.3390/antibiotics10040460
Hong J, Kang D, Kim D. Performance Evaluation of the Newly Developed In Vitro Rapid Diagnostic Test for Detecting OXA-48-Like, KPC-, NDM-, VIM- and IMP-Type Carbapenemases: The RESIST-5 O.K.N.V.I. Multiplex Lateral Flow Assay. Antibiotics. 2021; 10(4):460. https://doi.org/10.3390/antibiotics10040460
Chicago/Turabian StyleHong, Junsung, Dayoung Kang, and Dokyun Kim. 2021. "Performance Evaluation of the Newly Developed In Vitro Rapid Diagnostic Test for Detecting OXA-48-Like, KPC-, NDM-, VIM- and IMP-Type Carbapenemases: The RESIST-5 O.K.N.V.I. Multiplex Lateral Flow Assay" Antibiotics 10, no. 4: 460. https://doi.org/10.3390/antibiotics10040460
APA StyleHong, J., Kang, D., & Kim, D. (2021). Performance Evaluation of the Newly Developed In Vitro Rapid Diagnostic Test for Detecting OXA-48-Like, KPC-, NDM-, VIM- and IMP-Type Carbapenemases: The RESIST-5 O.K.N.V.I. Multiplex Lateral Flow Assay. Antibiotics, 10(4), 460. https://doi.org/10.3390/antibiotics10040460






