Denture-Soaking Solution Containing Piper betle Extract-Loaded Polymeric Micelles; Inhibition of Candida albicans, Clinical Study, and Effects on Denture Base Resin
Abstract
1. Introduction
2. Results and Discussion
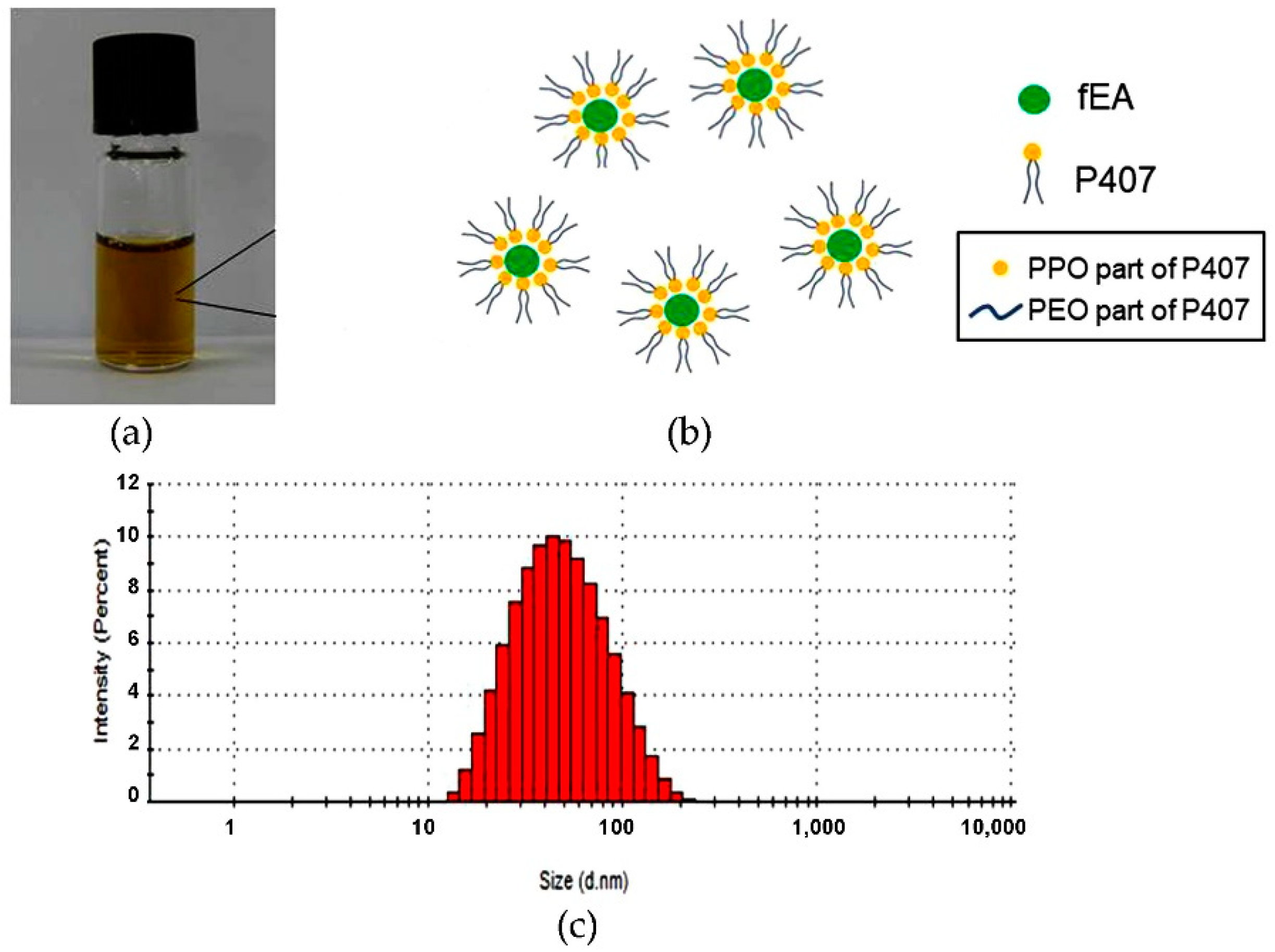
2.1. Development of PMF
2.2. In Vitro Antifungal Activity
Minimum Inhibitory Concentration (MIC) and Minimum Fungicidal Concentration (MFC)
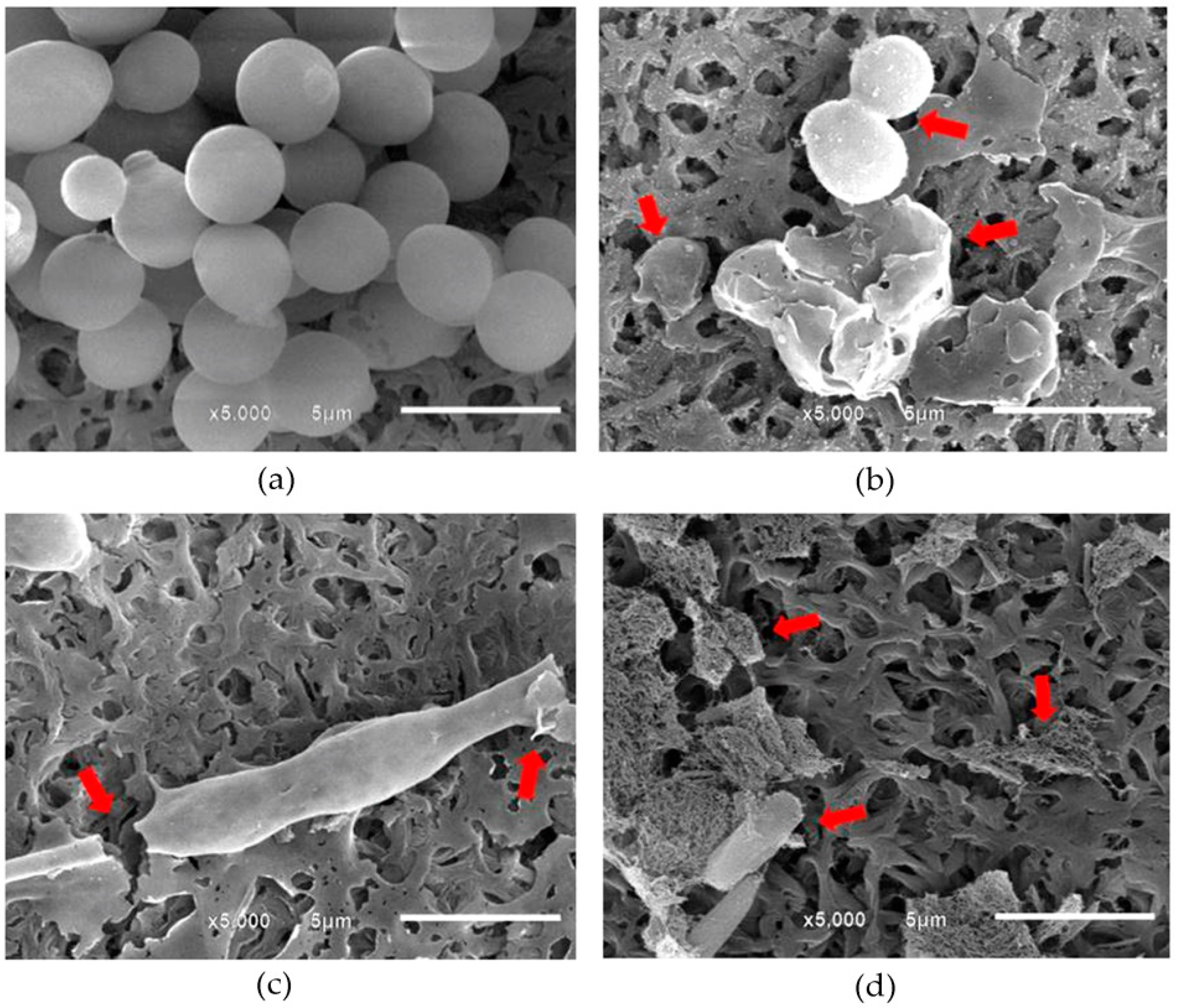
2.3. Visualization of Morphological Alterations
2.4. Preparation and Characterization of PMFS
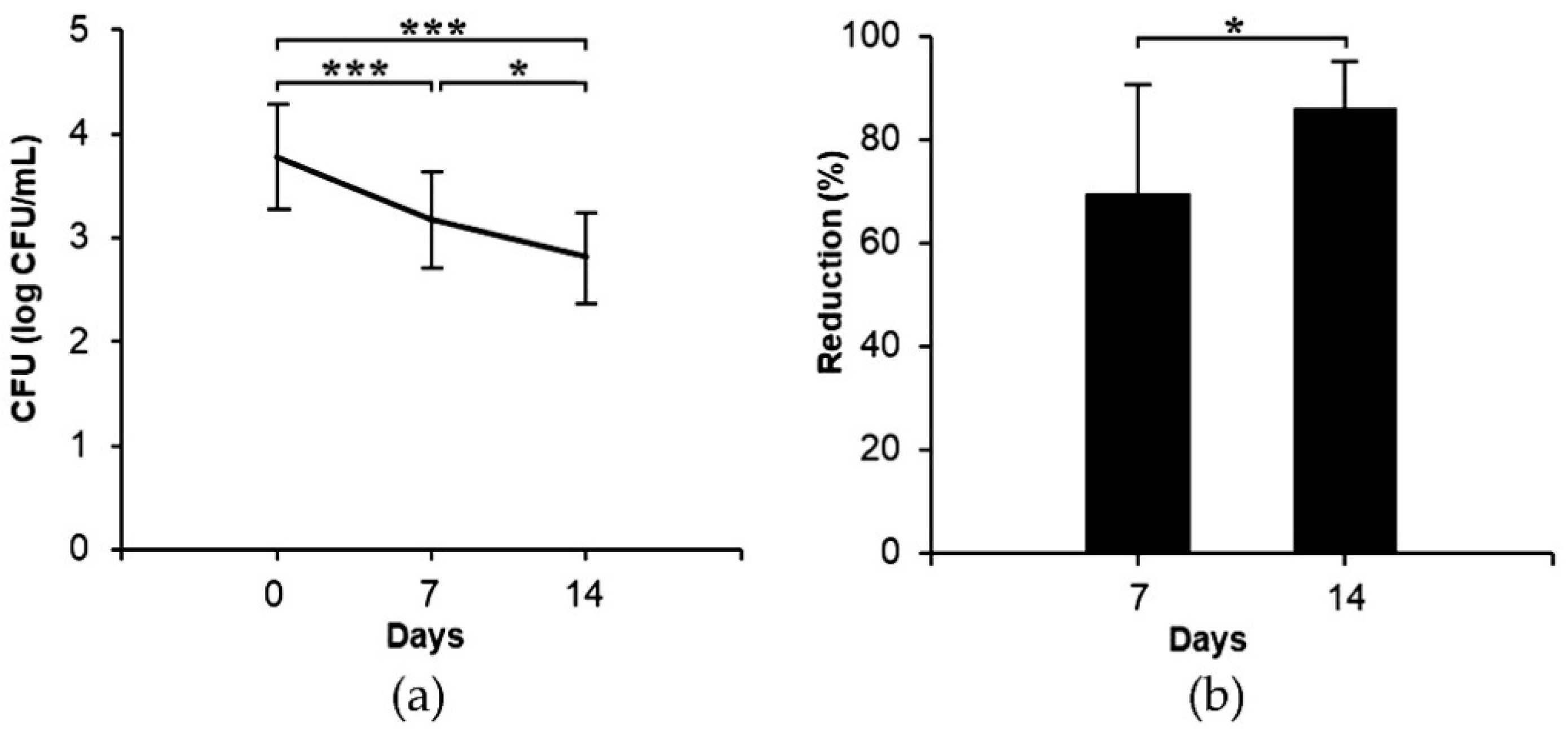
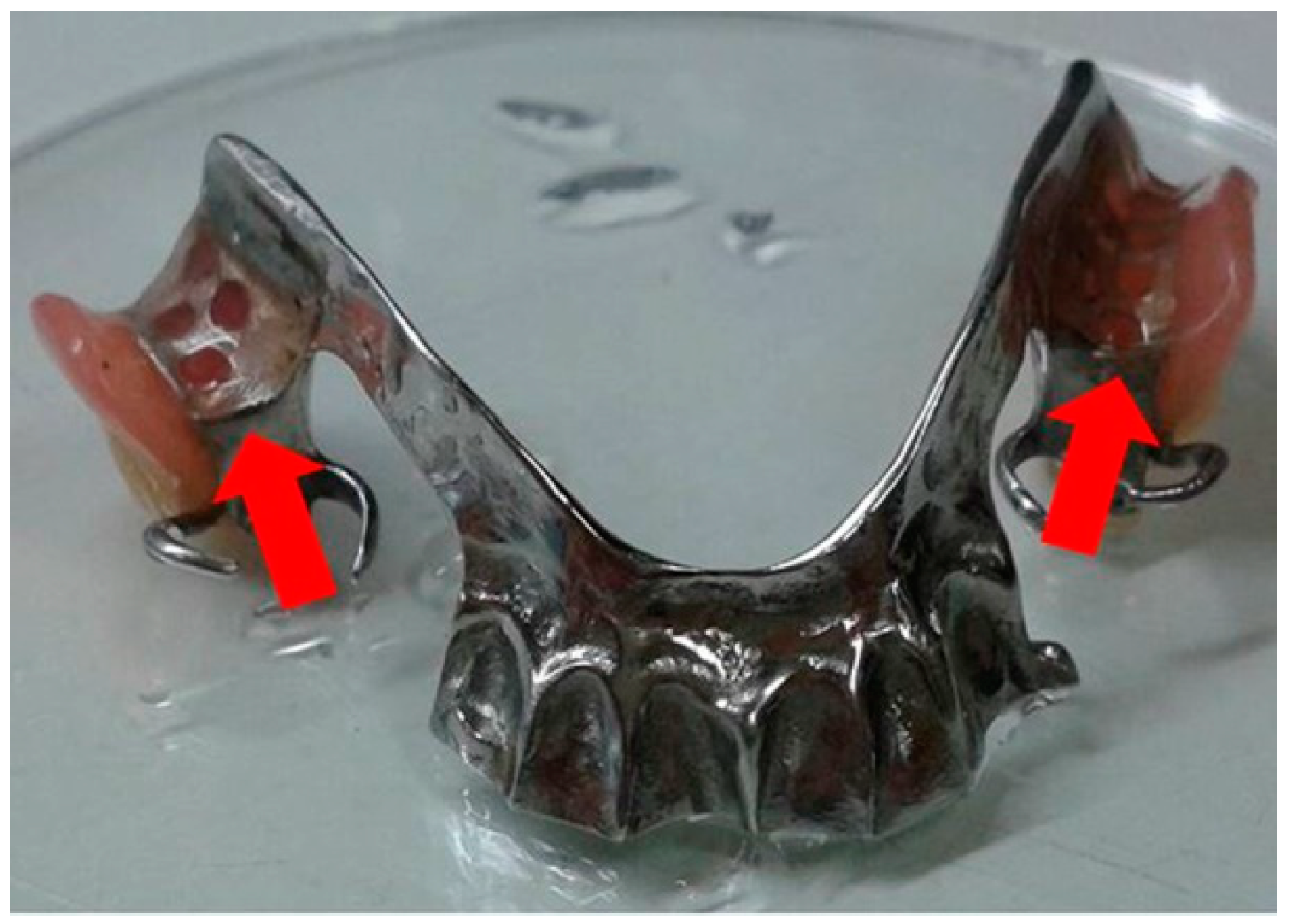
2.5. Clinical Study
2.6. Effects of the PMFS on Mechanical Properties of Acrylic Resin
3. Materials and Methods
3.1. Preparation of fEA
3.2. Development of PMF
3.3. Particle Analysis
3.4. Turbidity Measurement
3.5. Candida Strains and Growth Condition
3.6. In Vitro Antifungal Activity
3.7. Visualization of Morphological Alteration
3.8. Preparation and Characterization of PMFS
3.9. Clinical Study
3.10. Effects of PMFS on Physical and Mechanical Properties of Acrylic Resin
3.10.1. Surface Hardness Testing
3.10.2. Surface Roughness Testing
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dar-Odeh, N.S.; Shehabi, A.A. Oral candidosis in patients with removable dentures. Mycoses 2003, 46, 187–191. [Google Scholar] [CrossRef]
- Gleiznys, A.; Zdanavičienė, E.; Žilinskas, J. Candida albicans importance to denture wearers. A literature review. Stomatologija 2015, 17, 54–66. [Google Scholar]
- Delaloye, J.; Calandra, T. Invasive candidiasis as a cause of sepsis in the critically ill patient. Virulence 2014, 5, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Salerno, C.; Pascale, M.; Contaldo, M.; Esposito, V.; Busciolano, M.; Milillo, L.; Guida, A.; Petruzzi, M.; Serpico, R. Candida-associated denture stomatitis. Med. Oral Patol. Oral Cir. Bucal 2011, 16, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Gendreau, L.; Loewy, Z.G. Epidemiology and etiology of denture stomatitis. J. Prosthodont. 2011, 20, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Barani, K.; Manipal, S.; Prabu, D.; Ahmed, A.; Adusumilli, P.; Jeevika, C. Anti-fungal activity of Morinda citrifolia (noni) extracts against Candida albicans: An in vitro study. Indian J. Dent. Res. 2014, 25, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Gharechahi, J.; Asadzadeh, N.; Shahabian, F.; Gharechahi, M. Flexural strength of acrylic resin denture bases processed by two different methods. J. Dent. Res. Dent. Clin. Dent. Prospect. 2014, 8, 148–152. [Google Scholar]
- Pattanaik, S.; BVJ, V.; Pattanaik, B.; Sahu, S.; Lodam, S. Denture stomatitis: A literature review. J. Indian Acad. Oral Med. Radiol. 2010, 22, 136–140. [Google Scholar] [CrossRef]
- Pellizzaro, D.; Polyzois, G.; Machado, A.L.; Giampaolo, E.T.; Sanitá, P.V.; Vergani, C.E. Effectiveness of mechanical brushing with different denture cleansing agents in reducing in vitro Candida albicans biofilm viability. Braz. Dent. J. 2012, 23, 547–554. [Google Scholar] [CrossRef]
- Lamfon, H.; Porter, S.R.; McCullough, M.; Pratten, J. Susceptibility of Candida albicans biofilms grown in a constant depth film fermentor to chlorhexidine, fluconazole and miconazole: A longitudinal study. J. Antimicrob. Chemother. 2004, 53, 383–385. [Google Scholar] [CrossRef] [PubMed]
- Depaola, L.G.; Spolarich, A.E. Safety and efficacy of antimicrobial mouthrinses in clinical practice. J. Dent. Hyg. 2007, 81, 1–16. [Google Scholar]
- Zanatta, F.B.; Antoniazzi, R.P.; Rosing, C.K. Staining and calculus formation after 0.12% chlorhexidine rinses in plaque free and plaque covered surfaces: A randomized trial. J. Appl. Oral Sci. 2010, 18, 515–521. [Google Scholar] [CrossRef]
- Samaranayake, L.P.; McCourtie, J.; MacFarlane, T.W. Factors affecting the in-vitro adherence of Candida albicans to acrylic surfaces. Arch. Oral Biol. 1980, 25, 611–615. [Google Scholar] [CrossRef]
- Puttipan, R.; Wanachantararak, P.; Khongkhunthian, S.; Okonogi, S. Effects of Caesalpinia sappan on pathogenic bacteria causing dental caries and gingivitis. Drug Discov. Ther. 2017, 11, 316–322. [Google Scholar] [CrossRef]
- Villinski, J.R.; Bergeron, C.; Cannistra, J.C.; Gloer, J.B.; Coleman, C.M.; Ferreira, D.; Azelmat, J.; Grenier, D.; Gafner, S. Pyrano-isoflavans from Glycyrrhiza uralensis with antibacterial activity against Streptococcus mutans and Porphyromonas gingivalis. J. Nat. Prod. 2014, 77, 521–526. [Google Scholar] [CrossRef]
- Oliveira, S.A.C.; Zambrana, J.R.M.; Di Iorio, F.B.R.; Pereira, C.A.; Jorge, A.O.C. The antimicrobial effects of Citrus limonum and Citrus aurantium essential oils on multi-species biofilms. Braz. Oral Res. 2014, 28, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Phumat, P.; Khongkhunthian, S.; Wanachantararak, P.; Okonogi, S. Potential of Piper betle extracts on inhibition of oral pathogens. Drug Discov. Ther. 2017, 11, 307–315. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shoji, Y.; Nakashima, H. Nutraceutics and delivery systems. J. Drug Target. 2004, 12, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug solubility: Importance and enhancement techniques. ISRN Pharm. 2012, 2012, 195727. [Google Scholar] [CrossRef] [PubMed]
- Kulthe, S.S.; Choudhari, Y.M.; Inamdar, N.N.; Mourya, V. Polymeric micelles: Authoritative aspects for drug delivery. Des. Monomers Polym. 2012, 15, 465–521. [Google Scholar] [CrossRef]
- Cagel, M.; Tesan, F.C.; Bernabeu, E.; Salgueiro, M.J.; Zubillaga, M.B.; Moretton, M.A.; Chiappetta, D.A. Polymeric mixed micelles as nanomedicines: Achievements and perspectives. Eur. J. Pharm. Biopharm. 2017, 113, 211–228. [Google Scholar] [CrossRef]
- Sousa, F.; Ferreira, D.; Reis, S.; Costa, P. Current insights on antifungal therapy: Novel nanotechnology approaches for drug delivery systems and new drugs from natural sources. Pharmaceuticals 2020, 13, 248. [Google Scholar] [CrossRef] [PubMed]
- Oerlemans, C.; Bult, W.; Bos, M.; Storm, G.; Nijsen, J.F.W.; Hennink, W.E. Polymeric micelles in anticancer therapy: Targeting, imaging and triggered release. Pharm. Res. 2010, 27, 2569–2589. [Google Scholar] [CrossRef] [PubMed]
- Khonkarn, R.; Mankhetkorn, S.; Talelli, M.; Hennink, W.E.; Okonogi, S. Cytostatic effect of xanthone-loaded mPEG-b-p(HPMAm-Lac 2) micelles towards doxorubicin sensitive and resistant cancer cells. Colloids Surf. B Biointerfaces 2012, 94, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Bodratti, A.M.; Alexandridis, P. Formulation of poloxamers for drug delivery. J. Funct. Biomater. 2018, 9, 11. [Google Scholar] [CrossRef]
- Tima, S.; Anuchapreeda, S.; Ampasavate, C.; Berkland, C.; Okonogi, S. Stable curcumin-loaded polymeric micellar formulation for enhancing cellular uptake and cytotoxicity to FLT3 overexpressing EoL-1 leukemic cells. Eur. J. Pharm. Biopharm. 2017, 114, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Anantaworasakul, P.; Okonogi, S. Encapsulation of Sesbania grandiflora extract in polymeric micelles to enhance its solubility, stability, and antibacterial activity. J. Microencapsul. 2017, 34, 73–81. [Google Scholar] [CrossRef]
- Caesar, L.K.; Cech, N.B. Synergy and antagonism in natural product extracts: When 1 + 1 does not equal 2. Nat. Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef]
- Hussein, Y.H.A.; Youssry, M. Polymeric micelles of biodegradable diblock copolymers: Enhanced encapsulation of hydrophobic drugs. Materials 2018, 11, 688. [Google Scholar] [CrossRef]
- Sezgin, Z.; Yüksel, N.; Baykara, T. Preparation and characterization of polymeric micelles for solubilization of poorly soluble anticancer drugs. Eur. J. Pharm. Biopharm. 2006, 64, 261–268. [Google Scholar] [CrossRef]
- Phumat, P.; Khongkhunthian, S.; Wanachantararak, P.; Okonogi, S. Effects of Piper betle fractionated extracts on inhibition of Streptococcus mutans and Streptococcus intermedius. Drug Discov. Ther. 2018, 12, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Kitchener, B.G.B.; Wainwright, J.; Parsons, A.J. A review of the principles of turbidity measurement. Prog. Phys. Geogr. 2017, 41, 620–642. [Google Scholar] [CrossRef]
- Anderson, C.W. Turbidity. In Techniques of Water-Resources Investigations, 1st ed.; Anderson, C.W., Ed.; U.S. Geological Survey: Reston, VA, USA, 2005; Volume 1, pp. 1–55. [Google Scholar]
- Liu, J.; Xiao, Y.; Allen, C. Polymer-drug compatibility: A guide to the development of delivery systems for the anticancer agent, ellipticine. J. Pharm. Sci. 2004, 93, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.; Taher, M.; Mandal, U.K. uma. Experimental design and optimization of raloxifene hydrochloride loaded nanotransfersomes for transdermal application. Int. J. Nanomed. 2014, 9, 4331–4346. [Google Scholar]
- Bhattacharjee, S. DLS and zeta potential-What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems-A review (Part 1). Trop. J. Pharm. Res. 2013, 12, 255–264. [Google Scholar]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems-A review (Part 2). Trop. J. Pharm. Res. 2013, 12, 265–273. [Google Scholar]
- Shaarani, S.; Hamid, S.S.; Kaus, N.H.M. The Influence of pluronic F68 and F127 nanocarrier on physicochemical properties, in vitro release, and antiproliferative activity of thymoquinone drug. Pharmacogn. Res. 2017, 9, 12–20. [Google Scholar]
- Xie, S.; Tao, Y.; Pan, Y.; Qu, W.; Cheng, G.; Huang, L.; Chen, D.; Wang, X.; Liu, Z.; Yuan, Z. Biodegradable nanoparticles for intracellular delivery of antimicrobial agents. J. Control. Release 2014, 187, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Rodríguez, A.C.; Torrado-Durán, S.; Molero, G.; García-Rodríguez, J.J.; Torrado-Santiago, S. Efficacy and toxicity evaluation of new amphotericin B micelle systems for brain fungal infections. Int. J. Pharm. 2015, 494, 17–22. [Google Scholar] [CrossRef]
- Keepers, T.R.; Gomez, M.; Celeri, C.; Nichols, W.W.; Krause, K.M. Bactericidal activity, absence of serum effect, and time-kill kinetics of ceftazidime-avibactam against β-lactamase-producing Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2014, 58, 5297–5305. [Google Scholar] [CrossRef] [PubMed]
- Letscher-Bru, V.; Herbrecht, R. Caspofungin: The first representative of a new antifungal class. J. Antimicrob. Chemother. 2003, 51, 513–521. [Google Scholar] [CrossRef]
- Georgopapadakou, N.H.; Walsh, T.J. Antifungal agents: Chemotherapeutic targets and immunologic strategies. Antimicrob. Agents Chemother. 1996, 40, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Zarychanski, R.; Pisipati, A.; Kumar, A.; Kethireddy, S.; Bow, E.J. Fungicidal versus fungistatic therapy of invasive Candida infection in non-neutropenic adults: A meta-analysis. Mycology 2018, 9, 116–128. [Google Scholar] [CrossRef]
- Bradley, S.G.; Jones, L.A. Mechnisms of action of antibiotics. Ann. N. Y. Acad. Sci. 1960, 89, 122–133. [Google Scholar] [CrossRef]
- Hammond, S.M. Biological activity of polyene antibiotics. Prog. Med. Chem. 1977, 14, 105–179. [Google Scholar] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, P.; Chen, Z.; Cai, S.; Jing, T.; Fan, H.; Mo, F.; Zhang, J.; Lin, R. Preparation, characterization, and evaluation of amphotericin B-loaded MPEG-PCL-g-PEI micelles for local treatment of oral Candida albicans. Int. J. Nanomed. 2017, 12, 4269–4283. [Google Scholar] [CrossRef] [PubMed]
- Daneshmand, S.; Golmohammadzadeh, S.; Jaafari, M.R.; Movaffagh, J.; Rezaee, M.; Sahebkar, A.; Malaekeh-Nikouei, B. Encapsulation challenges, the substantial issue in solid lipid nanoparticles characterization. J. Cell. Biochem. 2018, 119, 4251–4264. [Google Scholar] [CrossRef]
- Amini, Y.; Amel Jamehdar, S.; Sadri, K.; Zare, S.; Musavi, D.; Tafaghodi, M. Different methods to determine the encapsulation efficiency of protein in PLGA nanoparticles. Biomed. Mater. Eng. 2017, 28, 613–620. [Google Scholar] [CrossRef]
- Moffa, E.B.; Izumida, F.E.; Jorge, J.H.; Mussi, M.C.M.; Siqueira, W.L.; Giampaolo, E.T. Effectiveness of chemical disinfection on biofilms of relined dentures: A randomized clinical trial. Am. J. Dent. 2016, 29, 15–19. [Google Scholar] [PubMed]
- Quirynen, M.; Bollen, C.M.L. The influence of surface roughness and surface-free energy on supra- and subgingival plaque formation in man: A review of the literature. J. Clin. Periodontol. 1995, 22, 1–14. [Google Scholar] [CrossRef]
- Radford, D.R.; Challacombe, S.J.; Walter, J.D. Denture plaque and adherence of Candida albicans to denture-base materials in vivo and in vitro. Crit. Rev. Oral Biol. Med. 1999, 10, 99–116. [Google Scholar] [CrossRef] [PubMed]
- Bollenl, C.M.L.; Lambrechts, P.; Quirynen, M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: A review of the literature. Dent. Mater. 1997, 13, 258–269. [Google Scholar] [CrossRef]
- Da Silva, F.C.; Kimpara, E.T.; Mancini, M.N.G.; Balducci, I.; Jorge, A.O.C.; Koga-Ito, C.Y. Effectiveness of six different disinfectants on removing five microbial species and effects on the topographic characteristics of acrylic resin. J. Prosthodont. 2008, 17, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.J.F.; da Costa, A.R.; Souza, C.F.; Tosin, F.F.S.; Garcia-Rojas, E.E. Complex coacervation between lysozyme and pectin: Effect of pH, salt, and biopolymer ratio. Int. J. Biol. Macromol. 2018, 107, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Phumat, P.; Khongkhunthian, S.; Wanachantararak, P.; Okonogi, S. Comparative inhibitory effects of 4-allylpyrocatechol isolated from Piper betle on Streptococcus intermedius, Streptococcus mutans, and Candida albicans. Arch. Oral Biol. 2020, 113, 1–10. [Google Scholar] [CrossRef]
- Naksuriya, O.; Shi, Y.; Van Nostrum, C.F.; Anuchapreeda, S.; Hennink, W.E.; Okonogi, S. HPMA-based polymeric micelles for curcumin solubilization and inhibition of cancer cell growth. Eur. J. Pharm. Biopharm. 2015, 94, 501–512. [Google Scholar] [CrossRef]
- Swaney, A.C.; Paffenbarger, G.C.; Caul, H.J.; Sweeney, W.T. American dental association specification No. 12 for denture base resin: Second revision. J. Am. Dent. Assoc. 1953, 46, 54–66. [Google Scholar] [CrossRef]
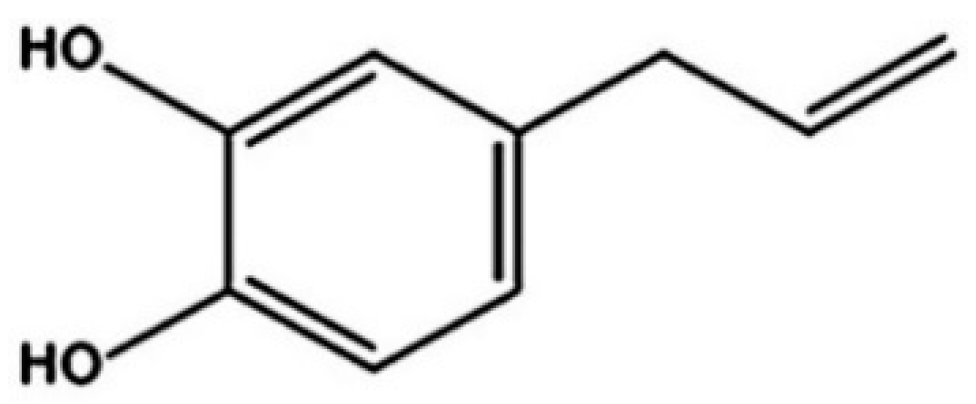
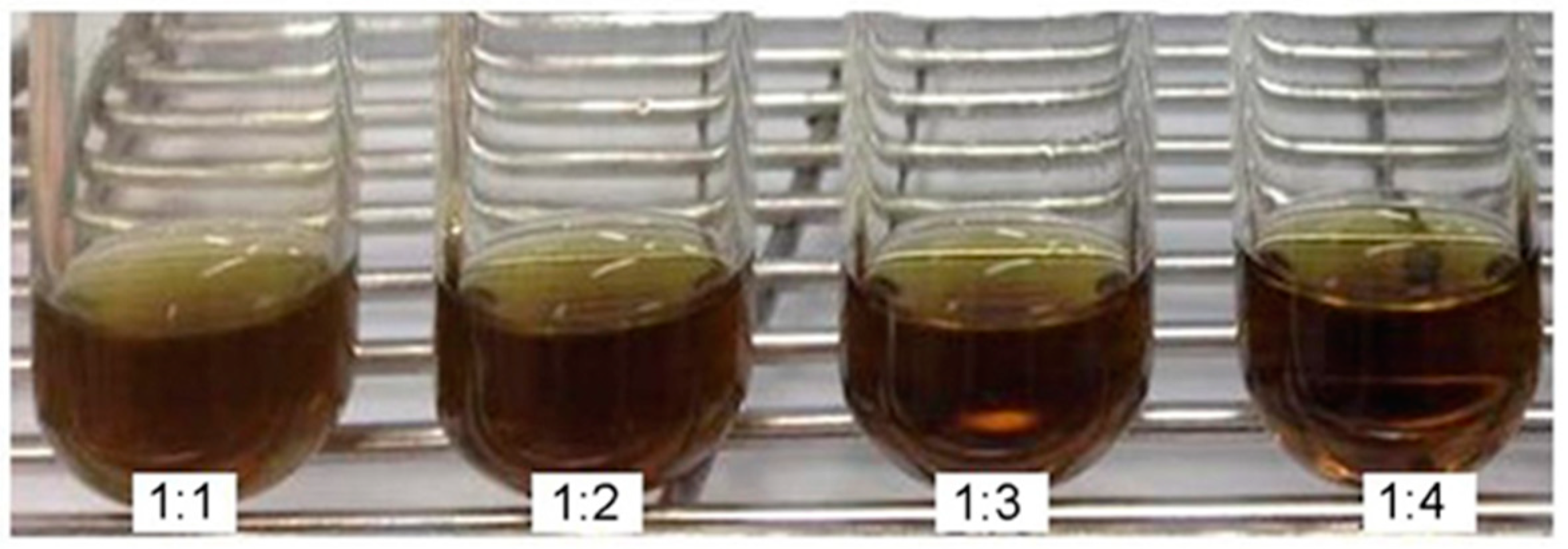
| fEA:P407. Ratio and Controls. | Transmittance * (%) | Turbidity * (%) |
|---|---|---|
| 1:1 | 27.99 ± 0.02 d | 72.01 ± 0.02 c |
| 1:2 | 29.82 ± 0.01 c | 70.18 ± 0.01 b |
| 1:3 | 35.34 ± 0.01 b | 64.66 ± 0.01 a |
| 1:4 | 35.51 ± 0.01 b | 64.49 ± 0.01 a |
| P407 solution | 99.46 ± 0.02 a | 0.95 ± 0.02 a |
| Deionized water | 99.45 ± 0.05 a | 0.55 ± 0.05 a |
| fEA in ethanol | 9.28 ± 0.01 e | 90.72 ± 0.01 e |
| fEA:P407 (Weight Ratios) | Particles Size * (nm) | PdI * | Zeta Potential * (mV) |
|---|---|---|---|
| 1:1 | 42.26 ± 0.09 b | 0.32 ± 0.01 c | −3.54 ± 0.56 c |
| 1:2 | 41.11 ± 0.28 a | 0.27 ± 0.02 b,c | −4.42 ± 0.11 a,b |
| 1:3 | 45.85 ± 2.27 c | 0.20 ± 0.01 a | −5.18 ± 0.08 a |
| 1:4 | 55.26 ± 1.05 d | 0.34 ± 0.02 a,b | −4.67 ± 0.72 a,b |
| Samples | C. albicans ATCC 10231 | C. albicans ATCC 90028 | ||||
|---|---|---|---|---|---|---|
| MIC | MFC | MFC/MIC Ratio | MIC | MFC | MFC/MIC Ratio | |
| PMF | 1.0 | 1.0 | 1.0 | 1.0 | 1.5 | 1.5 |
| fEA | 1.0 | 2.0 | 2.0 | 1.0 | 2.0 | 2.0 |
| Nystatin | 0.0006 | 0.0024 | 4.0 | 0.0031 | 0.0124 | 4.0 |
| Solution | Vicker’s Hardness * | Surface Roughness * (µm) | ||
|---|---|---|---|---|
| Before | After | Before | After | |
| PMFS | 19.65 ± 1.19 a | 18.03 ± 0.78 a | 0.14 ± 0.02 a | 0.15 ± 0.03 a |
| 0.5% NaOCl | 19.46 ± 0.80 a | 19.96 ± 0.84 a | 0.14 ± 0.02 a | 0.16 ± 0.03 a |
| Distilled water | 19.29 ± 0.73 a | 18.52 ± 0.74 a | 0.14 ± 0.02 a | 0.15 ± 0.03 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okonogi, S.; Phumat, P.; Khongkhunthian, S.; Suttiat, K.; Chaijareenont, P. Denture-Soaking Solution Containing Piper betle Extract-Loaded Polymeric Micelles; Inhibition of Candida albicans, Clinical Study, and Effects on Denture Base Resin. Antibiotics 2021, 10, 440. https://doi.org/10.3390/antibiotics10040440
Okonogi S, Phumat P, Khongkhunthian S, Suttiat K, Chaijareenont P. Denture-Soaking Solution Containing Piper betle Extract-Loaded Polymeric Micelles; Inhibition of Candida albicans, Clinical Study, and Effects on Denture Base Resin. Antibiotics. 2021; 10(4):440. https://doi.org/10.3390/antibiotics10040440
Chicago/Turabian StyleOkonogi, Siriporn, Pimpak Phumat, Sakornrat Khongkhunthian, Kullapop Suttiat, and Pisaisit Chaijareenont. 2021. "Denture-Soaking Solution Containing Piper betle Extract-Loaded Polymeric Micelles; Inhibition of Candida albicans, Clinical Study, and Effects on Denture Base Resin" Antibiotics 10, no. 4: 440. https://doi.org/10.3390/antibiotics10040440
APA StyleOkonogi, S., Phumat, P., Khongkhunthian, S., Suttiat, K., & Chaijareenont, P. (2021). Denture-Soaking Solution Containing Piper betle Extract-Loaded Polymeric Micelles; Inhibition of Candida albicans, Clinical Study, and Effects on Denture Base Resin. Antibiotics, 10(4), 440. https://doi.org/10.3390/antibiotics10040440






