Antibacterial Mechanisms and Efficacy of Sarecycline in Animal Models of Infection and Inflammation
Abstract
1. Introduction
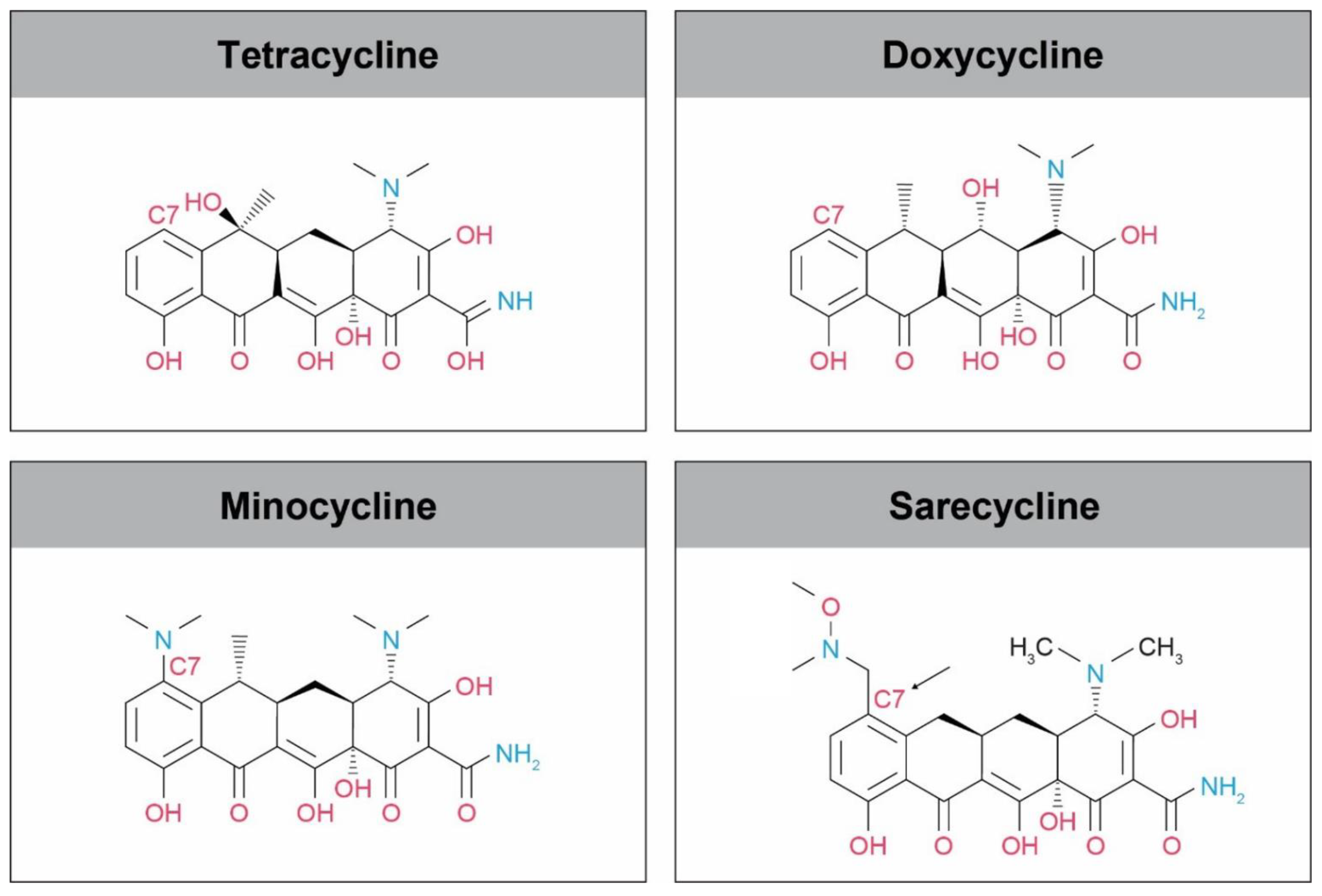
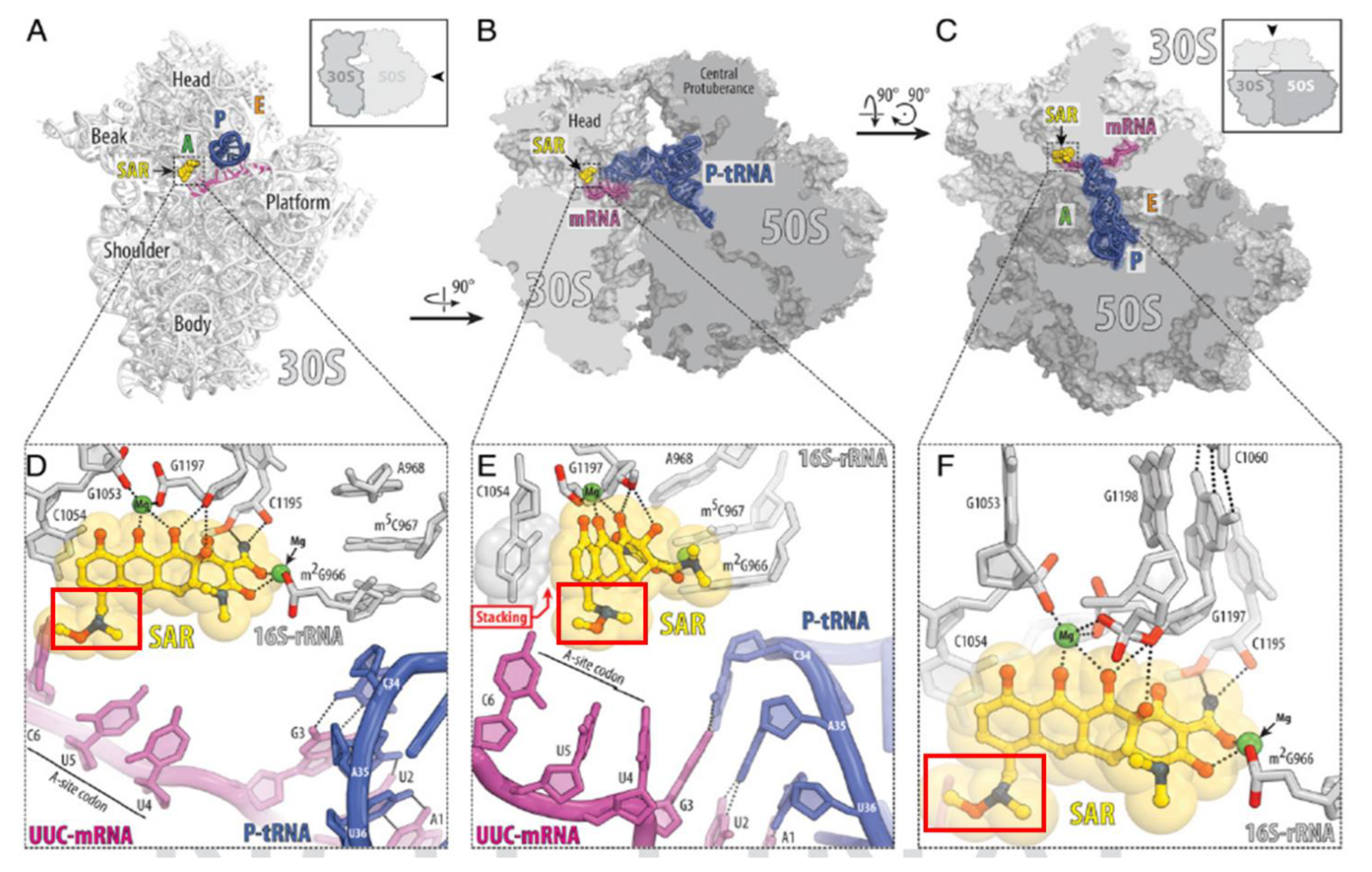
2. Sarecycline Structure and Mechanism of Action
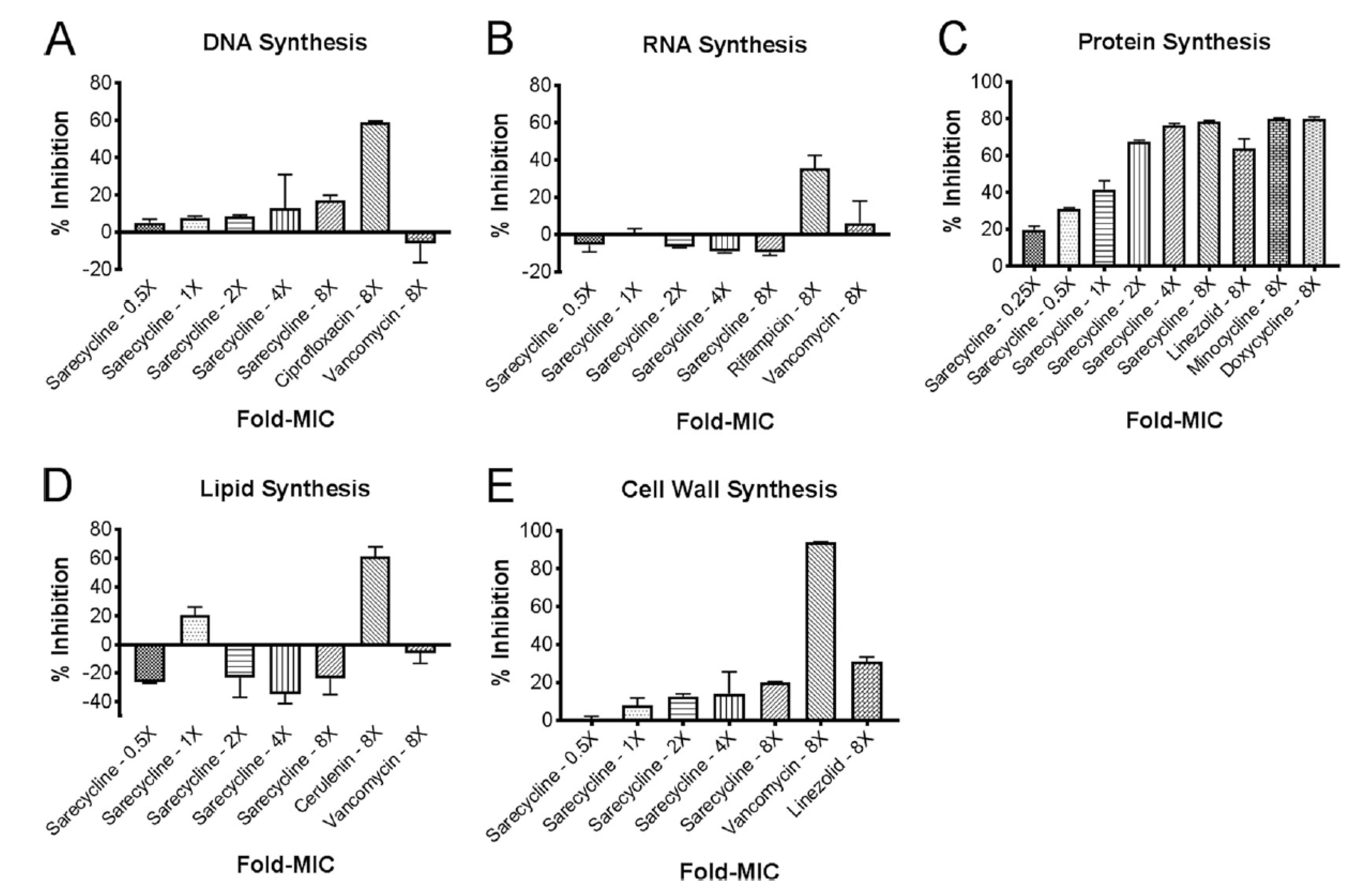
3. In vitro Inhibition of Bacterial Biosynthetic Endpoints
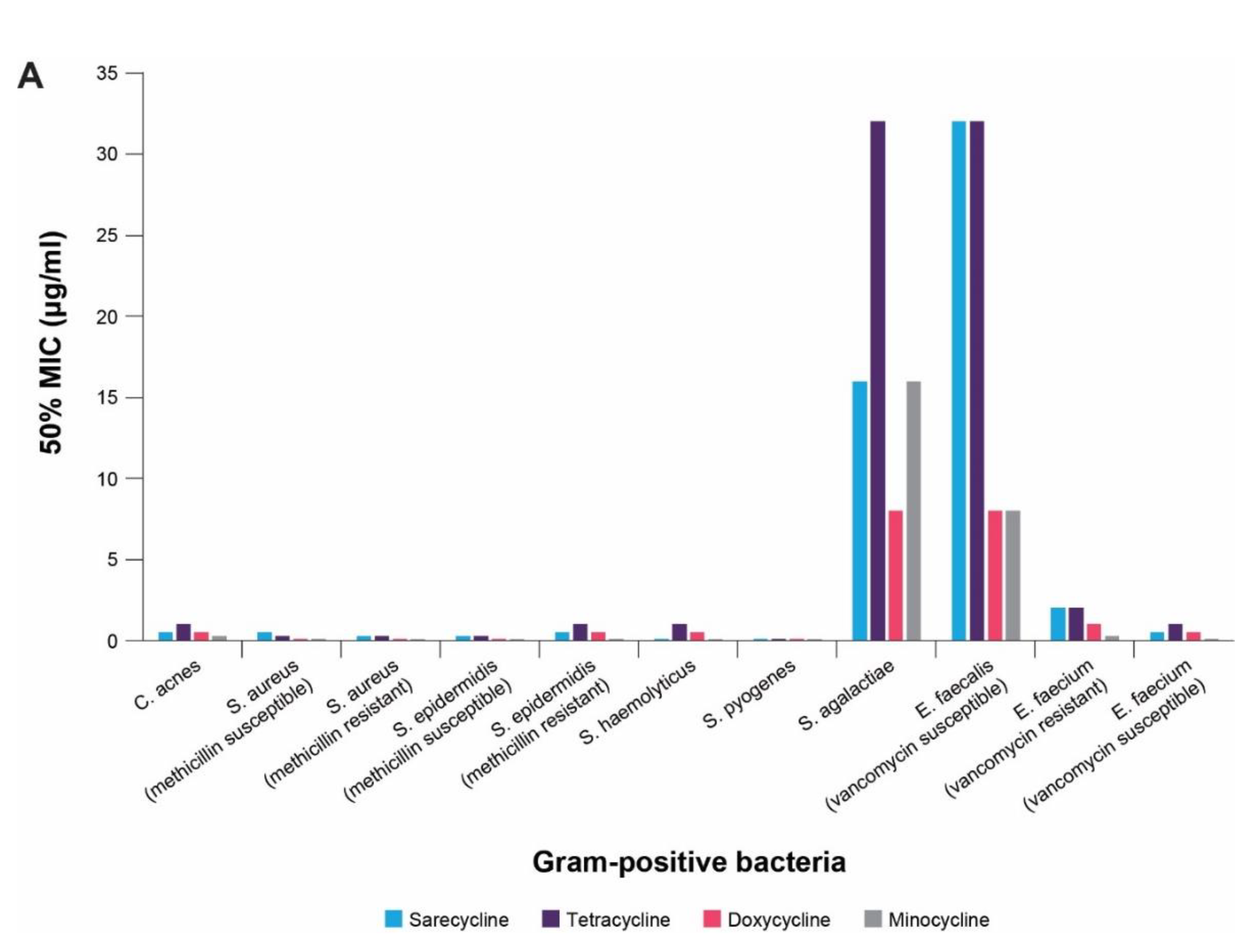
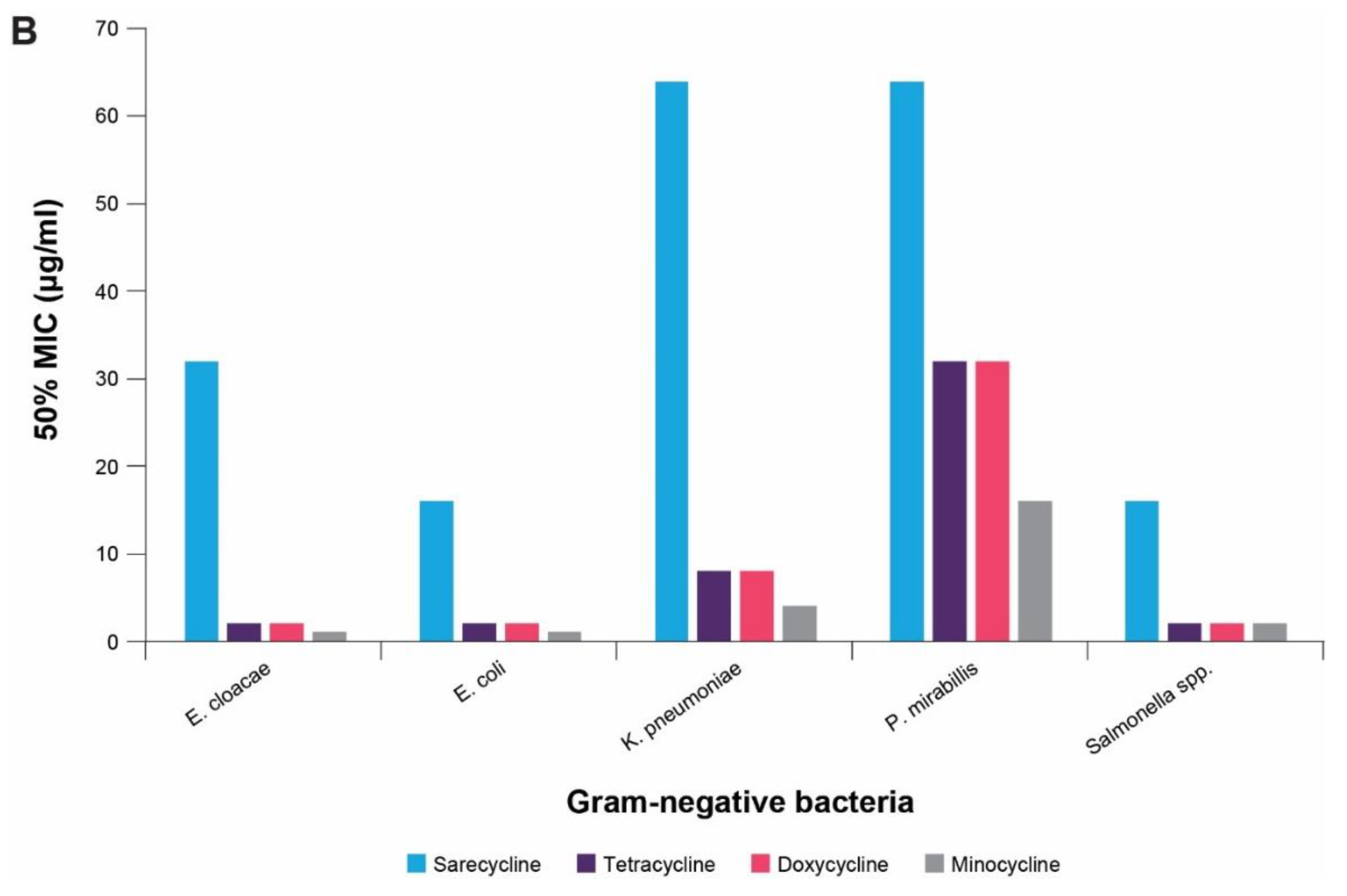
4. In vitro Antibacterial Effects
5. In vivo Antibacterial Efficacy
6. Antimicrobial Resistance Profile
7. In-Vivo Anti-Inflammatory Effects
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farrah, G.; Tan, E. The use of oral antibiotics in treating acne vulgaris: A new approach. Dermatol. Ther. 2016, 29, 377–384. [Google Scholar] [CrossRef]
- Barbieri, J.S.; Bhate, K.; Hartnett, K.P.; Fleming-Dutra, K.E.; Margolis, D.J. Trends in Oral Antibiotic Prescription in Dermatology, 2008 to 2016. JAMA Dermatol. 2019, 155, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Del Rosso, J.Q.; Webster, G.F.; Rosen, T.; Thiboutot, D.; Leyden, J.J.; Gallo, R.; Walker, C.; Zhanel, G.; Eichenfield, L. Status Report from the Scientific Panel on Antibiotic Use in Dermatology of the American Acne and Rosacea Society: Part 1: Antibiotic Prescribing Patterns, Sources of Antibiotic Exposure, Antibiotic Consumption and Emergence of Antibiotic Resistance, Impact of Alterations in Antibiotic Prescribing, and Clinical Sequelae of Antibiotic Use. J. Clin. Aesthetic Dermatol. 2016, 9, 18–24. [Google Scholar]
- Talkington, K. The U.S. Is Not Prepared to Combat ‘Existential Threat’ of Antibiotic-Resistant Superbugs. PEW Charitable Trusts. Available online: https://www.pewtrusts.org/en/research-and-analysis/articles/2020/07/27/the-us-is-not-prepared-to-combat-existential-threat-of-antibiotic-resistant-superbugs (accessed on 8 November 2020).
- Hobson, C.; Chan, A.N.; Wright, G.D. The Antibiotic Resistome: A Guide for the Discovery of Natural Products as Antimicrobial Agents. Chem. Rev. 2021, 121, 3464–3494. [Google Scholar] [CrossRef]
- Francino, M.P. Antibiotics and the Human Gut Microbiome: Dysbioses and Accumulation of Resistances. Front. Microbiol. 2016, 6, 1543. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.G.; Bustetter, L.A.; Gorbach, S.L.; Onderdonk, A.B. Comparative Effect of Tetracycline and Doxycycline on the Occurrence of Resistant Escherichia coli in the Fecal Flora. Antimicrob. Agents Chemother. 1975, 7, 55–57. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alkhawaja, E.; Hammadi, S.; Abdelmalek, M.; Mahasneh, N.; Alkhawaja, B.; Abdelmalek, S.M. Antibiotic resistant Cutibacterium acnes among acne patients in Jordan: A cross sectional study. BMC Dermatol. 2020, 20, 17. [Google Scholar] [CrossRef] [PubMed]
- Sheffer-Levi, S.; Rimon, A.; Lerer, V.; Shlomov, T.; Coppenhagen-Glazer, S.; Rakov, C.; Zeiter, T.; Nir-Paz, R.; Hazan, R.; Molcho-Pessach, V. Antibiotic Susceptibility of Cutibacterium acnes Strains Isolated from Israeli Acne Patients. Acta Derm. Venereol. 2020, 100, adv00295. [Google Scholar] [CrossRef]
- Holland, D.; Jeremy, A.; Roberts, S.G.; Seukeran, D.; Layton, A.; Cunliffe, W. Inflammation in acne scarring: A comparison of the responses in lesions from patients prone and not prone to scar. Br. J. Dermatol. 2004, 150, 72–81. [Google Scholar] [CrossRef]
- Kircik, L.H. Doxycycline and minocycline for the management of acne: A review of efficacy and safety with emphasis on clinical implications. J. Drugs Dermatol. JDD 2010, 9, 1407–1411. [Google Scholar]
- Thompson, K.G.; Rainer, B.M.; Antonescu, C.; Florea, L.; Mongodin, E.F.; Kang, S.; Chien, A.L. Minocycline and Its Impact on Microbial Dysbiosis in the Skin and Gastrointestinal Tract of Acne Patients. Ann. Dermatol. 2020, 32, 21–30. [Google Scholar] [CrossRef]
- Lee, T.W.; Russell, L.; Deng, M.; Gibson, P.R. Association of doxycycline use with the development of gastroenteritis, irritable bowel syndrome and inflammatory bowel disease in Australians deployed abroad. Intern. Med. J. 2013, 43, 919–926. [Google Scholar] [CrossRef]
- Margolis, D.J.; Fanelli, M.; Hoffstad, O.; Lewis, J.D. Potential Association Between the Oral Tetracycline Class of Antimicrobials Used to Treat Acne and Inflammatory Bowel Disease. Am. J. Gastroenterol. 2010, 105, 2610–2616. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.H.; Örtqvist, A.K.; Cao, Y.; Simon, T.G.; Roelstraete, B.; Song, M.; Joshi, A.D.; Staller, K.; Chan, A.T.; Khalili, H.; et al. Antibiotic use and the development of inflammatory bowel disease: A national case-control study in Sweden. Lancet Gastroenterol. Hepatol. 2020, 5, 986–995. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Hekmatjah, J.; Kircik, L.H. Oral Tetracyclines and Acne: A Systematic Review for Dermatologists. J. Drugs Dermatol. JDD 2020, 19, s6–s13. [Google Scholar] [PubMed]
- Ben-Ami, R.; Olshtain-Pops, K.; Krieger, M.; Oren, I.; Bishara, J.; Dan, M.; Wiener-Well, Y.; Weinberger, M.; Zimhony, O.; Chowers, M.; et al. Antibiotic Exposure as a Risk Factor for Fluconazole-Resistant Candida Bloodstream Infection. Antimicrob. Agents Chemother. 2012, 56, 2518–2523. [Google Scholar] [CrossRef] [PubMed]
- Spinillo, A.; Capuzzo, E.; Acciano, S.; De Santolo, A.; Zara, F. Effect of antibiotic use on the prevalence of symptomatic vulvovaginal candidiasis. Am. J. Obstet. Gynecol. 1999, 180, 14–17. [Google Scholar] [CrossRef]
- Barza, M.; Brown, R.B.; Shanks, C.; Gamble, C.; Weinstein, L. Relation Between Lipophilicity and Pharmacological Behavior of Minocycline, Doxycycline, Tetracycline, and Oxytetracycline in Dogs. Antimicrob. Agents Chemother. 1975, 8, 713–720. [Google Scholar] [CrossRef]
- Goulden, V.; Glass, D.; Cunliffe, W. Safety of long-term high-dose minocycline in the treatment of acne. Br. J. Dermatol. 1996, 134, 693–695. [Google Scholar] [CrossRef]
- Quinn, A.G.; Singer, S.B.; Buncic, J.R. Pediatric tetracycline-induced pseudotumor cerbri. J. AAPOS 1999, 3, 53–57. [Google Scholar] [CrossRef]
- Boucher, H.W.; Ambrose, P.G.; Chambers, H.F.; Ebright, R.H.; Jezek, A.; Murray, B.E.; Newland, J.G.; Ostrowsky, B.; Rex, J.H.; Infectious Diseases Society of America. White Paper: Developing Antimicrobial Drugs for Resistant Pathogens, Narrow-Spectrum Indications, and Unmet Needs. J. Infect. Dis. 2017, 216, 228–236. [Google Scholar] [CrossRef]
- Administration UFaD. Drug Approval Package: Seysara (sarecycline). US Department of Health and Human Services. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/209521Orig1s000TOC.cfm (accessed on 6 March 2021).
- Almirall. SEYSARA™ (Sarecycline) Tablets for Oral Use; Almirall, LLC: Exton, PA, USA, 2020. [Google Scholar]
- Moore, A.Y.; Del Rosso, J.; Johnson, J.L.; Grada, A. Sarecycline: A Review of Preclinical and Clinical Evidence. Clin. Cosmet. Investig. Dermatol. 2020, 13, 553–560. [Google Scholar] [CrossRef]
- Batool, Z.; Lomakin, I.B.; Polikanov, Y.S.; Bunick, C.G. Sarecycline interferes with tRNA accommodation and tethers mRNA to the 70S ribosome. Proc. Natl. Acad. Sci. USA 2020, 117, 20530–20537. [Google Scholar] [CrossRef]
- Chopra, I.; Roberts, M. Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef] [PubMed]
- Batool, Z.; Lomakin, I.; Polikanov, Y.; Bunick, C. 576 Crystal structure of sarecycline bound to the 70S bacterial ribosome reveals structural differences from other tetracyclines at atomic resolution. J. Investig. Dermatol. 2020, 140, S79. [Google Scholar] [CrossRef]
- Chukwudi, C.U. rRNA Binding Sites and the Molecular Mechanism of Action of the Tetracyclines. Antimicrob. Agents Chemother. 2016, 60, 4433–4441. [Google Scholar] [CrossRef]
- Zhanel, G.; Critchley, I.; Lin, L.-Y.; Alvandi, N. Microbiological Profile of Sarecycline, a Novel Targeted Spectrum Tetracycline for the Treatment of Acne Vulgaris. Antimicrob. Agents Chemother. 2018, 63. [Google Scholar] [CrossRef] [PubMed]
- Pato, M.L. Tetracycline Inhibits Propagation of Deoxyribonucleic Acid Replication and Alters Membrane Properties. Antimicrob. Agents Chemother. 1977, 11, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Abdi, F.; Kashani, H.H.; Naeini, F.F.; Narimani, T.; Khorvash, F. Staphylococcus aureus in acne pathogenesis: A case-control study. N. Am. J. Med Sci. 2012, 4, 573–576. [Google Scholar] [CrossRef]
- Fanelli, M.; Kupperman, E.; Lautenbach, E.; Edelstein, P.H.; Margolis, D.J. Antibiotics, Acne, and Staphylococcus aureus Colonization. Arch. Dermatol. 2011, 147, 917–921. [Google Scholar] [CrossRef]
- Moore, A.; Green, L.J.; Bruce, S.; Sadick, N.; Tschen, E.; Werschler, P.; Cook-Bolden, F.E.; Dhawan, S.S.; Forsha, D.; Gold, M.H.; et al. Once-Daily Oral Sarecycline 1.5 mg/kg/day Is Effective for Moderate to Severe Acne Vulgaris: Results from Two Identically Designed, Phase 3, Randomized, Double-Blind Clinical Trials. J. Drugs Dermatol. JDD 2018, 17, 987–996. [Google Scholar] [CrossRef]
- Pariser, D.M.; Green, L.J.; Lain, E.L.; Schmitz, C.; Chinigo, A.S.; McNamee, B.; Berk, D.R. Safety and Tolerability of Sarecycline for the Treatment of Acne Vulgaris: Results from a Phase III, Multicenter, Open-Label Study and a Phase I Phototoxicity Study. J. Clin. Aesthetic Dermatol. 2019, 12, E53–E62. [Google Scholar] [CrossRef]
- Nguyen, F.; Starosta, A.L.; Arenz, S.; Sohmen, D.; Dönhöfer, A.; Wilson, D.N. Tetracycline antibiotics and resistance mechanisms. Biol. Chem. 2014, 395, 559–575. [Google Scholar] [CrossRef]
- Grossman, T.H. Tetracycline Antibiotics and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025387. [Google Scholar] [CrossRef]
- Dönhöfer, A.; Franckenberg, S.; Wickles, S.; Berninghausen, O.; Beckmann, R.; Wilson, D.N. Structural basis for TetM-mediated tetracycline resistance. Proc. Natl. Acad. Sci. USA 2012, 109, 16900–16905. [Google Scholar] [CrossRef]
- Li, W.; Atkinson, G.C.; Thakor, N.S.; Allas, Ü.; Lu, C.-C.; Chan, K.-Y.; Tenson, T.; Schulten, K.; Wilson, K.S.; Hauryliuk, V.; et al. Mechanism of tetracycline resistance by ribosomal protection protein Tet(O). Nat. Commun. 2013, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mays, R.M.; Gordon, R.A.; Wilson, J.M.; Silapunt, S. New antibiotic therapies for acne and rosacea. Dermatol. Ther. 2012, 25, 23–37. [Google Scholar] [CrossRef]
- Perret, L.J.; Tait, C.P. Non-antibiotic properties of tetracyclines and their clinical application in dermatology. Australas. J. Dermatol. 2014, 55, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.O.; Ceballos, G.; Villarreal, F.J. Tetracycline compounds with non-antimicrobial organ protective properties: Possible mechanisms of action. Pharmacol. Res. 2011, 63, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Webster, G.; Del Rosso, J.Q. Anti-Inflammatory Activity of Tetracyclines. Dermatol. Clin. 2007, 25, 133–135. [Google Scholar] [CrossRef]
- Madke, B.; Pradhan, S.; Kabra, P.; Singh, A.L. Anti-inflammatory and immunomodulatory effects of antibiotics and their use in dermatology. Indian J. Dermatol. 2016, 61, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Cornet, S.; Spinnewyn, B.; Delaflotte, S.; Charnet, C.; Roubert, V.; Favre, C.; Hider, H.; Chabrier, P.E.; Auguet, M. Lack of evidence of direct mitochondrial involvement in the neuroprotective effect of minocycline. Eur. J. Pharmacol. 2004, 505, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Bastos, L.F.S.; Merlo, L.A.; Rocha, L.T.S.; Coelho, M.M. Characterization of the antinociceptive and anti-inflammatory activities of doxycycline and minocycline in different experimental models. Eur. J. Pharmacol. 2007, 576, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Leyden, J.J.; Sniukiene, V.; Berk, D.R.; Kaoukhov, A. Efficacy and Safety of Sarecycline, a Novel, Once-Daily, Narrow Spectrum Antibiotic for the Treatment of Moderate to Severe Facial Acne Vulgaris: Results of a Phase 2, Dose-Ranging Study. J. Drugs Dermatol. JDD 2018, 17, 333–338. [Google Scholar] [PubMed]
| Antibacterial Agent | S. aureus RN450-1 | E. coli PBS1478 | ||
|---|---|---|---|---|
| MIC (μg/mL) | PD50 (mg/kg) | MIC (μg/mL) | PD50 (mg/kg) | |
| Sarecycline | ≤0.06 | 0.25 | 4 | >40 |
| Doxycycline | ≤0.06 | 0.3 | 0.5 | 5.72 |
| Minocycline | ≤0.06 | 0.03 | 1 | 6.95 |
| Antibacterial Agent | MIC (μg/mL) | ED50 (mg/kg) |
|---|---|---|
| Sarecycline | ≤0.06 | 8.23 |
| Doxycycline | ≤0.06 | 8.31 |
| Inflammatory Mechanism of Action |
|---|
| Inhibition of bacterial products stimulating inflammation |
| Suppression of neutrophil migration and chemotaxis |
| Inhibition of T-lymphocyte activation and proliferation |
| Inhibition of phospholipase A2 |
| Inhibition of MMP |
| Inhibition of mast cell activation |
| Reactive oxygen species scavenging |
| Suppression of pro-inflammatory cytokine release (TNFα, IL-1β, IL-6, IL-8) |
| Inhibition of granuloma formulation in vitro |
| Inhibition of expression of nitric oxide synthase |
| Inhibition of angiogenesis in mouse models |
| Compound | Mean Percent Inflammation Compared to Untreated Controls | |||||||
|---|---|---|---|---|---|---|---|---|
| 150 mg/kg | 100 mg/kg | 75 mg/kg | 50 mg/kg | 25 mg/kg | 10 mg/kg | 5 mg/kg | 1 mg/kg | |
| Sarecycline | 25.8 | 53.1 | 55.7 | 52.0 | 59.0 | 65.2 | 77.8 | 103.3 |
| Doxycycline | - | 36.0 | 67.6 | - | - | - | - | - |
| Minocycline | - | 20.5 | 53.9 | 32.9 | 47.2 | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bunick, C.G.; Keri, J.; Tanaka, S.K.; Furey, N.; Damiani, G.; Johnson, J.L.; Grada, A. Antibacterial Mechanisms and Efficacy of Sarecycline in Animal Models of Infection and Inflammation. Antibiotics 2021, 10, 439. https://doi.org/10.3390/antibiotics10040439
Bunick CG, Keri J, Tanaka SK, Furey N, Damiani G, Johnson JL, Grada A. Antibacterial Mechanisms and Efficacy of Sarecycline in Animal Models of Infection and Inflammation. Antibiotics. 2021; 10(4):439. https://doi.org/10.3390/antibiotics10040439
Chicago/Turabian StyleBunick, Christopher G., Jonette Keri, S. Ken Tanaka, Nika Furey, Giovanni Damiani, Jodi L. Johnson, and Ayman Grada. 2021. "Antibacterial Mechanisms and Efficacy of Sarecycline in Animal Models of Infection and Inflammation" Antibiotics 10, no. 4: 439. https://doi.org/10.3390/antibiotics10040439
APA StyleBunick, C. G., Keri, J., Tanaka, S. K., Furey, N., Damiani, G., Johnson, J. L., & Grada, A. (2021). Antibacterial Mechanisms and Efficacy of Sarecycline in Animal Models of Infection and Inflammation. Antibiotics, 10(4), 439. https://doi.org/10.3390/antibiotics10040439









