Molecular Detection of Antibiotic Resistance Genes in Shiga Toxin-Producing E. coli Isolated from Different Sources
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Antibiotic Susceptibility Testing
2.3. Genomic DNA Extraction
2.4. Detection of Antibiotic Resistance Genes
3. Results
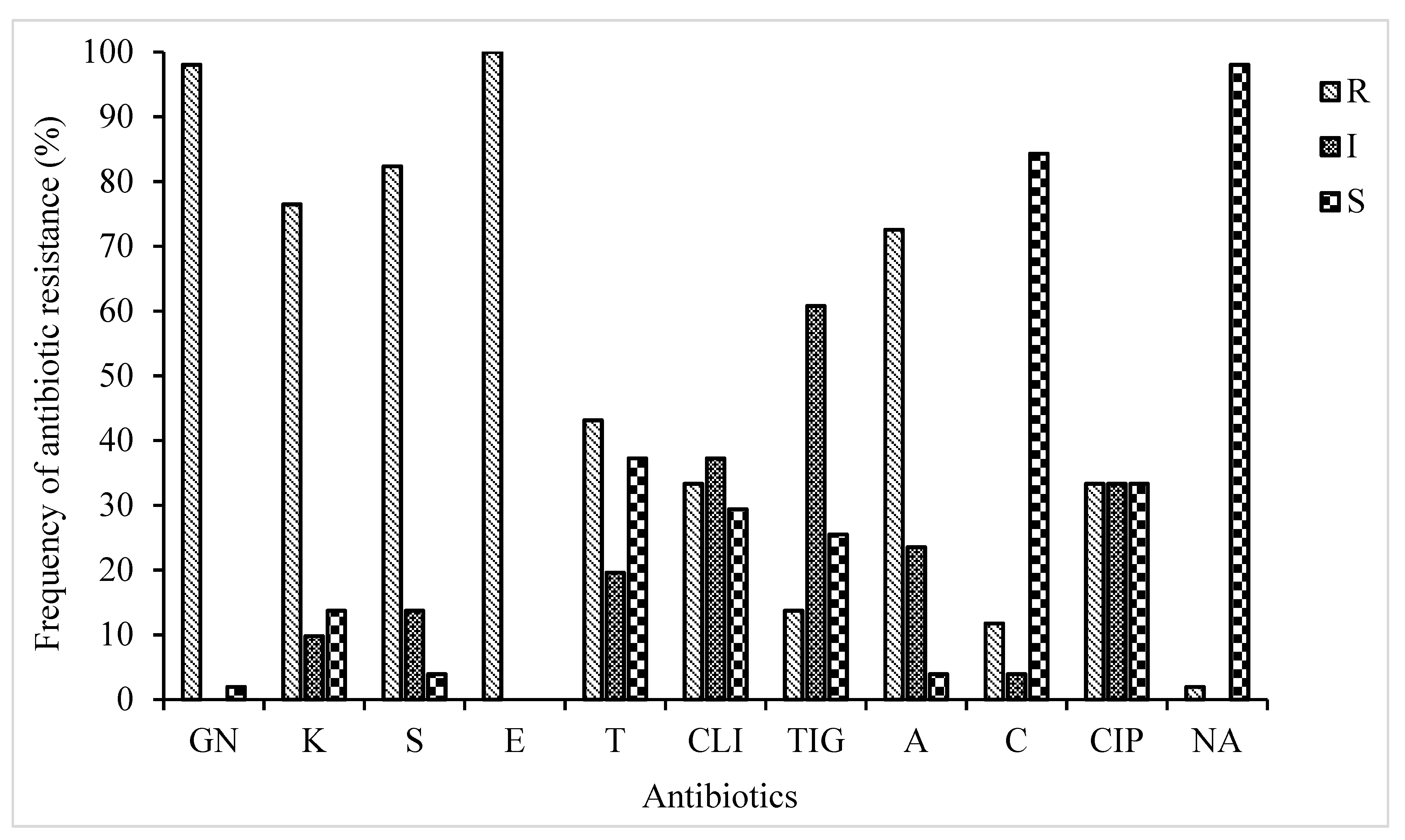
3.1. Antibiotic Resistance and Suceptibility Phenotype Characteristics
3.2. Antibiotic Resistance Genes
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hughes, A.C.; Zhang, Y.; Bai, X.; Xiong, Y.; Wang, Y.; Yang, X.; Xu, Q.; He, X. Structural and Functional Characterization of Stx2k, a New Subtype of Shiga Toxin 2. Microorganisms 2019, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Galindo-Méndez, M. Antimicrobial Resistance in Escherichia coli. In E. coli Infection; IntechOpen: London, UK, 2020. [Google Scholar]
- Karama, M.; Mainga, A.O.; Cenci-Goga, B.T.; Malahlela, M.; El-Ashram, S.; Kalake, A. Molecular profiling and antimicrobial resistance of Shiga toxin-producing Escherichia coli O26, O45, O103, O121, O145 and O157 isolates from cattle on cow-calf operations in South Africa. Sci. Rep. 2019, 9, 11930. [Google Scholar] [CrossRef] [PubMed]
- Rubab, M.; Oh, D.H. Virulence Characteristics and Antibiotic Resistance Profiles of Shiga Toxin-Producing Escherichia coli Isolates from Diverse Sources. Antibiotics 2020, 9, 587. [Google Scholar] [CrossRef]
- Sandvig, K.; Grimmer, S.; Lauvrak, S.U.; Torgersen, M.L.; Skretting, G.; van Deurs, B.; Iversen, T.G. Pathways followed by ricin and Shiga toxin into cells. Histochem. Cell Biol. 2002, 117, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.M.; Wilson, M.E.; Johnson, K.E.; Thorpe, C.M.; Sears, C.L. The emerging clinical importance of non-O157 Shiga toxin—producing Escherichia coli. Clin. Infect. Dis. 2006, 43, 1587–1595. [Google Scholar] [CrossRef]
- Adamu, M.S.; Ugochukwu, I.C.I.; Idoko, S.I.; Kwabugge, Y.A.; Sa’ad Abubakar, N.; Ameh, J.A. Virulent gene profile and antimicrobial susceptibility pattern of Shiga toxin-producing Escherichia coli (STEC) from humans in Maiduguri, Borno State, North-Eastern Nigeria. Comp. Clin. Pathol. 2018, 27, 341–351. [Google Scholar] [CrossRef]
- Melton-Celsa, A.R. Shiga toxin (Stx) classification, structure, and function. In Enterohemorrhagic Escherichia Coli Other Shiga Toxin-Prod. E. Coli; ASM Press: Washington, DC, USA, 2015; pp. 37–53. [Google Scholar]
- Galarce, N.; Sánchez, F.; Fuenzalida, V.; Ramos, R.; Escobar, B.; Lapierre, L.; Paredes-Osses, E.; Arriagada, G.; Alegría-Morán, R.; Lincopán, N.; et al. Phenotypic and Genotypic Antimicrobial Resistance in Non-O157 Shiga Toxin-Producing Escherichia coli Isolated From Cattle and Swine in Chile. Front. Vet. Sci. 2020, 7, 367. [Google Scholar] [CrossRef]
- Titilawo, Y.; Obi, L.; Okoh, A. Antimicrobial resistance determinants of Escherichia coli isolates recovered from some rivers in Osun State, South-Western Nigeria: Implications for public health. Sci. Total Environ. 2015, 523, 82–94. [Google Scholar] [CrossRef]
- Osińska, A.; Korzeniewska, E.; Harnisz, M.; Niestępski, S. The prevalence and characterization of antibiotic-resistant and virulent Escherichia coli strains in the municipal wastewater system and their environmental fate. Sci. Total Environ. 2017, 577, 367–375. [Google Scholar] [CrossRef]
- González-Escalona, N.; Kase, J.A. Virulence gene profiles and phylogeny of Shiga toxin-positive Escherichia coli strains isolated from FDA regulated foods during 2010-2017. PLoS ONE 2019, 14, e0214620. [Google Scholar] [CrossRef]
- Torkan, S.; Bahadoranian, M.A.; Khamesipour, F.; Anyanwu, M. Detection of virulence and antimicrobial resistance genes in Escherichia coli isolates from diarrhoiec dogs in Iran. Arch. Med. Vet. 2016, 48, 181–190. [Google Scholar] [CrossRef][Green Version]
- Van, T.T.H.; Chin, J.; Chapman, T.; Tran, L.T.; Coloe, P.J. Safety of raw meat and shellfish in Vietnam: An analysis of Escherichia coli isolations for antibiotic resistance and virulence genes. Int. J. Food Microbiol. 2008, 124, 217–223. [Google Scholar] [CrossRef]
- Chandy, S.J.; Naik, G.S.; Balaji, V.; Jeyaseelan, V.; Thomas, K.; Lundborg, C.S. High cost burden and health consequences of antibiotic resistance: The price to pay. J. Infect. Dev. Ctries. 2014, 8, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Alsan, M.; Schoemaker, L.; Eggleston, K.; Kammili, N.; Kolli, P.; Bhattacharya, J. Out-of-pocket health expenditures and antimicrobial resistance in low-income and middle-income countries: An economic analysis. Lancet Infect. Dis. 2015, 15, 1203–1210. [Google Scholar] [CrossRef]
- Szmolka, A.; Nagy, B. Multidrug resistant commensal Escherichia coli in animals and its impact for public health. Front. Microbiol. 2013, 4, 258. [Google Scholar] [CrossRef]
- Velez, R.; Sloand, E. Combating antibiotic resistance, mitigating future threats and ongoing initiatives. J. Clin. Nurs. 2016, 25, 1886–1889. [Google Scholar] [CrossRef]
- Briñas, L.; Zarazaga, M.; Sáenz, Y.; Ruiz-Larrea, F.; Torres, C. β-Lactamases in ampicillin-resistant Escherichia coli isolates from foods, humans, and healthy animals. Antimicrob. Agents Chemother. 2002, 46, 3156–3163. [Google Scholar] [CrossRef]
- Bhardwaj, D.K.; Taneja, N.K.; Dp, S.; Chakotiya, A.; Patel, P.; Taneja, P.; Sachdev, D.; Gupta, S.; Sanal, M.G. Phenotypic and genotypic characterization of biofilm forming, antimicrobial resistant, pathogenic Escherichia coli isolated from Indian dairy and meat products. Int. J. Food Microbiol. 2021, 336, 108899. [Google Scholar] [CrossRef]
- Dehdashti, S.; Ghanbarpour, R.; Hajikolaei, M.R.H. Molecular detection of Shiga toxin–producing and antibiotic-resistant Escherichia coli isolates from buffaloes in southwest of Iran. Trop. Anim. Health Prod. 2019, 51, 1725–1736. [Google Scholar] [CrossRef]
- Hu, M.; Guo, J.; Cheng, Q.; Yang, Z.; Chan, E.W.C.; Chen, S.; Hao, Q. Crystal structure of Escherichia coli originated MCR-1, a phosphoethanolamine transferase for colistin resistance. Sci. Rep. 2016, 6, 38793. [Google Scholar] [CrossRef]
- Karama, M.; Cenci-Goga, B.T.; Malahlela, M.; Smith, A.M.; Keddy, K.H.; El-Ashram, S.; Kabiru, L.M.; Kalake, A. Virulence Characteristics and Antimicrobial Resistance Profiles of Shiga Toxin-Producing Escherichia coli Isolates from Humans in South Africa: 2006–2013. Toxins 2019, 11, 424. [Google Scholar] [CrossRef]
- Pan, Y.; Hu, B.; Bai, X.; Yang, X.; Cao, L.; Liu, Q.; Sun, H.; Li, J.; Zhang, J.; Jin, D.; et al. Antimicrobial Resistance of Non-O157 Shiga Toxin-Producing Escherichia coli Isolated from Humans and Domestic Animals. Antibiotics 2021, 10, 74. [Google Scholar] [CrossRef]
- Rao, T.S.; Gill, J.; GVVPS, R.K.; Ghatak, S.J. Multi drug resistance patterns of Shiga toxin “producing Escherichia coli (STEC) and non” STEC isolates from meats, RTE meat foods, drinking water and human diarrhoeic samples of Punjab, India. Arch. Clin. Microbiol. 2011, 2, 1–12. [Google Scholar]
- Chirila, F.; Tabaran, A.; Fit, N.; Nadas, G.; Mihaiu, M.; Tabaran, F.; Cătoi, C.; Reget, O.L.; Dan, S.D.J.M. Concerning increase in antimicrobial resistance in Shiga toxin-producing Escherichia coli isolated from young animals during 1980–2016. Microbes Environ. 2017, 32, 252–259. [Google Scholar] [CrossRef]
- Oporto, B.; Ocejo, M.; Alkorta, M.; Marimón, J.M.; Montes, M.; Hurtado, A. Zoonotic approach to Shiga toxin-producing Escherichia coli: Integrated analysis of virulence and antimicrobial resistance in ruminants and humans. Epidemiol. Infect. 2019, 147, e164. [Google Scholar] [CrossRef] [PubMed]
- Dawangpa, A.; Lertwatcharasarakul, P.; Ramasoota, P.; Boonsoongnern, A.; Ratanavanichrojn, N.; Sanguankiat, A.; Phatthanakunanan, S.; Tulayakul, P. Genotypic and phenotypic situation of antimicrobial drug resistance of Escherichia coli in water and manure between biogas and non-biogas swine farms in central Thailand. J. Environ. Manag. 2021, 279, 111659. [Google Scholar] [CrossRef] [PubMed]
- Gow, S.P.; Waldner, C.L.; Harel, J.; Boerlin, P.J.A.; Microbiology, E. Associations between antimicrobial resistance genes in fecal generic Escherichia coli isolates from cow-calf herds in western Canada. Appl. Environ. Microbiol. 2008, 74, 3658–3666. [Google Scholar] [CrossRef] [PubMed]
- Schlüter, A.; Szczepanowski, R.; Pühler, A.; Top, E.M. Genomics of IncP-1 antibiotic resistance plasmids isolated from wastewater treatment plants provides evidence for a widely accessible drug resistance gene pool. FEMS Microbiol. Rev. 2007, 31, 449–477. [Google Scholar] [CrossRef] [PubMed]
- Tennstedt, T.; Szczepanowski, R.; Braun, S.; Pühler, A.; Schlüter, A. Occurrence of integron-associated resistance gene cassettes located on antibiotic resistance plasmids isolated from a wastewater treatment plant. FEMS Microbiol. Ecol. 2003, 45, 239–252. [Google Scholar] [CrossRef]
- Iyer, A.; Barbour, E.; Azhar, E.; El Salabi, A.A.; Hassan, H.M.A.; Qadri, I.; Chaudhary, A.; Abuzenadah, A.; Kumosani, T.; Damanhouri, G.; et al. Transposable Elements in Escherichia coli Antimicrobial Resistance. Adv. Biosci. Biotechnol. 2013, 4, 29273. [Google Scholar]
- Luitz, E.A.; McCarty, M.J.; Mollenkopf, D.F.; Funk, J.A.; Gebreyes, W.A.; Wittum, T.E. Ceftiofur use in finishing swine barns and the recovery of fecal Escherichia coli or Salmonella spp. resistant to ceftriaxone. Foodborne Pathog. Dis. 2011, 8, 1229–1234. [Google Scholar] [CrossRef]
- Tadesse, D.A.; Zhao, S.; Tong, E.; Ayers, S.; Singh, A.; Bartholomew, M.J.; McDermott, P.F. Antimicrobial drug resistance in Escherichia coli from humans and food animals, United States, 1950–2002. Emerg. Infect. Dis. 2012, 18, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, J.; Das, S.; Fatima, Z.; Hameed, S. Multidrug Resistance: An Emerging Crisis. Interdiscip. Perspect. Infect. Dis. 2014, 2014, 541340. [Google Scholar] [CrossRef] [PubMed]
- Mir, R.A.; Kudva, I.T. Antibiotic-resistant Shiga toxin-producing Escherichia coli: An overview of prevalence and intervention strategies. Zoonoses Public Health 2019, 66, 1–13. [Google Scholar] [CrossRef] [PubMed]

| Serogroup | Serotype | No. of Isolates | Source |
|---|---|---|---|
| O26 | O26:H11 | 7 | H |
| O45 | O45:NM, O45:H2, O45:H12 | 7 | Co (calf), H, Go |
| O103 | O103:H2, O103:H6, O103:H11, O103:H25 | 7 | H |
| O104 | O104:H2, O104:H4, O104:H7, O104:H21 | 7 | H, Ca, Co |
| O111 | O111:H-, O111:NM, O111:H8 | 7 | H |
| O121 | O121:NM, O121:H19 | 6 | H |
| O145 | O145:H-, O145:NM | 7 | H, Co |
| O157 | O157:H7 | 3 | H, Gb |
| Total | 51 |
| Target Gene | Primers | Oligonucleotide Sequence (5′→3′) | Size (bp) | Reference |
|---|---|---|---|---|
| Aminoglycosides resistance | ||||
| aac(3)–I | aac(3)–I–F | CTTCAGGATGGCAAGTTGGT | 286 | [15] |
| aac(3)–I –R | TCATCTCGTTCTCCGCTCAT | |||
| aadA1 | aadA1–F | TATCCAGCTAAGCGCGAACT | 447 | |
| aadA1–R | ATTTGCCGACTACCTTGGTC | |||
| β-Lactams resistance | ||||
| ampC | ampC–F | AATGGGTTTTCTACGGTCTG | 191 | [20] |
| ampC–R | GGGCAGCAAATGTGGAGCAA | |||
| blaSHV | blaSHV–F | TCGCCTGTGTATTATCTCCC | 768 | [15] |
| blaSHV–R | CGCAGATAAATCACCACAATG | |||
| blaCMY | blaCMY–F | TGGCCAGAACTGACAGGCAAA | 462 | |
| blaCMY–R | TTTCTCCTGAACGTGGCTGGC | |||
| Macrolides resistance | ||||
| ere(A) | ere(A)–F | GCCGGTGCTCATGAACTTGAG | 419 | [15] |
| ere(A)–R | CGACTCTATTCGATCAGAGGC | |||
| Tetracycline resistance | ||||
| Tet(A) | Tet(A)–F | GGTTCACTCGAACGACGTCA | 577 | [15] |
| Tet(A)–R | CTGTCCGACAAGTTGCATGA | |||
| No. of Antibiotics | Multidrug Resistance Profile | No. of Bacterial Strain | Total. No (%) |
|---|---|---|---|
| 2 | A, E | 1 | 1 (1.96) |
| 3 | A, E, GN | 1 | 2 (3.92) |
| GN, CIP, E | 1 | ||
| 4 | GN, A, E, K | 1 | 10 (19.6) |
| GN, E, S, CLI, | 1 | ||
| GN, A, E, S | 3 | ||
| GN, E, CLI, CIP | 1 | ||
| GN, A, E, CIP | 2 | ||
| GN, E, K, S | 2 | ||
| 5 | GN, K, E, S, T | 1 | 11 (21.5) |
| GN, K, E, S, CIP | 2 | ||
| GN, K, E, S, A | 5 | ||
| GN, K, E, S, C | 1 | ||
| GN, KN, E, CIP, A | 1 | ||
| GN, S, E, CLI, A | 1 | ||
| 6 | GN, K, E, A, S, T | 5 | 11 (21.5) |
| GN, K, E, A, S, CLI | 1 | ||
| GN, K, E, A, S, TIG | 2 | ||
| GN, K, E, A, CLI, CIP, | 1 | ||
| GN, K, E, S, CLI, T | 1 | ||
| GN, K, E, S, TIG, T | 1 | ||
| 7 | GN, A, E, T, K, S, CHL | 1 | 7 (13.7) |
| GN, A, E, T, K, S, CLI | 1 | ||
| GN, A, E, T, S, CLI, CIP | 1 | ||
| GN, A, E, T, K, S, CIP | 1 | ||
| GN, E, K, S, CLI, CIP, TIG | 1 | ||
| GN, E, K, T, S, CLI, CIP, | 1 | ||
| GN, A, E, K, S, CLI, CIP | 1 | ||
| 8 | GN, A, E, K, T, S, C, TIG | 1 | 9 (17.6) |
| GN, CIP, A, T, E, K, S, CIP | 1 | ||
| GN, K, A, E, T, S, CLI, CIP | 2 | ||
| GN, K, A, E, T, S, CLI, TIG | 1 | ||
| GN, K, A, E, T, S, CLI, C | 1 | ||
| GN, K, E, T, S, CLI, CIP, C | 1 | ||
| GN, K, A, E, T, S NA, TIG | 1 | ||
| GN, K, A, E, T, S, C, TIG | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubab, M.; Oh, D.-H. Molecular Detection of Antibiotic Resistance Genes in Shiga Toxin-Producing E. coli Isolated from Different Sources. Antibiotics 2021, 10, 344. https://doi.org/10.3390/antibiotics10040344
Rubab M, Oh D-H. Molecular Detection of Antibiotic Resistance Genes in Shiga Toxin-Producing E. coli Isolated from Different Sources. Antibiotics. 2021; 10(4):344. https://doi.org/10.3390/antibiotics10040344
Chicago/Turabian StyleRubab, Momna, and Deog-Hwan Oh. 2021. "Molecular Detection of Antibiotic Resistance Genes in Shiga Toxin-Producing E. coli Isolated from Different Sources" Antibiotics 10, no. 4: 344. https://doi.org/10.3390/antibiotics10040344
APA StyleRubab, M., & Oh, D.-H. (2021). Molecular Detection of Antibiotic Resistance Genes in Shiga Toxin-Producing E. coli Isolated from Different Sources. Antibiotics, 10(4), 344. https://doi.org/10.3390/antibiotics10040344







