Pitfalls in the Immunochemical Determination of β-Lactam Antibiotics in Water
Abstract
1. Introduction
2. Results and Discussion
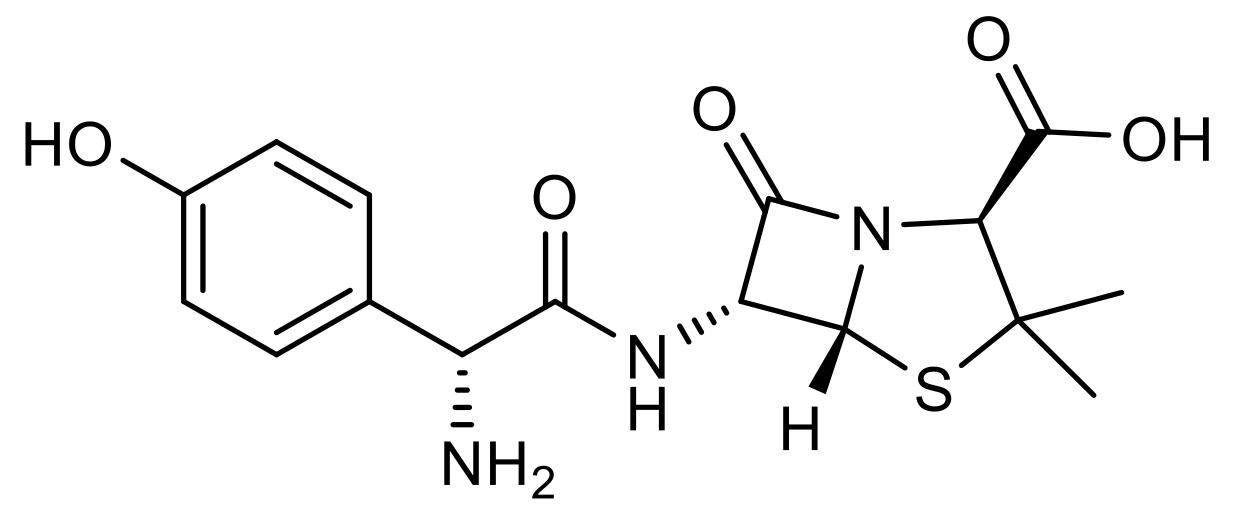
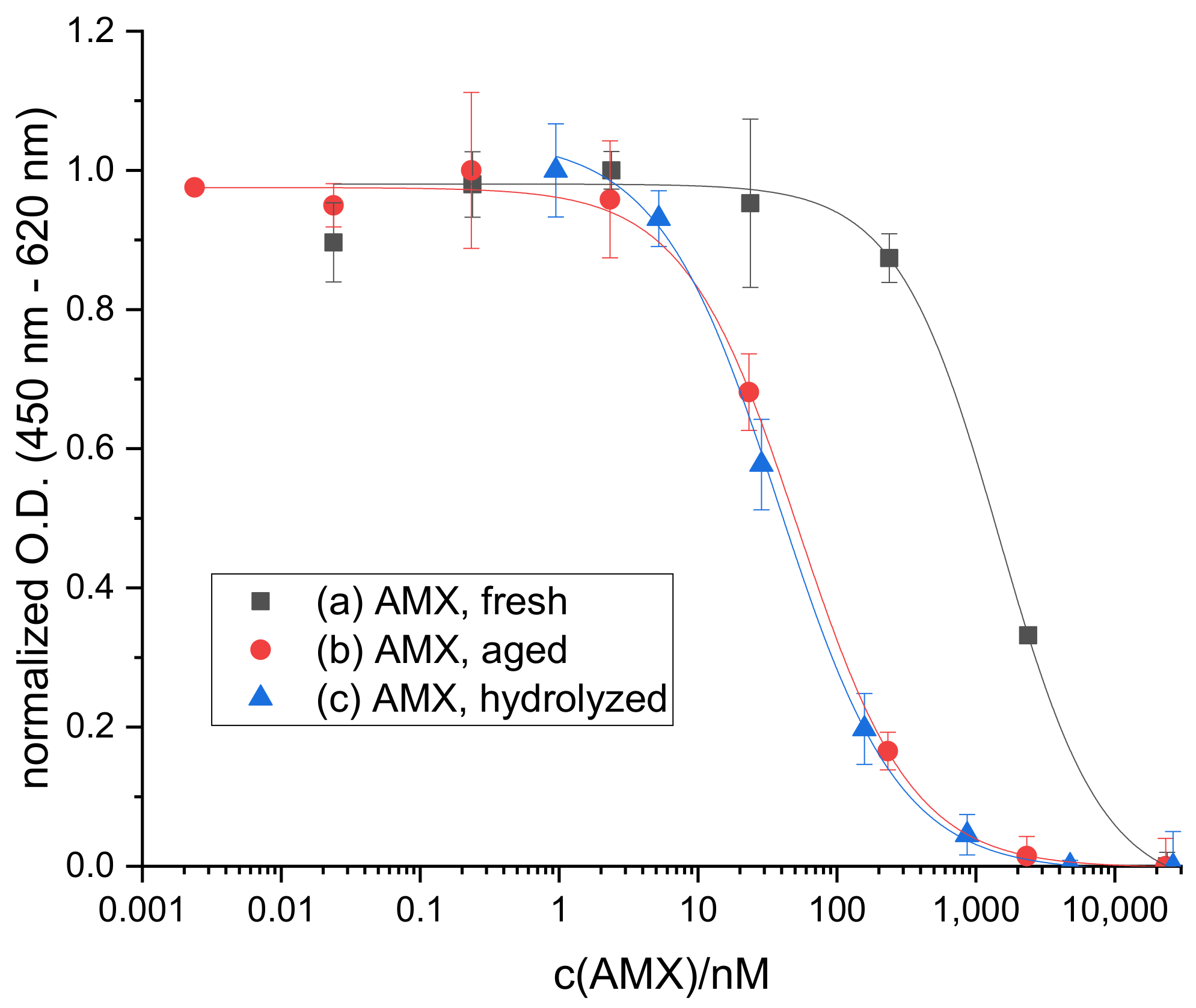
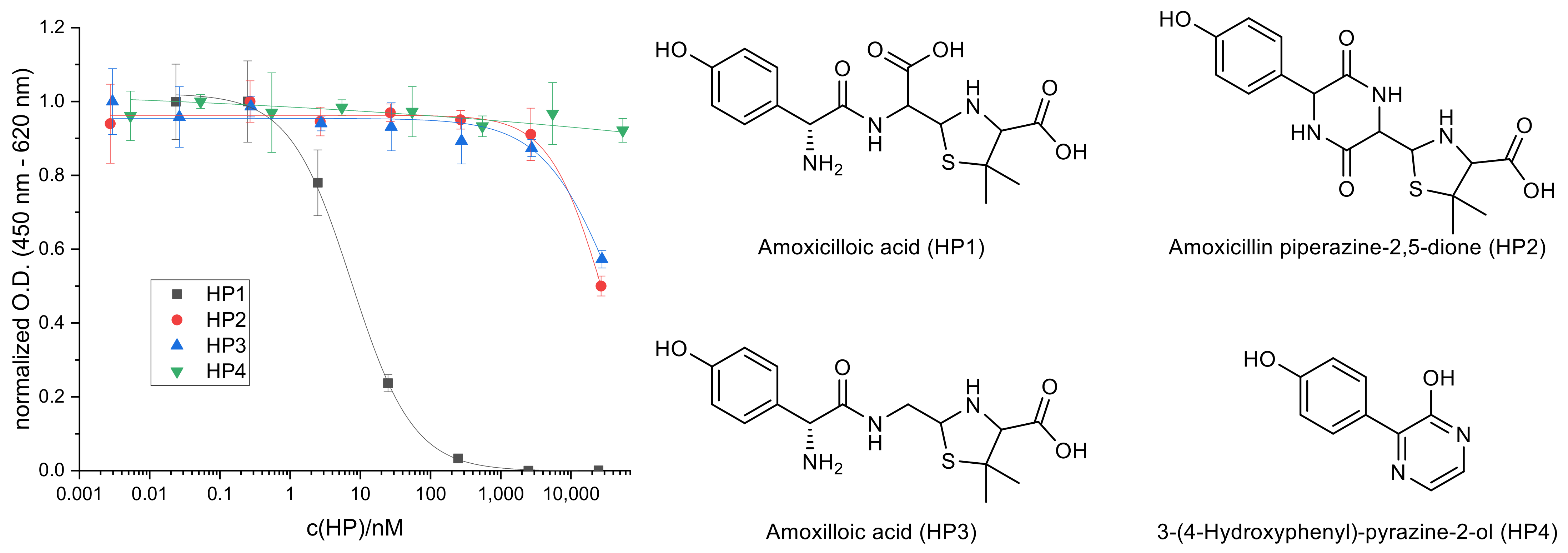
2.1. Hydrolysis of AMX
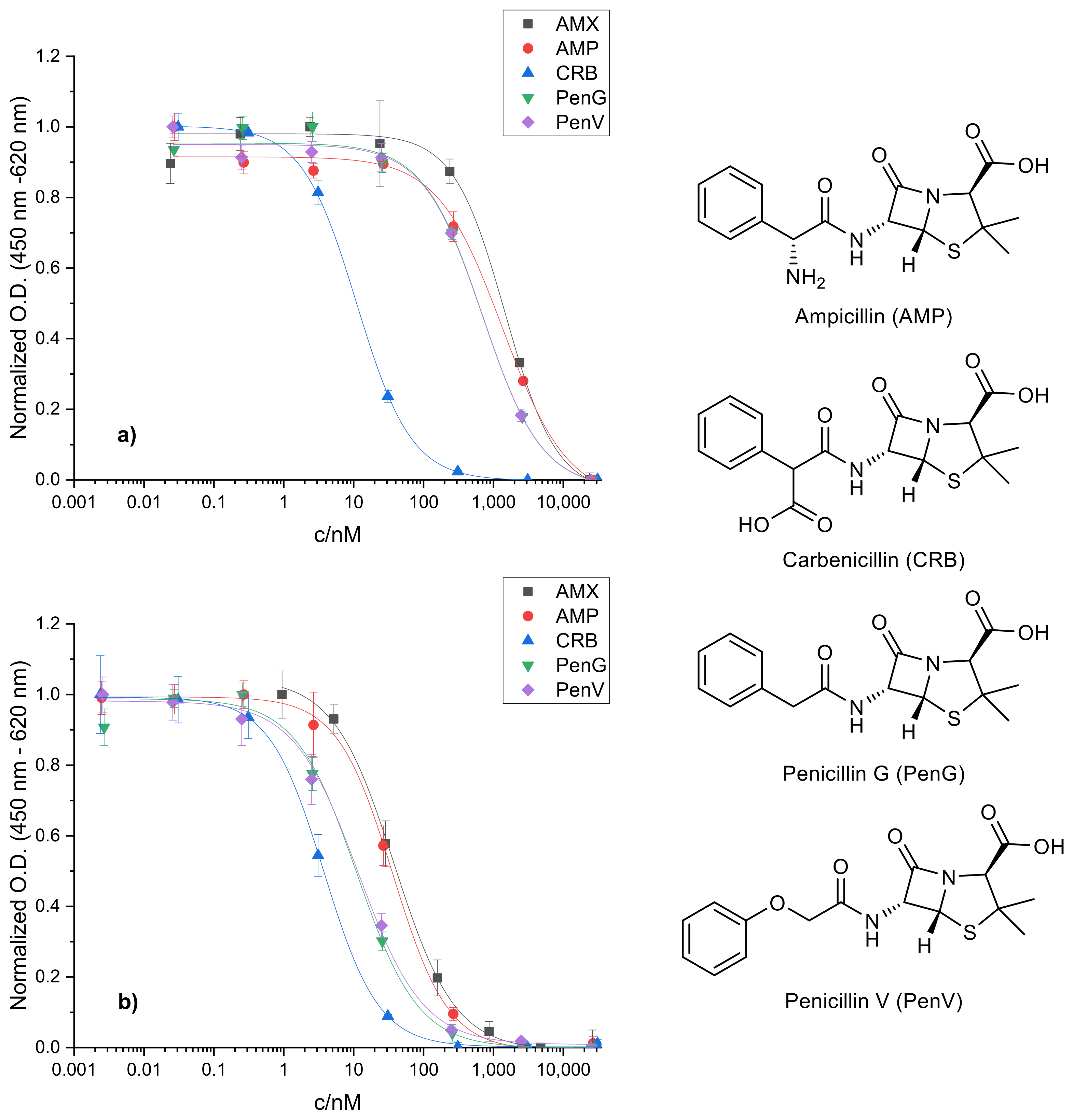
2.2. Cross-Reactivity of Related Penicillins
2.3. Sample Analysis
3. Materials and Methods
3.1. Chemicals
3.2. Materials and Equipment
3.3. Buffers
- Phosphate-buffered saline (PBS), pH 7.6: 10 mM sodium phosphate monobasic dihydrate, 70 mM sodium phosphate dibasic dihydrate, 145 mM sodium chloride.
- Washing buffer 60×, pH 7.6: 45 mM potassium phosphate monobasic, 375 mM potassium phosphate dibasic, 1.5 mM potassium sorbate, 3% Tween 20.
- Tris-buffered saline (Tris), pH 8.5: 10 mM tris(hydroxymethyl)aminomethane, 150 mM sodium chloride.
- Sample buffer, pH 7.6 or pH 8.5: 125 mM tris(hydroxymethyl)aminomethane, 187.5 mM sodium chloride, 13.375 mM ethylenediaminetetraacetic acid disodium salt dihydrate.
- Citrate buffer, pH 4.0, storage at 4 °C: 220 mM sodium citrate monobasic.
- TMB stock solution in N,N-dimethylacetamide, storage at 4 °C under argon: 8 mM tetrabutylammonium borohydride, 40 mM 3,3′,5,5′-tetramethylbenzidine (TMB).
3.4. Standards and Samples
3.5. Immunoassay Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ternes, T.A. Occurrence of drugs in German sewage treatment plants and rivers. Water Res. 1998, 32, 3245–3260. [Google Scholar] [CrossRef]
- Heberer, T. Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: A review of recent research data. Toxicol. Lett. 2002, 131, 5–17. [Google Scholar] [CrossRef]
- Cacace, D.; Fatta-Kassinos, D.; Manaia, C.M.; Cytryn, E.; Kreuzinger, N.; Rizzo, L.; Karaolia, P.; Schwartz, T.; Alexander, J.; Merlin, C.; et al. Antibiotic resistance genes in treated wastewater and in the receiving water bodies: A pan-European survey of urban settings. Water Res. 2019, 162, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Amador, P.; Fernandes, R.; Prudêncio, C.; Duarte, I. Prevalence of Antibiotic Resistance Genes in Multidrug-Resistant Enterobacteriaceae on Portuguese Livestock Manure. Antibiotics 2019, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- Müller, H.; Sib, E.; Gajdiss, M.; Klanke, U.; Lenz-Plet, F.; Barabasch, V.; Albert, C.; Schallenberg, A.; Timm, C.; Zacharias, N.; et al. Dissemination of multi-resistant Gram-negative bacteria into German wastewater and surface waters. FEMS Microbiol. Ecol. 2018, 94. [Google Scholar] [CrossRef]
- Varela, A.R.; André, S.; Nunes, O.C.; Manaia, C.M. Insights into the relationship between antimicrobial residues and bacterial populations in a hospital-urban wastewater treatment plant system. Water Res. 2014, 54, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.C.; Shaw, H.; Rhodes, V.; Hart, A. Review of Antimicrobial Resistance in the Environment and Its Relevance to Environmental Regulators. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef]
- Kraemer, S.A.; Ramachandran, A.; Perron, G.G. Antibiotic Pollution in the Environment: From Microbial Ecology to Public Policy. Microorganisms 2019, 7, 180. [Google Scholar] [CrossRef] [PubMed]
- Davey, P.; Ferech, M.; Ansari, F.; Muller, A.; Goossens, H. Outpatient antibiotic use in the four administrations of the UK: Cross-sectional and longitudinal analysis. J. Antimicrob. Chemother. 2008, 62, 1441–1447. [Google Scholar] [CrossRef]
- Fuentes, A.V.; Pineda, M.D.; Venkata, K.C.N. Comprehension of Top 200 Prescribed Drugs in the US as a Resource for Pharmacy Teaching, Training and Practice. Pharmacy 2018, 6, 43. [Google Scholar] [CrossRef]
- The Top 200 Drugs of 2020. Available online: https://clincalc.com/DrugStats/ (accessed on 10 February 2020).
- Center for Financing, Access and Cost Trends, Agency for Healthcare Research and Quality, Medical Expenditure Panel Survey, 1996–2017. Available online: https://www.meps.ahrq.gov/mepstrends/hc_pmed/ (accessed on 10 February 2020).
- Voigt, A.M.; Faerber, H.A.; Wilbring, G.; Skutlarek, D.; Felder, C.; Mahn, R.; Wolf, D.; Brossart, P.; Hornung, T.; Engelhart, S.; et al. The occurrence of antimicrobial substances in toilet, sink and shower drainpipes of clinical units: A neglected source of antibiotic residues. Int. J. Hyg. Environ. Health 2019, 222, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Andreozzi, R.; Caprio, V.; Ciniglia, C.; de Champdoré, M.; Lo Giudice, R.; Marotta, R.; Zuccato, E. Antibiotics in the Environment: Occurrence in Italian STPs, Fate, and Preliminary Assessment on Algal Toxicity of Amoxicillin. Environ. Sci. Technol. 2004, 38, 6832–6838. [Google Scholar] [CrossRef]
- Watkinson, A.J.; Murby, E.J.; Kolpin, D.W.; Costanzo, S.D. The occurrence of antibiotics in an urban watershed: From wastewater to drinking water. Sci. Total Environ. 2009, 407, 2711–2723. [Google Scholar] [CrossRef] [PubMed]
- Balakrishna, K.; Rath, A.; Praveenkumarreddy, Y.; Guruge, K.S.; Subedi, B. A review of the occurrence of pharmaceuticals and personal care products in Indian water bodies. Ecotoxicol. Environ. Saf. 2017, 137, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Christian, T.; Schneider, R.J.; Färber, H.A.; Skutlarek, D.; Meyer, M.T.; Goldbach, H.E. Determination of Antibiotic Residues in Manure, Soil, and Surface Waters. Acta Hydrochim. Hydrobiol. 2003, 31, 36–44. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. The occurrence of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs in surface water in South Wales, UK. Water Res. 2008, 42, 3498–3518. [Google Scholar] [CrossRef] [PubMed]
- Homem, V.; Alves, A.; Santos, L. Development and Validation of a Fast Procedure To Analyze Amoxicillin in River Waters by Direct-Injection LC–MS/MS. J. Chem. Educ. 2014, 91, 1961–1965. [Google Scholar] [CrossRef]
- Locatelli, M.A.F.; Sodré, F.F.; Jardim, W.F. Determination of Antibiotics in Brazilian Surface Waters Using Liquid Chromatography–Electrospray Tandem Mass Spectrometry. Arch. Environ. Contam. Toxicol. 2011, 60, 385–393. [Google Scholar] [CrossRef]
- Elizalde-Velázquez, A.; Gómez-Oliván, L.M.; Galar-Martínez, M.; Islas-Flores, H.; Dublán-García, O.; SanJuan-Reyes, N. Amoxicillin in the aquatic environment, its fate and environmental risk. In Environmental Health Risk—Hazardous Factors to Living Species; Larramendy, M.L., Soloneski, S., Eds.; IntechOpen: London, UK, 2016; pp. 247–267. [Google Scholar] [CrossRef]
- Hirte, K.; Seiwert, B.; Schüürmann, G.; Reemtsma, T. New hydrolysis products of the beta-lactam antibiotic amoxicillin, their pH-dependent formation and search in municipal wastewater. Water Res. 2016, 88, 880–888. [Google Scholar] [CrossRef]
- Deshpande, A.D.; Baheti, K.G.; Chatterjee, N.R. Degradation of β-lactam antibiotics. Curr. Sci. 2004, 87, 1684–1695. [Google Scholar] [CrossRef]
- Gozlan, I.; Rotstein, A.; Avisar, D. Amoxicillin-degradation products formed under controlled environmental conditions: Identification and determination in the aquatic environment. Chemosphere 2013, 91, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Nägele, E.; Moritz, R. Structure Elucidation of Degradation Products of the Antibiotic Amoxicillin with Ion Trap MSn and Accurate Mass Determination by ESI TOF. J. Am. Soc. Mass Spectrom. 2005, 16, 1670–1676. [Google Scholar] [CrossRef]
- Pérez-Parada, A.; Agüera, A.; Gómez-Ramos, M.D.M.; García-Reyes, J.F.; Heinzen, H.; Fernández-Alba, A.R. Behavior of amoxicillin in wastewater and river water: Identification of its main transformation products by liquid chromatography/electrospray quadrupole time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2011, 25, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Han, H.; Yin, H.; Han, L.; Huang, G. Variations in the fate and risk analysis of amoxicillin and its degradation products during pig manure aerobic composting. J. Hazard. Mater. 2018, 346, 234–241. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Implementing Decision (EU) 2018/840 of 5 June 2018 establishing a watch list for Union-wide monitoring in the field of water policy pursuant to Directive 2008/105/EC of the European Parliament and of the Council and repealing Commission Implementing Decision (EU) 2015/495. Off. J. Eur. Union 2018, L141, 9–12. [Google Scholar]
- Farré, M.; Ferrer, I.; Ginebreda, A.; Figueras, M.; Olivella, L.; Tirapu, L.; Vilanova, M.; Barceló, D. Determination of drugs in surface water and wastewater samples by liquid chromatography–mass spectrometry: Methods and preliminary results including toxicity studies with Vibrio fischeri. J. Chromatogr. A 2001, 938, 187–197. [Google Scholar] [CrossRef]
- Ahrer, W.; Scherwenk, E.; Buchberger, W. Determination of drug residues in water by the combination of liquid chromatography or capillary electrophoresis with electrospray mass spectrometry. J. Chromatogr. A 2001, 910, 69–78. [Google Scholar] [CrossRef]
- Berset, J.-D.; Brenneisen, R.; Mathieu, C. Analysis of llicit and illicit drugs in waste, surface and lake water samples using large volume direct injection high performance liquid chromatography—Electrospray tandem mass spectrometry (HPLC–MS/MS). Chemosphere 2010, 81, 859–866. [Google Scholar] [CrossRef] [PubMed]
- González-Mariño, I.; Quintana, J.B.; Rodríguez, I.; Cela, R. Determination of drugs of abuse in water by solid-phase extraction, derivatisation and gas chromatography–ion trap-tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 1748–1760. [Google Scholar] [CrossRef] [PubMed]
- Gros, M.; Petrović, M.; Barceló, D. Development of a multi-residue analytical methodology based on liquid chromatography–tandem mass spectrometry (LC–MS/MS) for screening and trace level determination of pharmaceuticals in surface and wastewaters. Talanta 2006, 70, 678–690. [Google Scholar] [CrossRef]
- Hill, S.L.; Burnett, D.; Lovering, A.L.; Stockley, R.A. Use of an enzyme-linked immunosorbent assay to assess penetration of amoxicillin into lung secretions. Antimicrob. Agents Chemother. 1992, 36, 1545. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mayorga, C.; Obispo, T.; Jimeno, L.; Blanca, M.; Moscoso Del Prado, J.; Carreira, J.; Garcia, J.J.; Juarez, C. Epitope mapping of β-lactam antibiotics with the use of monoclonal antibodies. Toxicology 1995, 97, 225–234. [Google Scholar] [CrossRef]
- Yeh, L.-C.; Lee, W.-M.; Koh, B.-W.; Chan, J.P.; Liu, C.-H.; Kao, J.-P.; Chou, C.-C. Development of Amoxicillin Enzyme-Linked Immunosorbent Assay and Measurements of Tissue Amoxicillin Concentrations in a Pigeon Microdialysis Model. Poult. Sci. 2008, 87, 577–587. [Google Scholar] [CrossRef]
- Grubelnik, A.; Padeste, C.; Tiefenauer, L. Highly Sensitive Enzyme Immunoassays for the Detection of β-Lactam Antibiotics. Food Agric. Immunol. 2001, 13, 161–169. [Google Scholar] [CrossRef]
- Komova, N.S.; Berlina, A.N.; Zherdev, A.V.; Dzantiev, B.B. Immune Recognition of Closed and Open Lactam Rings and Their Influence on Immunoassays of Ampicillin Antibiotics. Orient. J. Chem. 2020, 36, 21–25. [Google Scholar] [CrossRef]
- Nagakura, N.; Souma, S.; Shimizu, T.; Yanagihara, Y. Anti-ampicillin monoclonal antibodies and their cross-reactivities to various β-lactams. J. Antimicrob. Chemoth. 1991, 28, 357–368. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Y.; Wang, S. Development of an Enzyme-Linked Immunosorbent Assay To Detect Benzylpenicilloic Acid, a Degradation Product of Penicillin G in Adulterated Milk. J. Agric. Food Chem. 2010, 58, 8171–8175. [Google Scholar] [CrossRef]
- Ekins, R.P. The precision profile: Its use in assay design, assessment and quality control. In Immunoassays for Clinical Chemistry, 2nd ed.; Hunter, W.M., Corrie, J.E.T., Eds.; Churchill Livingstone: Edinburgh, UK, 1983; pp. 76–105. [Google Scholar]
- Gensmantel, N.P.; Proctor, P.; Page, M.I. Metal-ion catalysed hydrolysis of some β-lactam antibiotics. J. Chem. Soc. Perkin Trans. 1980, 2, 1725–1732. [Google Scholar] [CrossRef]
- Munro, A.C.; Chainey, M.G.; Woroniecki, S.R. Preparation and immunological cross-reactions of penicilloic and penilloic acids. J. Pharm. Sci. 1978, 67, 1197–1204. [Google Scholar] [CrossRef]
- Bundgaard, H.; Larsen, C. Piperazinedione formation from reaction of ampicillin with carbohydrates and alcohols in aqueous solution. Int. J. Pharm. 1979, 3, 1–11. [Google Scholar] [CrossRef]
- Frenzel, A.; Hust, M.; Schirrmann, T. Expression of Recombinant Antibodies. Front. Immunol. 2013, 4. [Google Scholar] [CrossRef] [PubMed]


| Sample | c(AMX)/nM | Recovery | |
|---|---|---|---|
| Spiked | Determined | ||
| P1 | 197 | 219 ± 8 | 111% |
| P2 | 26.2 | 16.3 ± 2.6 | 62% |
| P3 | 472 | 606 ± 41 | 128% |
| P4 | 94.5 | 105 ± 9 | 112% |
| P5 | 15.8 | 11.0 ± 5.6 | 70% |
| P6 | 115 | 103 ± 11 | 89% |
| P7 | 320 | 301 ± 13 | 94% |
| P8 | 226 | 189 ± 7 | 84% |
| P9 | 0 | 0 | 100% |
| P10 | 39.4 | 26.0 ± 8.1 | 66% |
| P11 | 415 | 422 ± 24 | 102% |
| P12 | 163 | 134 ± 16 | 82% |
| P13 | 155 | 106 ± 12 | 68% |
| P14 | 5.25 | 0 | 0% |
| P15 | 68.2 | 26.1 ± 14.2 | 38% |
| P16 | 378 | 268 ± 12 | 71% |
| P17 | 919 | 925 ± 93 | 101% |
| P18 | 52.5 | 32.9 ± 8.0 | 63% |
| P19 | 13.1 | 5.14 ± 4.8 | 39% |
| P20 | 186 | 173 ± 13 | 93% |
| P21 | 0 | 0 | 100% |
| P22 | 262 | 206 ± 48 | 78% |
| P23 | 84.0 | 41.0 ± 5.6 | 49% |
| P24 | 18.4 | 0 | 0% |
| Sample | c(AMX) Determined/nM | +/- | |
|---|---|---|---|
| With Hydrolysis | Without Hydrolysis | ||
| P1 | 219 | 333 | + |
| P2 | 16.3 | 28.3 | + |
| P3 | 606 | 1430 | + |
| P4 | 105 | 169 | + |
| P5 | 11.0 | 0 | - |
| P6 | 103 | 2.81 | - |
| P7 | 301 | 12.6 | - |
| P8 | 189 | 6.33 | - |
| P9 | 0 | 0 | 0 |
| P10 | 26.0 | 0 | - |
| P11 | 422 | 10.2 | - |
| P12 | 134 | 2.71 | - |
| P13 | 106 | 0 | - |
| P14 | 0 | 0 | 0 |
| P15 | 26.1 | 0 | - |
| P16 | 268 | 13.3 | - |
| P17 | 925 | 31.3 | - |
| P18 | 32.9 | 0 | - |
| P19 | 5.14 | 0 | - |
| P20 | 173 | 5.56 | - |
| P21 | 0 | 0 | 0 |
| P22 | 206 | 4.38 | - |
| P23 | 41.0 | 0 | - |
| P24 | 0 | 2.85 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ecke, A.; Schneider, R.J. Pitfalls in the Immunochemical Determination of β-Lactam Antibiotics in Water. Antibiotics 2021, 10, 298. https://doi.org/10.3390/antibiotics10030298
Ecke A, Schneider RJ. Pitfalls in the Immunochemical Determination of β-Lactam Antibiotics in Water. Antibiotics. 2021; 10(3):298. https://doi.org/10.3390/antibiotics10030298
Chicago/Turabian StyleEcke, Alexander, and Rudolf J. Schneider. 2021. "Pitfalls in the Immunochemical Determination of β-Lactam Antibiotics in Water" Antibiotics 10, no. 3: 298. https://doi.org/10.3390/antibiotics10030298
APA StyleEcke, A., & Schneider, R. J. (2021). Pitfalls in the Immunochemical Determination of β-Lactam Antibiotics in Water. Antibiotics, 10(3), 298. https://doi.org/10.3390/antibiotics10030298







